Experimental and In Silico Evaluation of New Heteroaryl Benzothiazole Derivatives as Antimicrobial Agents
Abstract
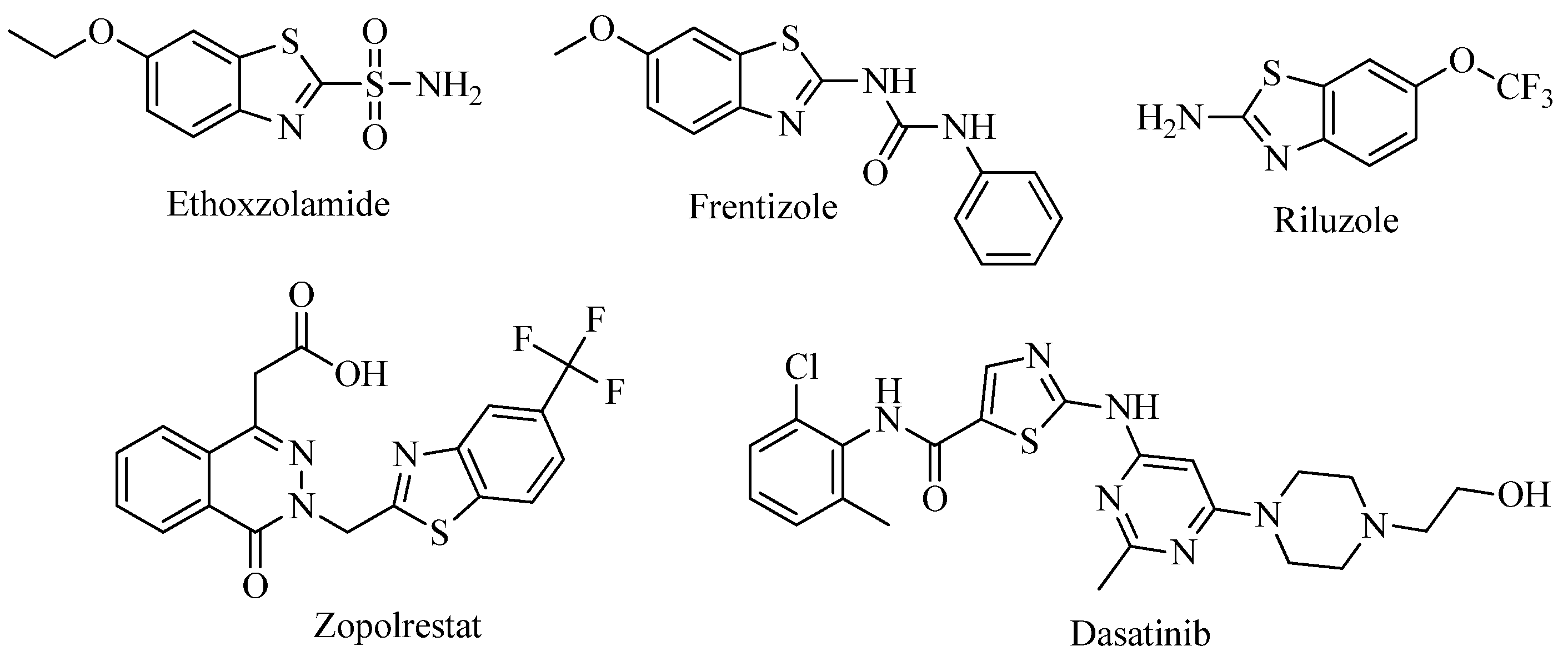
:1. Introduction
2. Results and Discussion
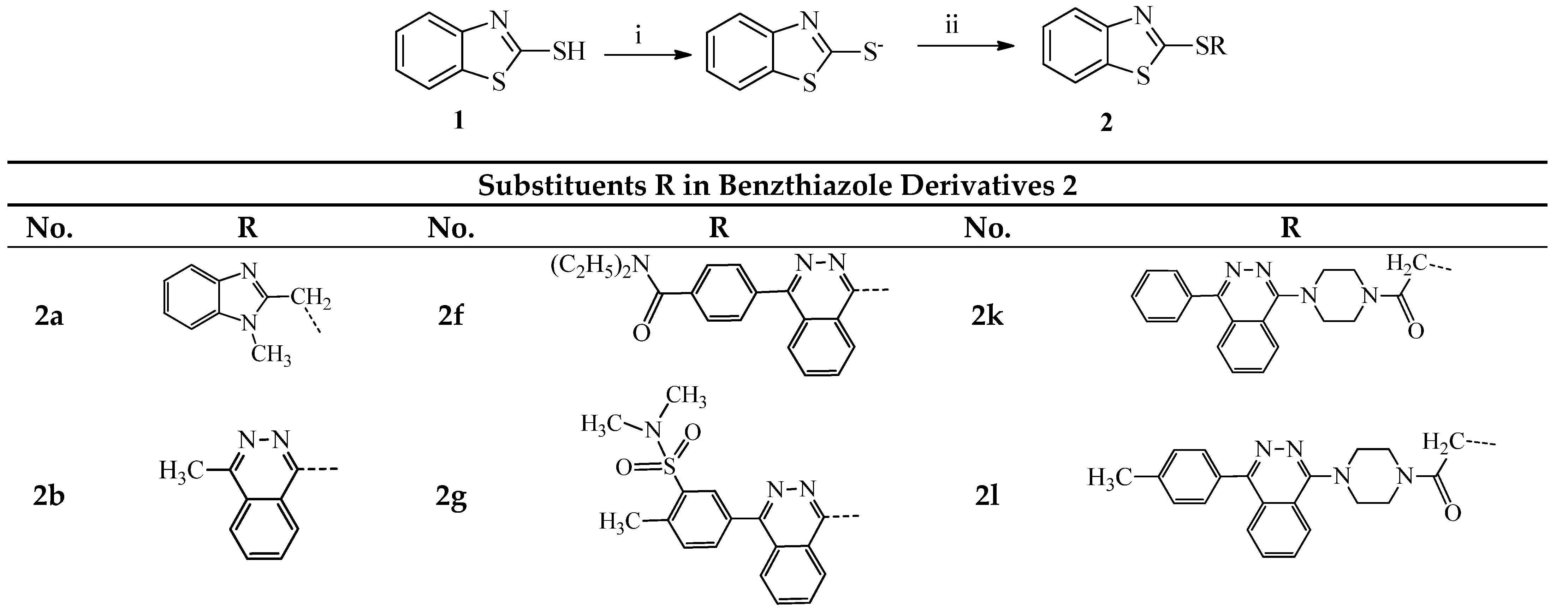
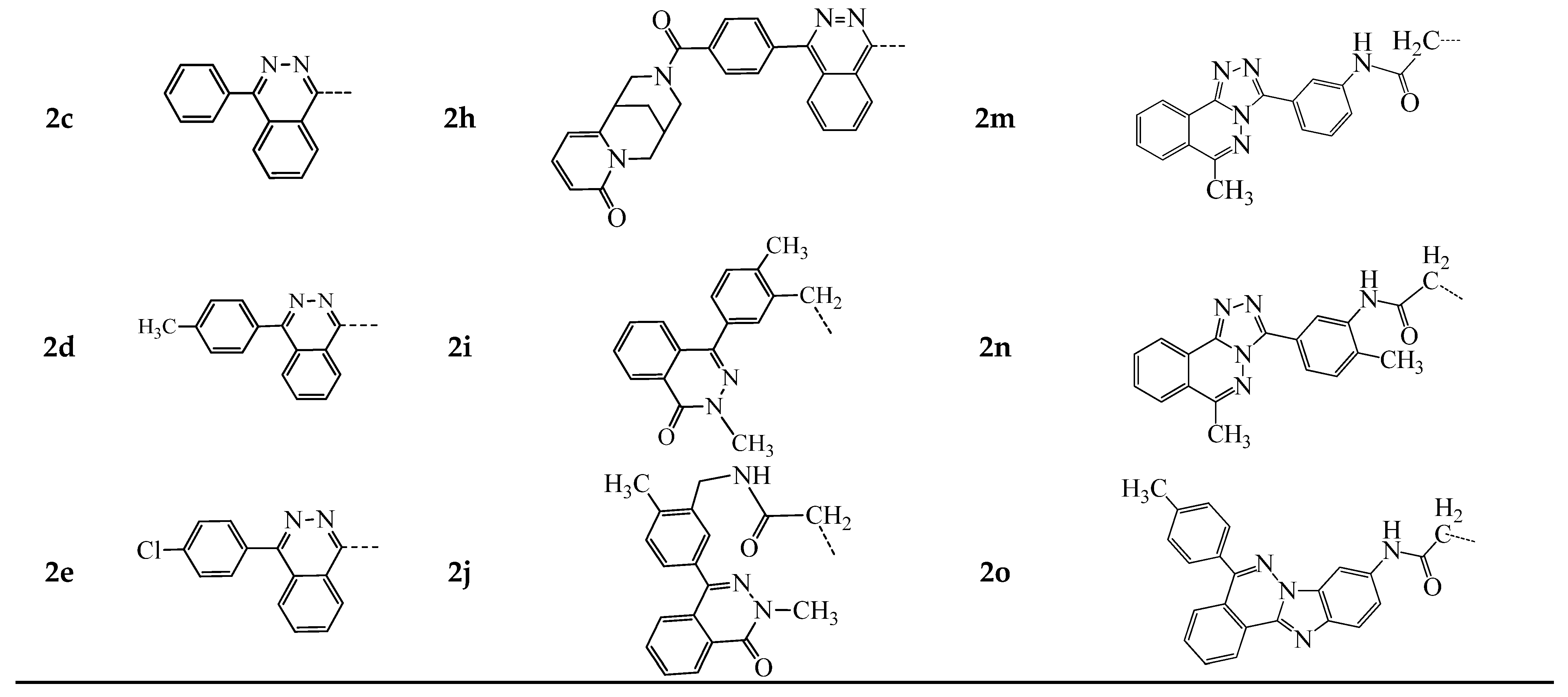
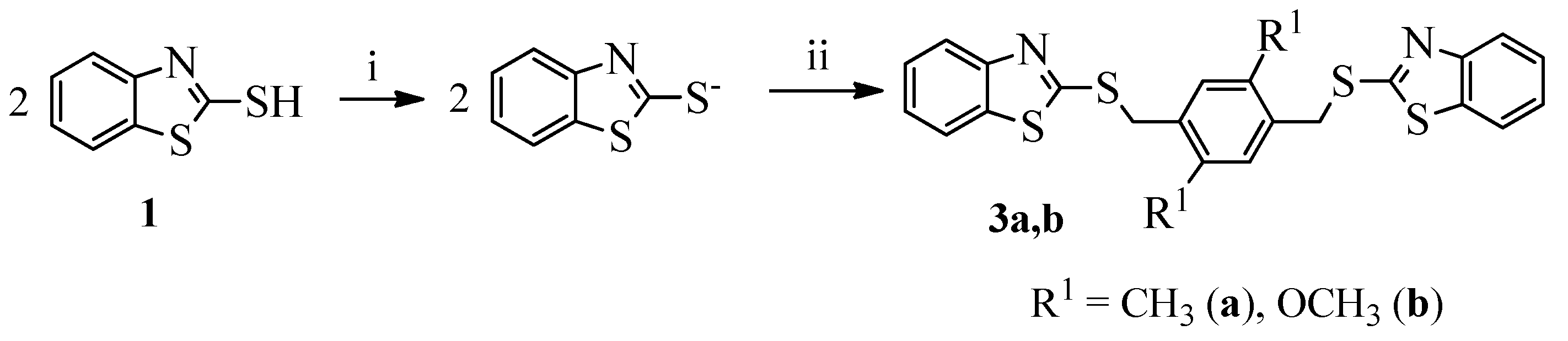
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Antibacterial Activity
2.2.2. Antifungal Activity
2.3. In Silico Studies—Molecular Docking
2.3.1. In Silico Studies to Antibacterial Targets
2.3.2. In Silico Studies to Antifungal Targets
2.4. Drug Likeness
3. Materials and Methods
3.1. Chemistry-General Information
3.1.1. General Procedure for the Synthesis of Compounds 2a–o and 3a,b
- 2-{[(1-Methyl-1H-benzo[d]imidazol-2-yl)methyl]thio}benzo[d]thiazole (2a). Yield 2.99 g (96%), colorless crystals, m.p. 90–92 °C (EtOAc). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 3.93 (s, 3H, Me), 4.98 (s, 2H, CH2), 7.14–7.25 (m, 2H, H-5′, H-6′), 7.29–7.48 (m, 3H, H-5, H-6, H-4′), 7.58 (d, J 7.6, 1H, H-7′), 7.85–7.88 (m, 2H, H-4, H-7). 13C NMR (100 MHz, DMSO-d6) δ 165.85 (S-C-S), 152.89 (C-12), 150.16 (C-4), 142.28 (C-15), 136.36 (C-14), 135.32 (C-5), 126.89 (C-8), 125.10 (C-7), 122.80 (C-18), 122.38 (C-19), 122.18 (C-6), 121.68 (C-9), 119.21 (C-17), 110.65 (C-20), 30.56 (CH3), 29.46. Found (%): C, 61.45; H, 4.00; N, 13.16; S, 20.72. Calc. for C16H13N3S2 (%): C, 61.71; H, 4.21; N, 13.49; S, 20.59.
- 2-(4-Methylphthalazin-1-yl)benzo[d]thiazole (2b). Yield 2.47 g (89%), colorless crystals, m.p. 163–165 °C (EtOH). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.99 (s, 3H, Me), 7.35–7.56 (m, 2H, H-5, H-6), 8.22–8.38 (m, 2H, H-6′), 8.02–8.15 (m, 3H, H-5′, H-7′, H-8′), 8.22–8.38 (m, 2H, H-4, H-7). 13C NMR (100 MHz, DMSO-d6) δ 161.14, 159.82, 155.43, 152.07 (C-4), 139.85 (C-10), 136.05 (C-5), 134.74 (C-16), 134.33 (C-17), 132.81 (C-14), 129.65 (C-15), 127.51 (C-8), 126.19 (C-19), 125.24 (C-7), 124.48 (C-18), 122.39 (2C, C-6, C-9), 30.24 (CH3). Found (%): C, 68.98; H, 3.68; N, 15.00; S, 11.72. Calc. for C16H11N3S (%): C, 69.29; H, 4.00; N, 15.15; S, 11.56.
- 2-(4-Phenylphthalazin-1-yl)benzo[d]thiazole (2c). Yield 3.12 g (92%), colorless crystals, m.p. 214–216 °C (methycellosolve). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 7.39–7.59 (m, 2H, H-5, H-6), 7.60–7.85 (m, 5H, H″–H-6″), 7.88–8.20, (m, 2H, 8.22–8.38 (m, 5H, H-7, H-5′–H-8′), 8.35–8.46 (m, 1H, H-4). 13C NMR (100 MHz, DMSO-d6) δ 161.10 (N=C-S), 159.83 (C-13), 155.32 (C-4), 152.01 (C-10), 139.82 (C-20), 136.03 (C-5), 134.72 (C-14), 134.33 (C-16), 132.81 (C-21), 129.60 (2C, C-25, C-6), 127.52 (C-23), 126.21 (2C, C-22, C-24), 125.23 (C-17), 331 124.29 (2C, C-7, C-8), 122.38 (2C, C-9, C-19), 121.79 (2C, C-15, C-18). Found (%): C, 74.10; H, 3.61; N, 12.11; S, 9.72. Calc. for C21H13N3S (%): C, 74.31; H, 3.86; N, 12.38; S, 9.45.
- 2-(4-(p-Tolyl)phthalazin-1-yl)benzo[d]thiazole (2d). Yield 3.11 g (88%), colorless crystals, m.p. 202–205 °C (DMF:EtOAc). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.91 (s, 6H, Me, H2O), 7.36–7.51 (m, 4H, H-2″, H-3″, H-5″, H-6″), 7.65 (d, 2H, H-5, H-6), 7.91–8.16 (m, 5H, H-5′–H-8′, H-7), 8.31–8.42 (m, 1H, H-4). 13C NMR (100 MHz, DMSO-d6) δ 167.82 (N-C-S), 159.10 (S-C=N), 158.86 (C-14), 148.14 (C-5), 144.38 (2C, C-13, C-21), 134.09 (2C, C-4, C-24), 132.54 (C-19), 130.07 (2C, C-22, C-26), 127.22 (3C, C-1, C-2, C-3), 126.57 (3C, C-12, C-23, C-25), 124.80 (2C, C-17, C-18), 123.10 (C-6), 116.08 (C-20), 18.87 (CH3). Found (%):C, 74.49; H, 4.00; N, 11.48; S, 9.29.Calc. for C22H15N3S (%): C, 74.76; H, 4.28; N, 11.89; S, 9.07.
- 2-[4-(4-Chlorophenyl)phthalazin-1-yl]benzo[d]thiazole (2e). Yield 3.14 g (84%), colorless crystals, m.p. 195–196 °C (methycellosolve). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 7.42 (td, J 7.5, 1.4, 1H, H-6), 7.50 (td, J 7.5, 1.4, 1H, H-5), 7.63–7.68 (m, 2H, H-3″, H-5″), 7.79 (d, J 7.5, 2H, H-5′, H-8′), 7.92–7.97 (m, 1H, H-7′), 8.02 (d, J 7.9, 1H, H-6), 8.03–8.17 (m, 3H, H-2″, H-6″, H-7), 8.40 (d, 1H, J 7.8, H-4). 13C NMR (100 MHz, DMSO-d6) δ 160.75 (N=C-S), 158.84 (C-13), 155.95 (C-4), 152.01 (C-10), 136.09 (C-20), 135.15 (C-5), 134.51 (C-Cl), 132.32 (3C, C-14, C-21, C-25), 129.21(3C, C-16, C-22, C-24), 127.30 (C-17), 127.02 (C-19), 126.01 (C-8), 125.81 (C-7), 125.08 (C-18), 124.45 (C-15), 122.47 (C-6), 122.39 (C-9). Found (%):C, 67.22; H, 3.01; Cl, 9.72; N, 11.00; S, 8.74. Calc. for C21H12ClN3S (%): C, 67.47; H, 3.24; Cl, 9.48; N, 11.24; S, 8.58.
- 4-[4-(Benzo[d]thiazol-2-yl)phthalazin-1-yl]-N,N-diethylbenzamide (2f). Yield 2.81 g (64%), colorless crystals, m.p. 180–181 °C (DMF). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 1.24 (t, J 7.0, 6H, 2CH3), 3.45 (s, 4H, 2CH2), 7.36–7.43 (m, 1H, H-6), 7.44–7.51 (m, 1H, H-5), 7.59 (d, J 7.8, 2H, H-5′, H-8′), 7.83 (d, J 7.9, 2H, H-2″, H-6″), 7.95 (t, J 8.7, 2H, H-6′, H-7′), 8.02–8.19 (m, 3H, H-3″, H-5″, H-7), 8.35–8.42 (m, 1H, H-4). 13C NMR (100 MHz, DMSO-d6) δ 166.63 (C=O), 165.61 (S-C=N), 152.83 (C-14), 142.40 (C-27), 141.68 (C-2), 139.63 (C-17), 136.58 (C-28), 135.21 (C-5), 133.47 (C-23), 132.26 (2C, C-4, C-6), 130.31 (2C, C-3, C-7), 129.58 (C-21), 128.68 (C-18), 127.98 (C-20), 126.84 (C-31), 125.35 (C-19), 124.97 (C-22), 124.20 (C-30), 124.14 (C-29), 122.36 (C-32), 21.35 (2C, CH3). Found (%): C, 71.00; H, 4.82; N, 12.49; S, 7.56. Calc. for C26H22N4OS (%): C, 71.21; H, 5.06; N, 12.78; S, 7.31.
- 5-(4-(Benzo[d]thiazol-2-yl)phthalazin-1-yl)-N,N,2-trimethylbenzenesulfonamide (2g). Yield 3.41 g (74%), colorless crystals, m.p. 163–165 °C (PrOH). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.74 (s, 3H, CH3), 2.83 (s, 6H, N(CH3)2), 7.44 (td, J 7.6, 1.3, 1H, H-6), 7.52 (td, J 7.6, 1.5, 1H, H-5), 7.69 (d, J 7.9, 1H, H-3″), 7.93–7.96 (m, 2H, H-6′, H-7′), 8.02–8.06 (m, 1H, H-4″), 8.09–8.18 (m, 4H, H-4, H-7, H-5′, H-8′), 8.38–8.43 (m, 1H, H-6″. 13C NMR (100 MHz, DMSO-d6) δ 167.20 (N-C-S), 159.42 (S-C=N), 152.60 (C-5), 146.22 (C-14), 137.35 (C-24), 137.12 (C-23), 134.93 (C-4), 133.42 (C-13), 132.63 (C-21), 132.05 (C-25), 130.50 (C-26), 128.92 (C-22), 128.43 (C-12), 128.31 (C-19), 127.66 (C-17), 126.82 (C-18), 126.58 (C-20), 126.42 (C-1), 124.74 (C-2), 121.95 (C-3), 121.02 (C-6), 36.80 (2C, N-(CH3)2), 19.88 (C-CH3). Found (%): C, 62.32; H, 4.12; N, 12.00; S, 14.26. Calc. for C24H20N4O2S2 (%): C, 62.59; H, 4.38; N, 12.16; S, 13.92.
- 3-{4-[4-(Benzo[d]thiazol-2-ylthio)phthalazin-1-yl]benzoyl}-1,2,3,4,5,6-hexahydro-8H-1,5-methanopyrido[1,2-a][1,5]diazocin-8-one (2h). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.01–2.14 (m, 2H, H-5), 2.50–2.57 (m, 1H, H-6, DMSO), 3.00 (s, 2H, H-7), 3.15–3.41 (m, 3H, H-4, H-1), 3.68–3.75 (m, 1H, H-2), 4.05–4.11 (m, 1H, H-2), 6.05 (d, 1H, H-11), 6.33 (dd, J 9.1, 1.3, 1H, H-9), 7.03–7.37 (m, 3H, H-5′, H-6′, H-10), 7.40–7.54 (m, 2H, H-6″, H-7″), 7.72 (d, J 7.8, 2H, H-5″, H-8″), 7.95 (d, J 8.0, 1H, H-5‴), 8.03 (d, J 7.9, 1H, H-3‴), 8.07–8.16 (m, 3H, H-4′, H-2‴, H-6‴), 8.40–8.42 (m, 1H, H-10). 13C NMR (100 MHz, DMSO-d6) δ 175.96 (2C, C=O, C-2), 156.54 (C=O), 153.10 (C-7), 147.82 (2C, C-14, C-19), 144.93 (C-4), 140.62 (C-27), 136.71 (C-24), 134.79 (C-21), 134.54 (C-26), 130.06 (3C, C-15, C-33, C-34), 129.18 (3C, C-31, C-32, C-39), 127.77 (2C, C-35, C-40), 126.52 (2C, C-17, C-36), 125.29 (2C, C-41, C-42), 122.75 (2C, C-28, C-38), 122.61 (C-37), 109.99 (C-25), 56.70 (C-22), 53.17 (2C, C-16, C-20), 40.65 (C-8), 32.37 (C-18), 21.58 (C-23). Found (%): C, 67.12; H, 4.20; N, 11.69; S, 11.22. Calc. for C33H25N5O2S2 (%): C, 67.44; H, 4.29; N, 11.92; S, 10.91.
- 4-[3-(Benzo[d]thiazol-2-ylthio)-4-methylphenyl]-2-methylphthalazin-1(2H)-one (2i). Yield 3.82 g (89%), colorless crystals, m.p. 111–112 °C (DMF). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.57 (s, 3H, CH3), 3.79 (s, 3H, NCH3), 4.74 (s, 1H, CH2), 7.30–7.44 (m, 4H, H-5′, H-5″, H-6″, H-8), 7.50–7.61 (m, 2H, H-6′, H-7), 7.67 (d, J 7.9, 1H, H-2′), 7.71–7.82 (m, 2H, H-6, H-7′), 7.83–7.88 (m, 1H, H-9), 8.36 (d, J 7.9, 1H, H-7″). 13C NMR (100 MHz, DMSO-d6) δ 166.13 (C-1), 158.62 (C=O), 153.01 (C-5), 145.86 (C-18), 138.40 (C-13), 135.19 (C-6), 134.94 (C-16), 133.56 (C-12), 132.91 (C-14), 132.18 (C-28), 131.14 (C-17), 131.10 (C-27), 129.29 (C-20), 128.90 (C-29), 127.75 (C-19), 126.86 (C-26), 126.73 (C-9), 126.71 (C-15), 125.08 (C-10), 122.31 (C-8), 121.69 (C-7), 39.50 (C-25), 35.33 (C-11), 19.23 (CH3). Found (%): C, 66.72; H, 4.18; N, 9.53; S, 15.27. Calc. for C24H19N3OS2 (%): C, 67.11; H, 4.46; N, 9.78; S, 14.93.
- 2-(Benzo[d]thiazol-2-ylthio)-N-[2-methyl-5-(3-methyl-4-oxo-3,4-dihydrophthalazin-1-yl)benzyl]acetamide (2j). Yield 4.09 g (84%), colorless crystals, m.p. 201–202 °C (DMF). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.41 (s, 3H, CH3), 3.77 (s, 3H, NCH3), 4.10 (s, 2H, SCH2), 4.43 (d, J 5.7, 2H, NCH2) 7.21–7.32 (m, 4H, H-5, H-6, H-3′, H-6″), 7.46 (d, J 1.7, H-7‴), 7.59–7.69 (m, 2H, H-7, H-6′), 7.72–7.77 (m, 3H, H-4, H-4, H-8″), 8.27–8.37 (m, 1H, H-5″), 8.56 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δ 167.17 (S-C-N), 166.46 (C=O), 158.55 (C=O), 152.66 (C-23), 146.23 (C-25), 137.36 (C-5), 137.00 (C-11), 134.95 (C-4), 133.42 (C-24), 132.65 (C-16), 132.07 (C-18), 130.51 (C-19), 128.95 (C-17), 128.42(C-3), 128.29 (C-2), 127.64 (C-30), 126.85 (C-6), 126.56 (C-31), 126.40 (C-33), 124.73 (C-32), 121.98 (C-20), 121.04 (C-21), 41.24 (CH2-NH), 36.81 (2C, N-CH3), 18.98 (C-CH3). Found (%): C, 64.00; H, 4.22; N, 11.18; S, 13.54. Calc. for C26H22N4O2S2 (%): C, 64.18; H, 4.56; N, 11.51; S, 13.18.
- 2-(Benzo[d]thiazol-2-ylthio)-1-[4-(4-phenylphthalazin-1-yl)piperazin-1-yl]ethan-1-one (2k). Yield 3.68 g (74%), colorless crystals, m.p. 162–163 °C (EtOH). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 3.53 (s, 2H, 2H-2′), 3.65 (s, 2H, 2H-6′), 3.88 (s, 2H, H-3′), 3.97 (s, 2H, H-5′), 4.57 (s, 2H, SCH2), 7.31 (t, J 7.6, H-4‴), 7.42 (t, J 7.6, H-6), 7.51–7.60 (m, 3H, H-5, H-3‴, H-5‴), 7.62–7.69 (m, 2H, H-6″, H-7″), 7.59–7.69 (m, 2H, H-7, H-6′), 7.99–7.80 (m, 5H, H-7, H-2‴, H-6‴,H-8″), 8.22 (d, J 8.2, 1H, H-4). 13C NMR (100 MHz, DMSO-d6) δ 161.14(S-C=N), 159.82 (C=O), 155.43 (C-20), 152.07 (2C, C-5, C-23), 139.85 (C-30), 136.05 (C-4), 134.74 (C-22), 134.33 (C-33), 132.81 (2C, C-11, C-28), 129.65 (4C, C-16, C-18, C-25, C-31), 127.51 (2C, C-27, C-2), 126.19 (C26), 125.24 (2C, C-1, C-29), 124.48 (2C, C-32, C-34), 122.39 (2C, C-19, C-15), 121.56 (3C, C-3, C-6, C-21). Found (%): C, 65.00; H, 4.39; N, 13.86; S, 12.99. Calc. for C27H23N5OS2 (%): C, 65.17; H, 4.66; N, 14.07; S, 12.88.
- 2-(Benzo[d]thiazol-2-ylthio)-1-(4-(4-(p-tolyl)phthalazin-1-yl)piperazin-1-yl)ethan-1-one (2l). Yield 3.53 g (69%), colorless crystals, m.p. 155–156 °C (CH3CN). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 3.02 (s, 3H, CH3), 3.51 (s, 2H, 2H-2′), 3.63 (s, 2H, 2H-6′), 3.87 (s, 2H, H-3′), 3.97 (s, 2H, H-5′), 4.57 (s, 2H, SCH2), 7.27–7.47 (m, 4H, H-3‴, H-4‴, H-5‴, H-6‴), 7.52–7.58 (m, 2H, H-5, H-6), 7.78–8.01 (m, 5H, H-7, H-5″–H-8″), 8.21 (d, J 8.1, 1H, H-4). 13C NMR (100 MHz, DMSO-d6) δ 166.50 (S-C-S), 165.94 (C=O), 155.37 (C-10), 152.95 (C-13), 147.79 (C-31), 139.59 (C-22), 135.21 (C-20), 134.37 (C-32), 131.49 (C-14), 129.79 (C-16), 127.77 (2C, C-24, C-25), 126.85 (C-17), 124.98 (C-18), 123.32 (C-15), 122.96 (C-35), 122.32 (3C, C-21, C-23, C-19), 121.51 (2C, C-33, C-34), 118.48 (C-36), 38.24 (4C, C-4, C-6, C-8, C-9), 20.20 (2C, C-2, CH3). Found (%): C, 65.48; H, 4.69; N, 13.46; S, 12.74. Calc. for C28H25N5OS2 (%): C, 65.73; H, 4.93; N, 13.69; S, 12.53.
- 2-(Benzo[d]thiazol-2-ylthio)-N-(3-(6-methyl-[1,2,4]triazolo[3,4-a]phthalazin-3-yl)phenyl)acetamide (2m). Yield 3.74 g (72%), colorless crystals, m.p. 235–237 °(DMFA). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.91 (s, 3H, Me), 4.38 (s, 2H, CH2), 7.27–7.36 (m, 1H, H-4″), 7.37–7.51 (m, 2H, H-5, H-6), 7.80–7.92 (m, 4H, H-7′–H-10′), 8.00 (t, J 7.6, 1H, H-5″), 8.17 (d, J 7.9, 2H, H-4, H-7), 8.61 (d, J 7.9, 1H, H-6″), 8.67 (d, J 2.0, 1H, H-2″), 10.48 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δ 166.50 (S-C=N), 165.95 (C=O), 155.37 (C-27), 152.95 (C-11), 147.79 (C-CH3), 143.79 (N-C-N), 139.58 (C-18), 135.21 (C-28), 134.37 (C-2), 131.49 (C-1), 129.79 (C-4), 127.77 (C-16), 127.43 (C-14), 126.85 (C-3), 124.98 (C-31), 123.32 (2C, C-6, C-15), 122.97 (C-17), 122.90 (C-5), 122.33 (C-32), 121.51 (C-33), 121.02 (C-30), 118.48 (C-19), 38.23 (CH2-C=O), 20.21 (CH3). Found (%): C, 62.00; H, 3.41; N, 17.21; S, 13.04. Calc. for C25H18N6OS2 (%): C, 62.22; H, 3.76; N, 17.41; S, 13.29.
- 2-(Benzo[d]thiazol-2-ylthio)-N-(2-methyl-5-(6-methyl-[1,2,4]triazolo[3,4-a]phthalazin-3-yl)phenyl)acetamide (2n). Yield 3.87 g (78%), colorless crystals, m.p. 251–253 °C (DMFA). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.36 (s, 3H, Me), 2.87 (s, 6H, Me, DMSO), 4.39 (s, 2H, CH2), 7.32–7.42 (m, 3H, H-5, H-6, H-5″), 7.84–7.89 (m, 3H, H-7, H-8′, H-9′), 7.96–8.03 (m, 1H, H-7), 8.13–8.18 (m, 2H, H-7′, H-10′), 8.61 (d, J 8.0, 1H, H-4″), 8.69 (s, 1H, H-2″), 9.67 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δ 166.53 (S-C=N), 165.92 (C=O), 155.35 (C-27), 152.92 (C-11), 147.80 (C-7), 143.77 (C-10), 139.56 (C-18), 135.18 (C-28), 134.35 (C-16), 131.46 (C-17), 129.80 (C-2), 127.76 (C-1), 127.32 (C-4), 126.91 (C-14), 124.95 (C-3), 123.30 (C-31), 122.99 (2C, C-6, C-32), 122.91 (C-15), 122.34 (C-5), 121.50 (C-33), 121.07 (C-30), 118.46 (C-19), 38.24 (C-23), 24.51 (CH3), 20.19 (CH3). Found (%): C, 62.56; H, 4.40; N, 17.11; S, 13.15. Calc. for C26H20N6OS2 (%): C, 62.88; H, 4.06; N, 16.92; S, 12.91.
- 2-(Benzo[d]thiazol-2-ylthio)-N-(5-(p-tolyl)benzo[4,5]imidazo[2,1-a]phthalazin-9-yl)acetamide (2o). Yield 4.68 g (88%), colorless crystals, m.p. 248–250 °C (DMFA). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 2.52 (s, 3H, Me), 4.40 (s, 2H, CH2), 7.33–7.44 (m, 3H, H-3″, H-5″, H-10′), 7.57–7.69 (m, 3H, H-2″, H-6″, H-11′), 7.76–8.03 (m, 6H, H-5, H-6, H-1′–H-4′), 8.26 (d, J 1.8, 1H, H-8′), 8.74–8.78 (m, 2H, H-4, H-7), 10.47 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6) δ 166.59 (S-C=N), 165.60 (2C, N=C-N, C=O), 152.98 (C-33), 142.42 (C-8), 141.68 (C-16), 139.63 (C-24), 136.57 (C-17), 135.21 (C-19), 133.47 (C-34), 132.25 (C-22), 130.20 (2C, C-23, C-25), 129.61 (2C, C-26, C-27), 128.68 (C-11), 127.97 (C-12), 126.86 (C-9), 125.35 (C-10), 124.97 (C-13), 124.21 (C-36), 124.12 (C-37), 122.35 (C-20), 121.51 (C-35), 116.21 (C-38), 111.51 (C-14), 109.66 (2C, C-18, C-21), 38.34(CH2-C=O), 21.44 (CH3). Found (%): C, 67.42; H, 4.21; N, 13.54; S, 12.36. Calc. for C30H21N5OS2 (%): C, 67.77; H, 3.98; N, 13.17; S, 12.06.
- 2,2′-{[(2,5-Dimethyl-1,4-phenylene)bis(methylene)]bis(sulfanediyl)}bis(benzo[d]thiazole) (3a). Yield 90%, colorless crystals, m.p. 170–171 °C (methylcellosolve), 1H NMR (200 MHz, DMSO-d6, δ, ppm): 2.32 (s, 6H, 2Me), 4.54 (s, 4H, 2CH2), 7.26 (s, 2H, H-3″, H-5″), 7.34–7.37 (m, 2H, H-5, H-5′), 7.43–7.47 (m, 2H, H-6, H-6′), 7.86 (d, J 1.1, H-7), 7.88 (d, J 1.1, H-7′), 7.92 (d, J 1.3, H-4), 7.93 (d, J 1.3, H-4′). 13C NMR (100 MHz, DMSO-d6) δ 166.60 (S-C=N), 165.72 (S-C-S), 152.92 (C-5), 142.40 (C-23), 141.62 (C-14), 136.56 (C-17), 135.23 (C-4), 133.24 (C-22), 130.20 (2C, C-13, C-16), 129.63 (2C, C-12, C-15), 127.97 (C-1), 126.86 (C-2), 125.35 (C-27), 124.97 (C-26), 124.23 (2C, C-3, C-25), 124.11 (2C, C-6, C-28), 37.15 (2C, C-11, C-18), 21.42 (2C, CH3). Found (%): C, 62.36; H, 4.56; N, 6.34; S, 27.81. Calc. for C24H20N2S4 (%): C, 62.03; H, 4.34; N, 6.03; S, 27.60.
- 2,2′-{[(2,5-Dimethoxy-1,4-phenylene)bis(methylene)]bis(sulfanediyl)}bis(benzo[d]thiazole) (3b). Yield 95%, colorless crystals, m.p. 184–186 °C (DMFA). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 3.79 (s, 6H, 2OMe), 4.56 (s, 4H, 2CH2), 7.18 (s, 2H, H-3″, H-6″), 7.30–7.36 (m, 2H, H-5, H-5′), 7.41–7.47 (m, 2H, H-6, H-6′), 7.84–7.92 (m, 4H, H-4, H-4′, H-7, H-7′). 13C NMR (100 MHz, DMSO-d6) δ 166.59 (S-C=N), 166.45 (S-C-S), 159.14 (2C, C-OCH3), 152.66 (C-5), 148.82 (C-23), 137.53 (C-4), 136.67 (C-22), 133.48 (C-15), 132.65 (C-12), 132.05 (2C, C-1, C-17), 128.44 (2C, C-2, C-26), 127.65 (2C, C-3, C-25), 124.78 (2C, C-6, C-28), 121.05 (2C, C-13, C-16), 53.28 (2C, O-CH3), 36.84 (2C, C-11, C-18). Found (%): C, 58.40; H, 4.29; N, 5.26; S, 25.49. Calc. for C24H20N2O2S4 (%): C, 58.04; H, 4.06; N, 5.64; S, 25.82.
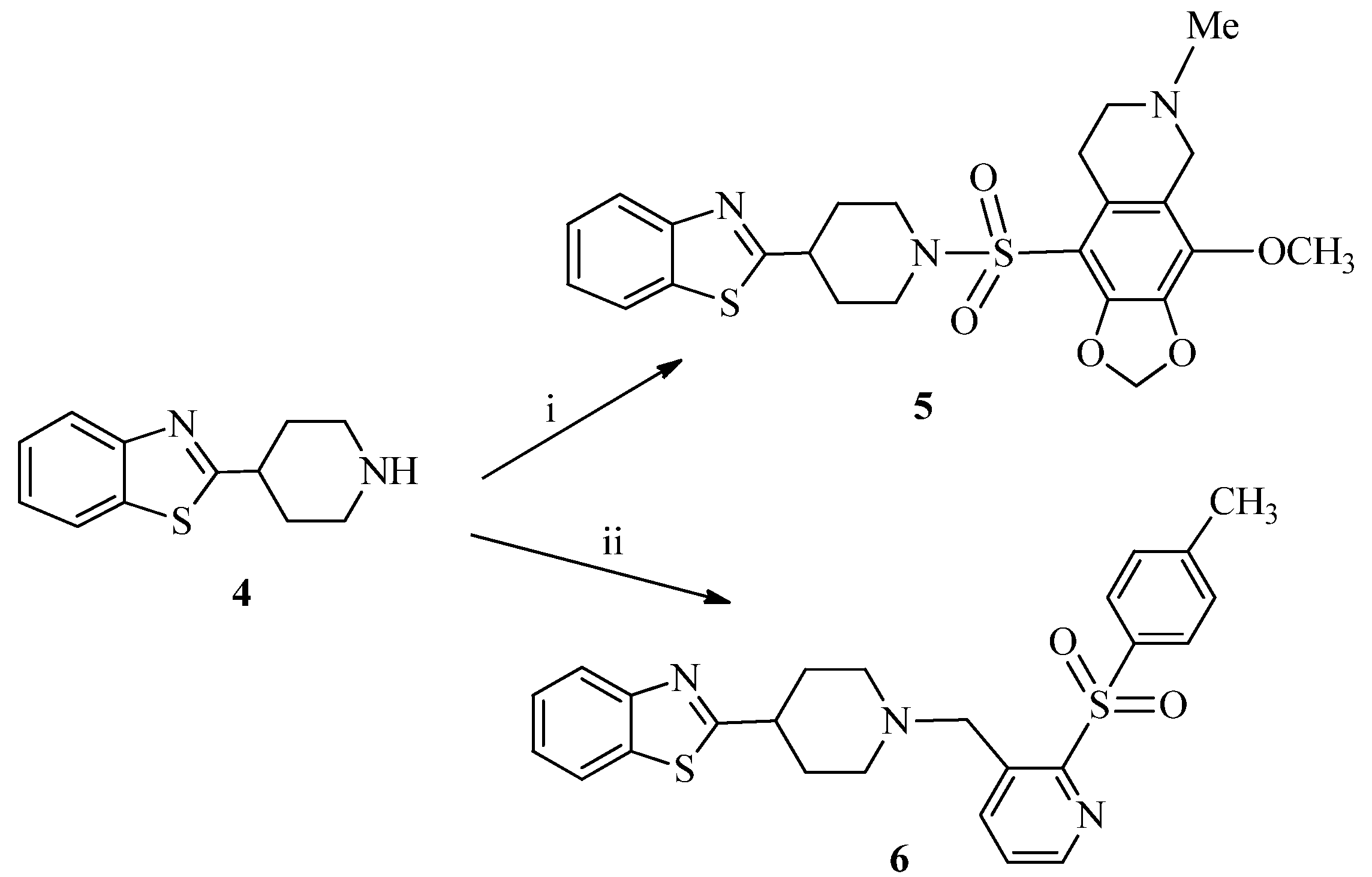
3.1.2. Synthesis of 9-((4-(Benzo[d]thiazol-2-yl)piperidin-1-yl)sulfonyl)-4-methoxy-6-methyl-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]isoquinoline (5)
3.1.3. Synthesis of 2-{1-[(2-Tosylpyridin-3-yl)methyl]piperidin-4-yl}benzo[d]thiazole (6)
3.1.4. Synthesis of N-[6-(4-Bromo-1H-pyrazol-1-yl)pyridin-3-yl]benzo[d]thiazole-6-carboxamide (8)
3.1.5. Synthesis of N-(6-Bromobenzo[d]thiazol-2-yl)-2-(4-methyl-1-oxophthalazin-2(1H)-yl) acetamide (10)
3.2. Biological Evaluation
3.2.1. Antibacterial Activity
3.2.2. Antifungal Activity
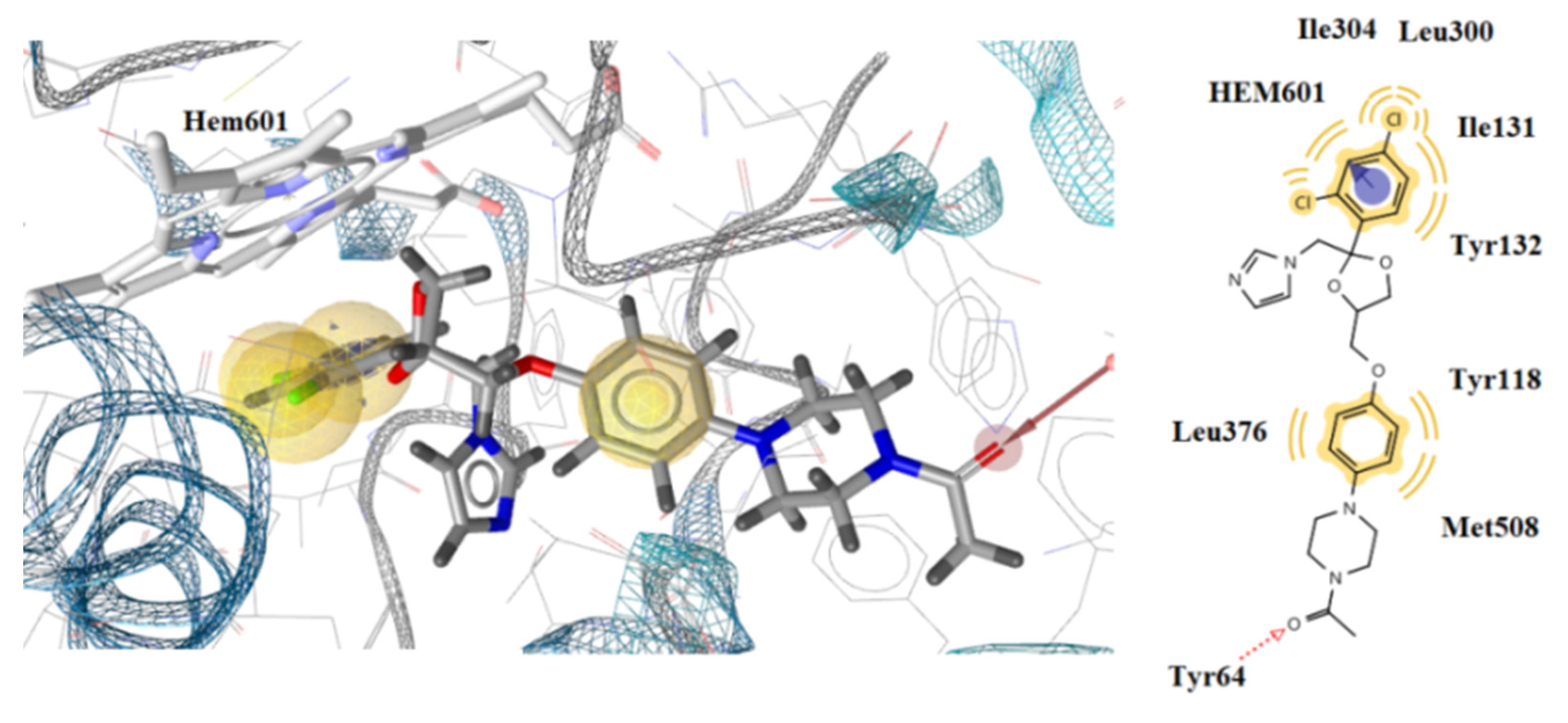
3.3. Docking Studies
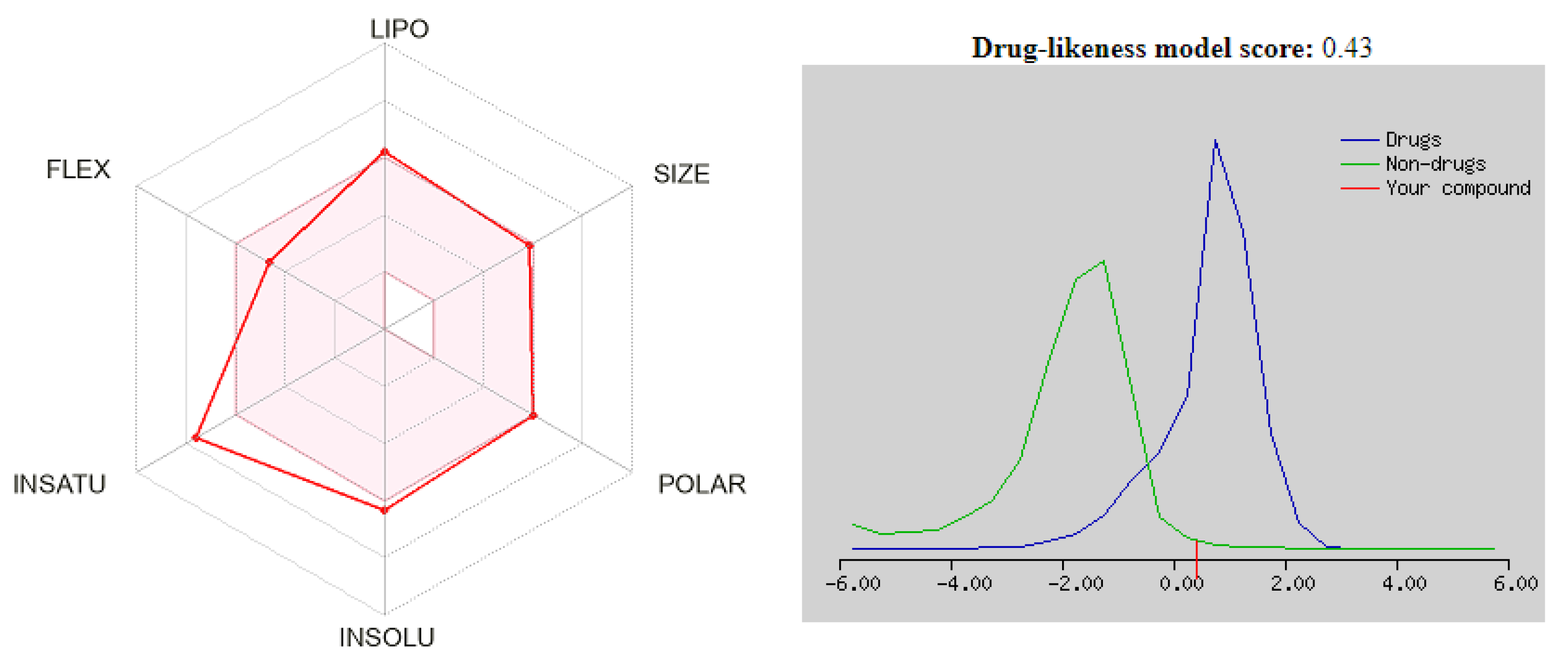
3.4. Drug Likeness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.J.; Li, C.S.; Cui, M.Y.; Song, Z.W.; Bai, X.Q.; Liang, C.W.; Wang, H.Y.; Zhang, T.Y. Synthesis, biological evaluation of benzothiazole derivatives bearing a 1,3,4-oxadiazole moiety as potential anti-oxidant and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2020, 30, 127237. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Petrou, A.; Tratrat, C.; Venugopala, K.N.; Harsha, N.S. Discovery of benzothiazole-based thiazolidinones as potential anti-inflammatory agents: Anti-inflammatory activity, soybean lipoxygenase inhibition effect and molecular docking studies. SAR QSAR Environ. Res. 2022, 33, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Ali, E.M.; Kandeel, M.; Venugopala, K.N.; Nair, A.B.; Greish, K.; El-Daly, M. Screening and Molecular Docking of Novel Benzothiazole Derivatives as Potential Antimicrobial Agents. Antibiotics 2020, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M.; Tratrat, C.; Kositsi, K.; Tsolaki, E.; Petrou, A.; Aldhubiab, B.; Attimarad, M.; Harsha, S.; Geronikaki, A.; Venugopala, K.N.; et al. New Benzothiazole-based Thiazolidinones as Potent Antimicrobial Agents. Design, synthesis and Biological Evaluation. Curr. Top. Med. Chem. 2018, 18, 75–87. [Google Scholar] [CrossRef]
- Nishad, K.; Shukla, S.; Chawla, A. Design and Synthesis of 2-Substituted Benzothiazole Derivatives as Antioxidant and Antimicrobial Agents. Curr. Bioact. Compd 2020, 16, 1242–1248. [Google Scholar] [CrossRef]
- Pankaj, S.; Govindasamy, J.; Mohd, H. Design, Synthesis and Biological Evaluation of Benzothiazole-thiophene Hybrids: A New Class of Potent Antimicrobial Agents. Anti-Infect. Agents 2018, 16, 57–63. [Google Scholar]
- Irfan, A.; Batool, F.; Zahra Naqvi, S.A.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Haider, K.; Shrivastava, N.; Pathak, A.; Dewangan, R.; Yahya, S. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Res. Chem. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Uremis, N.; Uremis, M.M.; Tolun, F.I.; Ceylan, M.; Doganer, A.; Kurt, A.H. Synthesis of 2-Substituted Benzothiazole Derivatives and Their In Vitro Anticancer Effects and Antioxidant Activities Against Pancreatic Cancer Cells. Anticancer Res. 2017, 37, 6381–6389. [Google Scholar]
- Venugopala, K.N.; Chandrashekharappa, S.; Pillay, M.; Bhandary, S.; Kandeel, M.; Mahomoodally, F.M.; Morsy, M.A.; Chopra, D.; Aldhubiab, B.E.; Attimarad, M.; et al. Synthesis and Structural Elucidation of Novel Benzothiazole Derivatives as Anti-tubercular Agents: In-silico Screening for Possible Target Identification. Med. Chem. 2019, 15, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Moodley, R.; Mashaba, C.; Rakodi, G.H.; Ncube, N.B.; Maphoru, M.V.; Balogun, M.O.; Jordan, A.; Warner, D.F.; Khan, R.; Tukulula, M. New Quinoline-Urea-Benzothiazole Hybrids as Promising Antitubercular Agents: Synthesis, In Vitro Antitubercular Activity, Cytotoxicity Studies, and In Silico ADME Profiling. Pharmaceuticals 2022, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Bhagdev, K.; Sarkar, S. Benzothiazole: As an Antidiabetic Agent. Ann. Rom. Soc. Cell Biol. 2021, 25, 20269–20285. [Google Scholar]
- Krátký, M.; Štěpánková, Š.; Vorčáková, K.; Vinšová, J. Synthesis and in vitro evaluation of novel rhodanine derivatives as potential cholinesterase inhibitors. Bioorg Chem. 2016, 68, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhot, Y.N.; Hassan, M.Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef]
- Bhagdev, K.; Sarkar, S. Benzothiazole Moiety and Its Derivatives as Antiviral Agents. Med. Sci. Forum 2021, 7, 9–16. [Google Scholar]
- Al-Masoudia, N.; Jafarb, N.; Abbasc, L.; Baqirb, S.; Pannecouque, C. Synthesis and anti-HIV activity of new Benzimidazole, Benzothiazole and Carbohyrazide derivatives of the anti-Inflammatory drug Indomethacin. Z. Naturforsch. 2011, 66b, 953–960. [Google Scholar] [CrossRef]
- Er, M.; Özera, A.; Şahin, D. Novel substituted benzothiazole and Imidazo[2,1-b][1,3,4]Thiadiazole derivatives: Synthesis, characterization, molecular docking study, and investigation of their in vitro antileishmanial and antibacterial activities. J. Mol. Str. 2019, 1194, 284–296. [Google Scholar] [CrossRef]
- Chikhale, R.; Thorat, S.; Pant, A.; Jadhav, A.; Thatipamula, K.C.; Bansode, R.; Bhargavi, G.; Karodia, N.; Rajasekharan, M.V.; Paradkar, A.; et al. Design, synthesis and pharmacological evaluation of pyrimidobenzothiazole-3-carboxylate derivatives as selective L-type calcium channel blockers. Bioorg. Med. Chem. 2015, 23, 6689–6713. [Google Scholar] [CrossRef]
- Djuidje, E.N.; Barbari, R.; Baldisserotto, A.; Durini, E.; Sciabica, S.; Balzarini, J.; Liekens, S.; Vertuani, S.; Manfredini, S. Benzothiazole Derivatives as Multifunctional Antioxidant Agents for Skin Damage: Structure-Activity Relationship of a Scaffold Bearing a Five-Membered Ring System. Antioxidants 2022, 11, 407. [Google Scholar] [CrossRef]
- Payaz, D.Ü.; Küçükbay, F.Z.; Küçükbay, H.; Angeli, A.; Supuran, C.T. Synthesis carbonic anhydrase enzyme inhibition and antioxidant activity of novel benzothiazole derivatives incorporating glycine, methionine, alanine, and phenylalanine moieties. J Enzyme Inhib. Med. Chem. 2019, 34, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Gangwar, M.; Nath, G.; Singh, S.K. Synthesis, DNA cleavage and antimicrobial activity of 4-thiazolidinones-benzothiazole conjugates. Indian J. Exp. Biol. 2014, 52, 1062–1070. [Google Scholar] [PubMed]
- Kamal, A.; Syed, M.A.; Mohammed, S.M. Therapeutic potential of benzothiazoles: A patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.; Payal, K.; Mohammad, A. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch. Pharm. Chem. Life Sci. 2019, 352, e180017. [Google Scholar]
- Mourad, A.K.; Makhlouf, A.A.; Soliman, A.; Mohamed, S.A. Phthalazines and phthalazine hybrids as antimicrobial agents: Synthesis and biological evaluation. J. Chem. Res. 2020, 44, 31–41. [Google Scholar] [CrossRef]
- El Rayes, S.M.; El Enany, G.; Ali, I.; Ibrahim, W.; Nafie, M.S. Synthesis of Novel Phthalazinedione-Based Derivatives with Promising Cytotoxic, Anti-bacterial, and Molecular Docking Studies as VEGFR2 Inhibitors. ACS Omega 2022, 7, 26800–26811. [Google Scholar] [CrossRef]
- Gouda, M.; Hussein, B. Synthesis and Anti-Oxidant Evaluation of Some Novel Sulfa Drugs. Lett. Drug Des. Dis. 2017, 14, 1425–1432. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Horishny, V.; Kartsev, V.; Matiychuk, V.; Geronikaki, A.; Anthi, P.; Pogodin, P.; Poroikov, V.; Ivanov, M.; Kostic, M.; Soković, M.D.; et al. 3-Amino-5-(indol-3-yl)methylene-4-oxo-2-thioxothiazolidine Derivatives as Antimicrobial Agents: Synthesis, Computational and Biological Evaluation. Pharmaceuticals 2020, 13, 229. [Google Scholar] [CrossRef]
- Horishny, V.; Kartsev, V.; Geronikaki, A.; Matiychuk, V.; Petrou, A.; Glamoclija, J.; Ciric, A.; Sokovic, M. 5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl)alkancarboxylic Acids as Antimicrobial Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies. Molecules. 2020, 25, 1964. [Google Scholar] [CrossRef] [Green Version]
- Kartsev, V.G.; Zubenko, A.A.; Divaeva, L.N.; Morkovnik, A.S.; Baryshnikov, T.K.; Shirinian, V.Z. New Structural Modifications of Cotarnine Alkaloid Derivatives: Cotarnone and Dihydrocotarnine. Russ. J. Gen. Chem. 2020, 90, 238–243. [Google Scholar] [CrossRef]
- Kartsev, V.; Lichitsky, B.; Geronikaki, A.; Petrou, A.; Smiljkovic, M.; Kostic, M.; Radanovic, O.; Soković, M. Design, synthesis and antimicrobial activity of usnic acid derivatives. Med. Chem. Comm. 2018, 9, 870–882. [Google Scholar] [CrossRef] [Green Version]
- Simakov, S.; Kartsev, V.; Petrou, A.; Nicolaou, I.; Geronikaki, A.; Ivanov, M.; Kostic, M.; Glamočlija, J.; Soković, M.; Talea, D.; et al. 4-(Indol-3-yl)thiazole-2-amines and 4-ιndol-3-yl)thiazole Acylamines as Νovel Antimicrobial Agents: Synthesis, In Silico and In Vitro Evaluation. Pharmaceuticals 2021, 14, 1096. [Google Scholar] [CrossRef]
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef] [Green Version]
- Kritsi, E.; Matsoukas, M.T.; Potamitis, C.; Detsi, A.; Ivanov, M.; Sokovic, M.; Zoumpoulakis, P. Novel Hit Compounds as Putative Antifungals: The Case of Aspergillus fumigatus. Molecules 2019, 24, 3853. [Google Scholar] [CrossRef]
- Fesatidou, M.; Zagaliotis, P.; Camoutsis, C.; Perou, A.; Eleftheriou, P.; Tratrtat, C.; Haroun, M.; Geronikaki, A.; Ciric, A.; Sokovic, M. 5-Adamantan thiadiazole-based thiazolidinones as antimicrobial agents. Design, synthesis, molecular docking and evaluation. Bioorg.Med.Chem. 2018, 26, 4664–4676. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Wars, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]

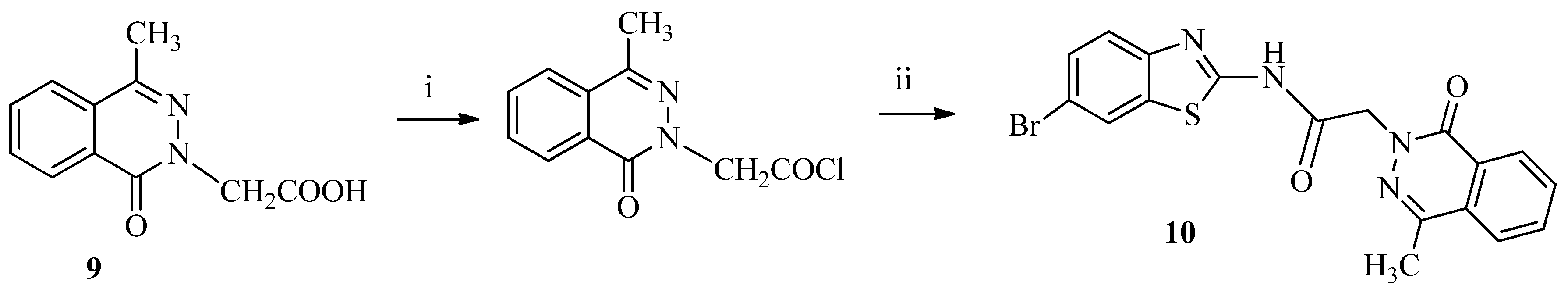



| No. | S.a. | B.c. | L.m. | E.c. | S.t. | En.cl. | |
|---|---|---|---|---|---|---|---|
| 2a | MIC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.35 ± 0.08 | 0.70 ± 0.19 | 0.70 ± 0.19 |
| MBC | 3.75 ± 0.00 | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2b | MIC | 1.41 ± 0.38 | 0.47 ± 0.00 | 0.47 ± 0.00 | >3.75 | 0.23 ± 0.00 | 0.47 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | >3.75 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| 2c | MIC | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 |
| MBC | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2d | MIC | 1.41 ± 0.38 | 0.35 ± 0.08 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2e | MIC | 1.41 ± 0.38 | 0.94 ± 0.00 | 0.70 ± 0.19 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 |
| MBC | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| 2f | MIC | 2.50 ± 0.88 | 1.41 ± 0.38 | 0.94 ± 0.00 | 0.70 ± 0.19 | 1.41 ± 0.38 | 2.50 ± 0.88 |
| MBC | 3.75 ± 0.00 | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | 3.75 ± 0.00 | |
| 2g | MIC | 1.41 ± 0.38 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.23 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2h | MIC | 0.94 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.47 ± 0.00 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2i | MIC | 1.41 ± 0.38 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.35 ± 0.08 | 0.70 ± 0.19 | 0.94 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | |
| 2j | MIC | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.47 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| 2k | MIC | 0.94 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | >3.75 | >3.75 | 0.23 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | >3.75 | >3.75 | 0.47 ± 0.00 | |
| 2l | MIC | 1.41 ± 0.38 | 0.94 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.94 ± 0.00 |
| MBC | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | |
| 2m | MIC | 1.41 ± 0.38 | >3.75 | 0.70 ± 0.19 | 0.47 ± 0.00 | 0.35 ± 0.08 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | >3.75 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| 2n | MIC | 1.41 ± 0.38 | 0.70 ± 0.19 | 0.70 ± 0.19 | >3.75 | 0.47 ± 0.00 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | >3.75 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 2o | MIC | 1.41 ± 0.38 | 0.70 ± 0.19 | 0.70 ± 0.19 | >3.75 | 0.47 ± 0.00 | 0.47 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | >3.75 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 3a | MIC | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.47 ± 0.00 | 0.70 ± 0.19 |
| MBC | 3.75 ± 0.00 | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | |
| 3b | MIC | 1.41 ± 0.38 | 0.70 ± 0.19 | 0.70 ± 0.19 | >3.75 | 0.70 ± 0.19 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | >3.75 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 5 | MIC | 1.41 ± 0.38 | 0.47 ± 0.00 | 0.70 ± 0.19 | 0.35 ± 0.08 | 0.70 ± 0.19 | 1.41 ± 0.38 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | |
| 6 | MIC | 0.94 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | >3.75 | 0.70 ± 0.19 | 0.47 ± 0.00 |
| MBC | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | >3.75 | 0.94 ± 0.00 | 0.94 ± 0.00 | |
| 8 | MIC | 2.50 ± 0.88 | 1.41 ± 0.38 | 1.41 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.94 ± 0.00 |
| MBC | 3.75 ± 0.00 | 1.88 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | |
| 10 | MIC | 1.41 ± 0.38 | 0.23 ± 0.00 | 0.70 ± 0.19 | 0.70 ± 0.19 | 0.23 ± 0.00 | 0.70 ± 0.19 |
| MBC | 1.88 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| Streptomycin | MIC | 0.10 ± 0.00 | 0.02 ± 0.00 | 0.15 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.02 ± 0.00 |
| MBC | 0.20 ± 0.01 | 0.05 ± 0.00 | 0.30 ± 0.01 | 0.20 ± 0.00 | 0.20 ± 0.01 | 0.05 ± 0.00 | |
| Ampicillin | MIC | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 |
| MBC | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.30 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.00 | 0.15 ± 0.01 |
| No. | A.f. | A.n. | A.v. | P.f. | T.v. | P.v.c. | |
|---|---|---|---|---|---|---|---|
| 2a | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.35 ± 0.08 | 0.11 ± 0.00 | 0.11 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | |
| 2b | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2c | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.23 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | |
| 2d | MIC | 0.17 ± 0.05 | 0.11 ± 0.00 | 0.17 ± 0.05 | 0.17 ± 0.05 | 0.08 ± 0.00 | 0.17 ± 0.05 |
| MFC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 | |
| 2e | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.35 ± 0.08 | 0.17 ± 0.05 | 0.35 ± 0.08 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2f | MIC | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.23 ± 0.00 |
| MFC | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2g | MIC | 0.47 ± 0.00 | 0.17 ± 0.05 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.17 ± 0.05 | 0.35 ± 0.08 |
| MFC | 0.94 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2h | MIC | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.35 ± 0.08 | 0.11 ± 0.00 | 0.70 ± 0.19 |
| MFC | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.94 ± 0.00 | |
| 2i | MIC | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.06 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.11 ± 0.00 | 0.47 ± 0.00 | |
| 2j | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2k | MIC | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.94 ± 0.00 | |
| 2l | MIC | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2m | MIC | 0.17 ± 0.05 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.35 ± 0.08 |
| MFC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 2n | MIC | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.23 ± 0.00 | 0.35 ± 0.08 |
| MFC | 0.94 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | |
| 2o | MIC | 0.17 ± 0.05 | 0.17 ± 0.05 | 0.11 ± 0.00 | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.23 ± 0.00 |
| MFC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 3a | MIC | 0.17 ± 0.05 | 0.11 ± 0.00 | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.11 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 3b | MIC | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.17 ± 0.05 | 0.17 ± 0.05 | 0.06 ± 0.00 | 0.17 ± 0.05 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 | |
| 5 | MIC | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.11 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.23 ± 0.00 | 0.47 ± 0.00 | |
| 6 | MIC | 0.23 ± 0.00 | 0.35 ± 0.08 | 0.23 ± 0.00 | 0.23 ± 0.00 | 0.06 ± 0.00 | 0.23 ± 0.00 |
| MFC | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.47 ± 0.00 | 0.11 ± 0.00 | 0.47 ± 0.00 | |
| 8 | MIC | 3.75 ± 0.00 | 3.75 ± 0.00 | 3.75 ± 0.00 | 3.75 ± 0.00 | 3.75 ± 0.00 | 3.75 ± 0.00 |
| MFC | >3.75 | >3.75 | >3.75 | >3.75 | >3.75 | >3.75 | |
| 10 | MIC | 1.88 ± 0.00 | 0.47 ± 0.00 | 0.70 ± 0.019 | 1.41 ± 0.38 | 0.70 ± 0.19 | 2.50 ± 0.88 |
| MFC | 3.75 ± 0.00 | 0.94 ± 0.00 | 0.94 ± 0.00 | 1.88 ± 0.00 | 0.94 ± 0.00 | 3.75 ± 0.00 | |
| Bifonazole | MIC | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.10 ± 0.00 | 0.20 ± 0.00 | 0.15 ± 0.00 | 0.10 ± 0.00 |
| MFC | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.25 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 | |
| Ketoconazole | MIC | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0.20 ± 0.00 | 1.00 ± 0.01 | 0.20 ± 0.00 |
| MFC | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 1.50 ± 0.00 | 0.30 ± 0.010 |
| Comp. | Est. Binding Energy (kcal/mol) | I-H E. coli MurB | Residues E. coli MurB | ||||
|---|---|---|---|---|---|---|---|
| E. coli Gyrase 1KZN | Thymidylate Kinase 4QGG | E. coli Primase 1DDE | E. coli MurA JV4T | E. coli MurB 2Q85 | |||
| 2a | −4.52 | - | - | −3.62 | −8.03 | 1 | Ser229 |
| 2b | −5.28 | - | −1.23 | −4.27 | −7.86 | 1 | Arg158 |
| 2c | −4.39 | −2.55 | - | −5.19 | −10.13 | 2 | Ser50, Ser229 |
| 2d | −5.19 | −1.03 | −2.26 | −6.52 | −9.64 | 2 | Arg158, Arg213 |
| 2e | −4.55 | - | −1.39 | −5.24 | −9.61 | 2 | Arg213, Ser229 |
| 2f | −4.37 | - | - | −5.37 | −6.53 | 1 | Arg213 |
| 2g | −5.37 | −1.54 | −2.33 | −6.72 | −10.02 | 2 | Arg158, Arg213 |
| 2h | −4.19 | - | - | −5.68 | −9.71 | 2 | Arg213, Ser229 |
| 2i | −5.63 | −1.28 | −1.30 | −5.22 | −9.34 | 2 | Ser50, Ser229 |
| 2j | −5.27 | - | - | −6.34 | −10.75 | 3 | Ser50, Ser116, Ile173 |
| 2k | −4.23 | - | - | −4.53 | −8.76 | 1 | Ser229 |
| 2l | −4.96 | - | −2.38 | −6.59 | −9.11 | 2 | Ser50, Ser229 |
| 2m | −5.23 | - | - | −4.56 | −7.80 | 1 | Arg158 |
| 2n | −4.31 | −1.85 | - | −3.11 | −7.30 | 1 | Arg213 |
| 2o | −3.62 | - | - | −2.54 | −7.35 | 1 | Arg213 |
| 3a | −4.35 | - | - | −3.64 | −8.45 | 1 | Ser229 |
| 3b | −2.32 | −1.68 | - | −4.52 | −7.18 | 1 | Arg158 |
| 5 | −5.12 | - | −1.30 | −5.57 | −9.53 | 2 | Arg213, Ser229 |
| 6 | −3.66 | - | - | −2.50 | −7.42 | 1 | Arg213 |
| 8 | −5.10 | −1.52 | −2.46 | −6.22 | −9.90 | 2 | Arg158, Arg213 |
| 10 | −4.28 | - | - | −3.55 | −6.92 | 1 | Arg213 |
| Naphthyl Tetronic Acid inhibitor | - | - | - | - | −8.82 | - | Asn233 |
| Est. Binding Energy(kcal/mol) | Residues Involved in H-Bond Formation | Residues Involved in Hydrophobic Interactions | Residues Involved in Aromatic Interactions | Interactions with HEM601 | ||
|---|---|---|---|---|---|---|
| N/N | DNA TopoIV 1S16 | CYP51 of C. albicans 5V5Z | ||||
| 2a | −1.38 | −9.85 | Tyr132 | Tyr118, Leu121, Thr311, Phe380, Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| 2b | −3.59 | −8.82 | Tyr132 | Tyr118, Tyr122, Ile304, Thr311, Hem601 | Tyr118 | Hydrophobic |
| 2c | −2.64 | −8.03 | - | Tyr118, Thr311, Leu376, Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| 2d | −3.57 | −11.32 | - | Tyr118, Leu121, Tyr122, Thr311, Leu376, Phe380, Met508, Hem601 | Tyr118, Hem601 | Hydrophobic, aromatic |
| 2e | −3.15 | −8.50 | Tyr118 | Tyr118, Leu376, Met508, Hem601 | Tyr118 | Hydrophobic |
| 2f | −1.29 | −7.46 | - | Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| 2g | - | −7.93 | - | Ile304, Thr311, Met508, Hem601 | - | Hydrophobic |
| 2h | −2.44 | −7.21 | - | Tyr118, Leu376, Met508, Hem601 | Tyr118 | Hydrophobic |
| 2i | - | −9.82 | Tyr132 | Tyr118, Phe380, Met508, Hem601 | - | Hydrophobic |
| 2j | −3.83 | −9.56 | - | Tyr118, Tyr122, Thr311, Leu376, Met508, Hem601 | Tyr118, Hem601 | Hydrophobic, aromatic |
| 2k | - | −7.39 | - | Tyr118, Met508, Hem601 | - | Hydrophobic |
| 2l | −1.27 | 9.52 | - | Tyr118, Tyr122, Leu376, Met508, Hem601 | Tyr122, Hem601 | Hydrophobic, aromatic |
| 2m | −3.21 | −10.02 | Tyr64 | Tyr118, Tyr122, Thr311, Leu376, Phe380, Hem601 | Tyr118 | Hydrophobic |
| 2n | - | −7.25 | - | Tyr118, Leu376, Met508, Hem601 | - | Hydrophobic |
| 2o | −2.50 | −10.25 | - | Tyr118, Leu121, Tyr122, Thr311, Hem601 | Hem601 | Hydrophobic, aromatic |
| 3a | −2.75 | −10.31 | - | Tyr118, Leu121, Leu376, Phe380, Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| 3b | −3.23 | −10.87 | - | Tyr118, Tyr122, Thr311, Leu376, Met508, Hem601 | Tyr118, Hem601 | Hydrophobic, aromatic |
| 5 | - | −8.62 | - | Tyr118, Tyr122, Thr311, Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| 6 | −2.45 | −9.10 | - | Tyr118, Tyr122, Ile304, Thr311, Leu376, Hem601 | Ile131, Hem601 | Hydrophobic, aromatic |
| 8 | −2.06 | −8.21 | - | Tyr118, Tyr122, Ile131, Leu376, Met508, Hem601 | - | Hydrophobic |
| 10 | −2.41 | −7.20 | - | Tyr118, Leu376, Met508, Hem601 | Tyr118 | Hydrophobic |
| ketoconazole | - | −8.23 | Tyr64 | Tyr118, Ile131, Tyr132, Leu300, Ile304, Leu376, Met508, Hem601 | Hem601 | Hydrophobic, aromatic |
| No. | MW | Number of HBA a | Number of HBD b | Log Po/w (iLOGP) c | Log S d | TPSA e | BBB Permeant f | Lipinski, Ghose, Veber, Egan, and Muegge Violations | Bioavailability Score | Drug-Likeness Model Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 311.42 | 2 | 0 | 2.81 | Poorly soluble | 84.25 | No | 0 | 0.55 | −0.08 |
| 2b | 309.41 | 3 | 0 | 3.19 | Poorly soluble | 92.21 | No | 0 | 0.55 | −0.19 |
| 2c | 371.48 | 3 | 0 | 3.69 | Poorly soluble | 92.21 | No | 0 | 0.55 | −0.35 |
| 2d | 385.50 | 3 | 0 | 3.86 | Poorly soluble | 92.21 | No | 0 | 0.55 | −0.12 |
| 2e | 405.92 | 3 | 0 | 4.03 | Poorly soluble | 92.21 | No | 0 | 0.55 | 0.20 |
| 2f | 470.61 | 4 | 0 | 4.30 | Poorly soluble | 112.52 | No | 2 * | 0.55 | 0.65 |
| 2g | 492.64 | 6 | 0 | 4.13 | Poorly soluble | 137.97 | No | 3 ** | 0.55 | −0.22 |
| 2h | 587.71 | 5 | 0 | 4.65 | Insoluble | 134.52 | No | 2 *** | 0.17 | 0.37 |
| 2i | 429.56 | 3 | 0 | 3.65 | Poorly soluble | 101.32 | No | 0 | 0.55 | 0.06 |
| 2j | 486.61 | 4 | 1 | 3.73 | Poorly soluble | 130.42 | No | 0 | 0.55 | 0.43 |
| 2k | 497.63 | 4 | 0 | 4.36 | Poorly soluble | 115.76 | No | 0 | 0.55 | 0.21 |
| 2l | 511.66 | 4 | 0 | 4.31 | Poorly soluble | 115.76 | No | 0 | 0.55 | 0.42 |
| 2m | 482.58 | 5 | 1 | 3.61 | Poorly soluble | 138.61 | No | 0 | 0.55 | 0.32 |
| 2n | 496.61 | 5 | 1 | 4.14 | Poorly soluble | 138.61 | No | 0 | 0.55 | 0.34 |
| 2o | 531.65 | 4 | 1 | 4.38 | Poorly soluble | 125.72 | No | 2 *** | 0.17 | −0.03 |
| 3a | 464.69 | 2 | 0 | 4.75 | Poorly soluble | 132.86 | No | 0 | 0.55 | −0.05 |
| 3b | 496.69 | 4 | 0 | 4.87 | Poorly soluble | 151.32 | No | 0 | 0.55 | 0.43 |
| 5 | 501.62 | 8 | 0 | 3.85 | Poorly soluble | 117.82 | No | 0 | 0.55 | 0.79 |
| 6 | 463.61 | 5 | 0 | 3.56 | Poorly soluble | 99.78 | No | 0 | 0.55 | 0.49 |
| 8 | 400.25 | 4 | 1 | 2.93 | Poorly soluble | 100.94 | No | 0 | 0.55 | −0.60 |
| 10 | 429.29 | 4 | 1 | 2.65 | Poorly soluble | 105.12 | No | 0 | 0.55 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubenko, A.; Kartsev, V.; Petrou, A.; Geronikaki, A.; Ivanov, M.; Glamočlija, J.; Soković, M.; Divaeva, L.; Morkovnik, A.; Klimenko, A. Experimental and In Silico Evaluation of New Heteroaryl Benzothiazole Derivatives as Antimicrobial Agents. Antibiotics 2022, 11, 1654. https://doi.org/10.3390/antibiotics11111654
Zubenko A, Kartsev V, Petrou A, Geronikaki A, Ivanov M, Glamočlija J, Soković M, Divaeva L, Morkovnik A, Klimenko A. Experimental and In Silico Evaluation of New Heteroaryl Benzothiazole Derivatives as Antimicrobial Agents. Antibiotics. 2022; 11(11):1654. https://doi.org/10.3390/antibiotics11111654
Chicago/Turabian StyleZubenko, Alexander, Victor Kartsev, Anthi Petrou, Athina Geronikaki, Marija Ivanov, Jasmina Glamočlija, Marina Soković, Lyudmila Divaeva, Anatolii Morkovnik, and Alexander Klimenko. 2022. "Experimental and In Silico Evaluation of New Heteroaryl Benzothiazole Derivatives as Antimicrobial Agents" Antibiotics 11, no. 11: 1654. https://doi.org/10.3390/antibiotics11111654






