Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Susceptibility Testing and Chequerboard Assays
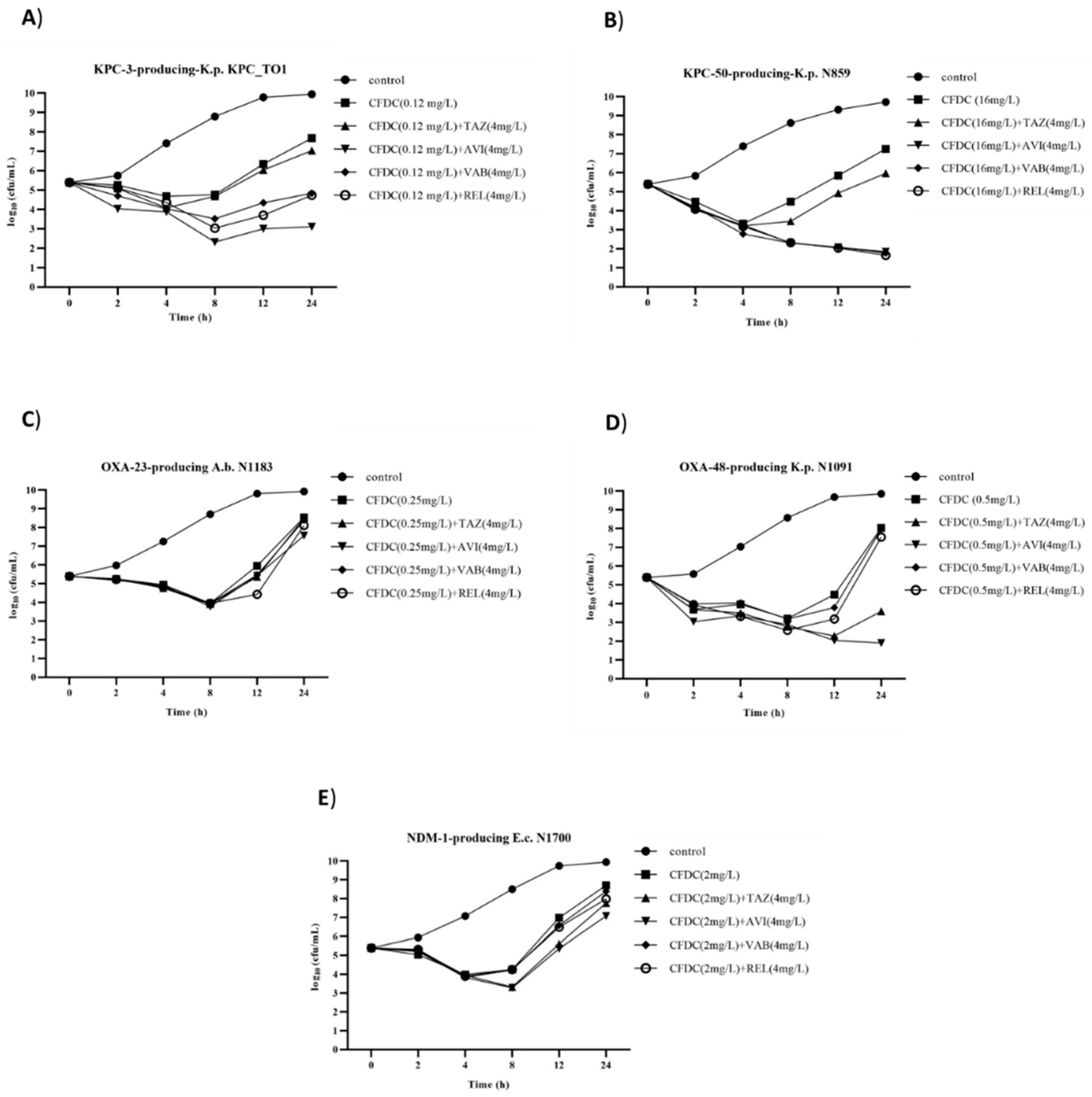
2.2. Time–Kill Assays
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Cefiderocol Susceptibility Testing
4.3. Chequerboard Assay
4.4. Time–Kill Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S538–S543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, M.R.; Abdelhamed, A.M.; Good, C.E.; Rhoads, D.D.; Hujer, K.M.; Hujer, A.M.; Domitrovic, T.N.; Rudin, S.D.; Richter, S.S.; van Duin, D.; et al. ARGONAUT-I: Activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, Including Carbapenem-Resistant Nonfermenters and Enterobacteriaceae with Defined Extended-Spectrum β-Lactamases and Carbapenemases. Antimicrob. Agents Chemother. 2018, 63, e01801-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Dobias, J.; Dénervaud-Tendon, V.; Poirel, L.; Nordmann, P. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2319–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, G.; Boattini, M.; Comini, S.; Iannaccone, M.; Casale, R.; Allizond, V.; Barbui, A.M.; Banche, G.; Cavallo, R.; Costa, C. Activity of ceftolozane-tazobactam, ceftazidime-avibactam, meropenem-vaborbactam, cefiderocol and comparators against Gram-negative organisms causing bloodstream infections in Northern Italy (2019–2021): Emergence of complex resistance phenotypes. J. Chemother. 2022, 34, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob. Agents Chemother. 2017, 61, e00093-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlowsky, J.A.; Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated by Clinical Laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int. J. Antimicrob. Agents 2019, 53, 456–466. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob. Agents Chemother. 2018, 62, e01968-17. [Google Scholar] [CrossRef] [Green Version]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Parsels, K.A.; Mastro, K.A.; Steele, J.M.; Thomas, S.J.; Kufel, W.D. Cefiderocol: A novel siderophore cephalosporin for multidrug-resistant Gram-negative bacterial infections. J. Antimicrob. Chemother. 2021, 76, 1379–1391. [Google Scholar] [CrossRef]
- Shields, R.K.; Iovleva, A.; Kline, E.G.; Kawai, A.; McElheny, C.L.; Doi, Y. Clinical Evolution of AmpC-Mediated Ceftazidime-Avibactam and Cefiderocol Resistance in Enterobacter cloacae Complex Following Exposure to Cefepime. Clin. Infect. Dis. 2020, 71, 2713–2716. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Beisken, S.; Bergman, Y.; Posch, A.E.; Cosgrove, S.E.; Tamma, P.D. Cefiderocol Activity Against Clinical Pseudomonas aeruginosa Isolates Exhibiting Ceftolozane-Tazobactam Resistance. Open Forum Infect. Dis. 2021, 8, ofab311. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Iannaccone, M.; Bondi, A.; Cavallo, R.; Costa, C. In vitro activity of cefiderocol against ceftazidime-avibactam susceptible and resistant KPC-producing Enterobacterales: Cross-resistance and synergistic effects. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of Reduced Susceptibility to Cefiderocol Among Isolates from the CREDIBLE-CR and APEKS-NP Clinical Trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Smoke, S.M.; Brophy, A.; Reveron, S.; Iovleva, A.; Kline, E.G.; Marano, M.; Miller, L.P.; Shields, R.K. Evolution and transmission of cefiderocol-resistant Acinetobacter baumannii during an outbreak in the burn intensive care unit. Clin. Infect. Dis. 2022, ciac647. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Amadesi, S.; Lazzarotto, T.; Ambretti, S. Genome characterization of a Klebsiella pneumoniae co-producing OXA-181 and KPC-121 resistant to ceftazidime/avibactam, meropenem/vaborbactam, imipenem/relebactam and cefiderocol isolated from a critically ill patient. J. Glob. Antimicrob. Resist. 2022, 30, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Sadek, M.; Kusaksizoglu, A.; Nordmann, P. Co-resistance to ceftazidime-avibactam and cefiderocol in clinical isolates producing KPC variants. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Masseron, A.; Prod’Hom, G.; Galperine, T.; Greub, G.; Poirel, L.; Nordmann, P. Phenotypic, biochemical and genetic analysis of KPC-41, a KPC-3 variant conferring resistance to ceftazidime-avibactam and exhibiting reduced carbapenemase activity. Antimicrob. Agents Chemother. 2019, 63, e01111-19. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Arcari, G.; Bibbolino, G.; Sacco, F.; Tomolillo, D.; Di Lella, F.M.; Trancassini, M.; Faino, L.; Venditti, M.; Antonelli, G.; et al. Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates. Antimicrob. Agents Chemother. 2021, 65, e0057421. [Google Scholar] [CrossRef]
- Poirel, L.; Ortiz de la Rosa, J.M.; Sadek, M.; Nordmann, P. Impact of Acquired Broad-Spectrum β-Lactamases on Susceptibility to Cefiderocol and Newly Developed β-Lactam/β-Lactamase Inhibitor Combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2022, 66, e00039-22. [Google Scholar] [CrossRef]
- Fröhlich, C.; Sørum, V.; Tokuriki, N.; Johnsen, P.J.; Samuelsen, Ø. Evolution of β-lactamase-mediated cefiderocol resistance. J. Antimicrob. Chemother. 2022, 77, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, R.A.; Bernabeu, S.; Emeraud, C.; Creton, E.; Vanparis, O.; Naas, T.; Jousset, A.B.; Dortet, L. Susceptibility of OXA-48-producing Enterobacterales to imipenem/relebactam, meropenem/vaborbactam and ceftazidime/avibactam. Int. J. Antimicrob. Agents 2022, 60, 106660. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Comini, S.; Bianco, G.; Iannaccone, M.; Casale, R.; Cavallo, R.; Costa, C. Activity of cefiderocol and synergy of novel β-lactam-β-lactamase inhibitor-based combinations against metallo-β-lactamase-producing gram-negative bacilli: Insights from a two-year study (2019–2020). J. Chemother. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type β-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e00877-21. [Google Scholar] [CrossRef]
- Ni, W.; Wang, Y.; Ma, X.; He, Y.; Zhao, J.; Guan, J.; Li, Y.; Gao, Z. In vitro and in vivo efficacy of cefiderocol plus tigecycline, colistin, or meropenem against carbapenem-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1451–1457. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Guidance Document on Broth Microdilution Testing of Cefiderocol. 2020. Available online: http://www.eucast.org (accessed on 31 October 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 31 October 2022).
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef]
- Document M26-A; Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. National Committee for Clinical Laboratory Standards (NCCLS): Wayne, PA, USA, 1999.
| Strain | Species | Sequence Typing | Carbapenemase Gene | Other β-Lactamases Genes | MIC (mg/L) | FICI and Interpretation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFDC | CFDC+TAZ | CFDC+AVI | CFDC+VAB | CDFC+REL | CFDC+TAZ | CFDC+AVI | CFDC+VAB | CDFC+REL | |||||
| Ambler class A | |||||||||||||
| BO318KP [a] | K. pneumoniae | ST-512 | blaKPC-3 | blaTEM-1, blaSHV-11 | 4 | 2 | 1 | 1 | 1 | 0.56 | 0.25 | 0.31 | 0.31 |
| N1118 [b] | K. pneumoniae | - | blaKPC-2 | blaSHV-11 | 0.12 | 0.12 | ≤0.007 | 0.015 | 0.015 | 1.06 | 0.18 | 0.24 | 0.24 |
| N2350 [b] | K. pneumoniae | - | blaKPC-3 | blaSHV-11, blaOXA-9 | 2 | 2 | ≤0.007 | 0.25 | 0.125 | 1.06 | 0.25 | 0.37 | 0.31 |
| CAZ156BO [a] | K. pneumoniae | ST-101 | blaKPC-3 | blaSHV-156 | 1 | 0.5 | 0.125 | 0.06 | 0.125 | 0.56 | 0.19 | 0.12 | 0.19 |
| BAT16KP [a] | K. pneumoniae | ST-512 | blaKPC-3 | blaTEM-1, blaSHV-11 | 1 | 1 | 0.06 | 0.25 | 0.06 | 1.03 | 0.12 | 0.31 | 0.12 |
| BAT15KP [a] | K. pneumoniae | ST-512 | blaKPC-3 | blaTEM-1, blaSHV-11 | 1 | 1 | 0.12 | 0.25 | 0.12 | 1.06 | 0.18 | 0.31 | 0.18 |
| KPC_TO5 [c] | K. pneumoniae | ST-512 | blaKPC-3 | blaTEM-1A, blaOXA-9, blaSHV-11 | 0.5 | 0.25 | 0.015 | 0.12 | 0.015 | 0.56 | 0.09 | 0.3 | 0.09 |
| KPC_TO1 [c] | K. pneumoniae | ST-512 | blaKPC-3 | blaTEM-1A, blaOXA-9, blaSHV-11 | 0.12 | 0.06 | ≤0.007 | 0.015 | 0.015 | 0.56 | 0.12 | 0.19 | 0.19 |
| KPB07 [a] | K. pneumoniae | ST-1519 | blaKPC-3 | blaTEM-1, blaOXA-9, blaSHV-11 | 0.25 | 0.25 | 0.015 | 0.06 | 0.06 | 1.06 | 0.18 | 0.30 | 0.30 |
| KPB09 [a] | K. pneumoniae | ST-1519 | blaKPC-3 | blaTEM-1, blaOXA-9, blaSHV-11 | 0.125 | 0.125 | ≤0.007 | 0.015 | 0.015 | 1.06 | 0.18 | 0.18 | 0.18 |
| KPB013 [a] | K. pneumoniae | ST-1519 | blaKPC-3 | blaOXA-9, blaSHV-11 | 0.125 | 0.125 | ≤0.007 | 0.015 | ≤0.007 | 1.06 | 0.18 | 0.18 | 0.12 |
| KPB02 [a] | K. pneumoniae | ST-1519 | blaKPC-36 | blaTEM-1, blaOXA-9, blaSHV-11 | 0.25 | 0.25 | ≤0.007 | 0.06 | 0.06 | 1.06 | 0.28 | 0.30 | 0.30 |
| KPC_TO3 [c] | K. pneumoniae | ST-512 | blaKPC-66 | blaTEM-1A, blaOXA-9, blaSHV-11 | 0.5 | ≤0.007 | ≤0.007 | 0.015 | 0.015 | 0.26 | 0.08 | 0.09 | 0.09 |
| BOT-EMOKP [a] | K. pneumoniae | ST-1519 | blaKPC-31, blaKPC-3 | blaTEM-1, blaOXA-9, blaSHV-11 | 8 | 2 | 0.5 | 0.25 | 0.5 | 0.31 | 0.12 | 0.09 | 0.12 |
| N435 [b] | K. pneumoniae | - | blaKPC-41 | blaSHV-11, blaTEM-1 | 4 | 0.5 | 0.12 | 0.12 | 0.5 | 0.13 | 0.09 | 0.09 | 0.19 |
| N859 [b] | K. pneumoniae | ST-258 | blaKPC-50 | blaSHV-11 | 16 | 16 | 4 | 1 | 4 | 1.06 | 0.31 | 0.12 | 0.31 |
| CAZ59BO [a] | K. pneumoniae | ST-512 | blaKPC-53 | blaTEM-1, blaSHV-11 | 2 | 0.5 | 0.125 | 0.125 | 0.125 | 0.31 | 0.13 | 0.13 | 0.13 |
| R90 [b] | P. aeruginosa | - | blaKPC-2 | blaTEM-1 | 0.25 | 0.25 | 0.03 | 0.06 | 0.06 | 1.06 | 0.18 | 0.30 | 0.30 |
| Ambler class B | |||||||||||||
| N590 [b] | E. coli | ST-167 | blaNDM-5 | blaCMY-42 | 4 | 4 | 2 | 4 | 2 | 1.06 | 0.56 | 1.06 | 0.56 |
| N1700 [b] | E. coli | ST-69 | blaNDM-1 | blaCMY-4, blaCTX-M-15, blaOXA-10, blaTEM-1B | 2 | 0.5 | 0.06 | 1 | 1 | 0.31 | 0.28 | 0.56 | 0.56 |
| N2352 [b] | E. coli | - | blaNDM-5 | blaCTX-M-15, blaOXA-1, blaTEM-190 | 0.5 | 0.25 | 0.25 | 0.25 | 0.5 | 0.56 | 0.62 | 0.56 | 1.06 |
| R2752 [b] | E.coli | - | blaVIM-34 | blaTEM-1 | 0.06 | 0.03 | 0.03 | 0.06 | 0.03 | 0.56 | 0.75 | 1.06 | 0.56 |
| N1491 [b] | E. cloacae | ST-78 | blaNDM-1 | blaACT-24, blaCTX-M-15, blaTEM-1, blaOXA-1 | 4 | 4 | 2 | 4 | 4 | 1.06 | 0.62 | 1.06 | 1.06 |
| N1692 [b] | K. pneumoniae | ST-147 | blaNDM-1 | blaCTX-M-15, blaOXA-140, blaOXA-9, blaSHV-11, blaTEM-1A | 8 | 4 | 2 | 4 | 4 | 0.56 | 0.31 | 0.56 | 0.56 |
| N1697 [b] | C. freundi | - | blaNDM-1 | blaOXA-1, blaSHV-12 | 2 | 2 | 1 | 1 | 1 | 0.62 | 0.56 | 0.56 | 0.56 |
| N1215 [b] | P. aeruginosa | - | blaVIM-2 | blaOXA-486, blaPDC-3, blaPER-1, blaOXA-4 | 0.25 | 0.125 | 0.125 | 0.25 | 0.06 | 0.56 | 0.56 | 1.06 | 0.30 |
| N1539 [b] | P. aeruginosa | ST-235 | blaNDM-1 | blaPAO, blaOXA-50 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 1.06 | 1.06 | 1.06 | 1.06 |
| N1244 [b] | P. aeruginosa | ST-111 | blaIMP-18 | blaPDC-3, blaOXA-2, blaOXA-50 | 0.03 | 0.015 | 0.015 | 0.03 | 0.03 | 0.56 | 0.56 | 1.06 | 1.06 |
| N1744 [b] | P. aeruginosa | ST-2613 | blaNDM-1 | blaOXA-488, blaPAO-like | 1 | 1 | 0.5 | 0.5 | 0.5 | 1.06 | 0.56 | 0.56 | 0.56 |
| Ambler class B+D | |||||||||||||
| N1898 [b] | A. baumannii | ST-52 | blaNDM-9, blaOXA-58 | blaADC-158, blaOXA-98 | 16 | 8 | 16 | 8 | 16 | 0.56 | 1.06 | 0.56 | 1.06 |
| N2004 [b] | A. baumannii | - | blaNDM-1, blaOXA-23 | blaOXA-66, blaADC-30 | 8 | 8 | 8 | 8 | 8 | 1.06 | 1.06 | 1.06 | 1.06 |
| Ambler class D | |||||||||||||
| N1067 [b] | E. coli | ST-38 | blaOXA-181 | blaCTX-M-27 | 0.25 | 0.03 | 0.015 | 0.06 | 0.03 | 0.18 | 0.12 | 0.30 | 0.18 |
| N1085 [b] | E. coli | ST-38 | blaOXA-244 | blaCTX-M-27 | 0.12 | 0.015 | 0.03 | 0.03 | 0.015 | 0.19 | 0.37 | 0.31 | 0.19 |
| N1091 [b] | K. pneumoniae | ST-11 | blaOXA-48 | blaCTX-M-15, blaSHV-11 | 0.5 | 0.06 | 0.015 | 0.12 | 0.06 | 0.09 | 0.18 | 0.30 | 0.18 |
| N612 [b] | A. baumannii | ST-2 | blaOXA-23 | blaADC-25like, blaOXA-66 | 0.25 | 0.12 | 0.12 | 0.12 | 0.25 | 0.56 | 0.56 | 0.56 | 1.06 |
| N774 [b] | A. baumannii | ST-2 | blaOXA-40 | blaADC-25like, blaOXA-66 | 1 | 0.5 | 0.5 | 1 | 0.5 | 0.56 | 0.62 | 0.56 | 1.06 |
| N1183 [b] | A. baumannii | ST-2 | blaOXA-23 | blaADC-25-like, blaOXA-66 | 0.25 | 0.12 | 0.12 | 0.25 | 0.12 | 0.56 | 0.56 | 1.06 | 1.06 |
| ACBB0432 [a] | A. baumannii | ST-195 | blaOXA-23 | blaTEM-1 | 0.25 | 0.25 | 0.125 | 0.125 | 0.25 | 1.06 | 0.56 | 0.56 | 1.06 |
| BO415CRAB [a] | A. baumannii | ST-195 | blaOXA-23 | blaTEM-1 | 0.12 | 0.12 | 0.06 | 0.12 | 0.12 | 1.06 | 0.56 | 1.06 | 1.06 |
| Reference | |||||||||||||
| ATCC 25922 | E. coli | - | - | - | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 1.06 | 1.25 | 1.06 | 1.06 |
| ATCC 700603 | K. pneumoniae | - | - | blaSHV-18 | 0.25 | 0.015 | 0.06 | 0.06 | 0.03 | 0.12 | 0.30 | 0.30 | 0.18 |
| ATCC BAA-2814 | K. pneumoniae | - | blaKPC | blaSHV-11, blaTEM-1 | 1 | 0.03 | 0.5 | 0.06 | 0.06 | 0.15 | 0.56 | 0.12 | 0.12 |
| ATCC 27853 | P. aeruginosa | - | - | - | 0.06 | 0.06 | 0.06 | 0.06 | 0.03 | 1.06 | 1.06 | 1.06 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, G.; Gaibani, P.; Comini, S.; Boattini, M.; Banche, G.; Costa, C.; Cavallo, R.; Nordmann, P. Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms. Antibiotics 2022, 11, 1681. https://doi.org/10.3390/antibiotics11121681
Bianco G, Gaibani P, Comini S, Boattini M, Banche G, Costa C, Cavallo R, Nordmann P. Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms. Antibiotics. 2022; 11(12):1681. https://doi.org/10.3390/antibiotics11121681
Chicago/Turabian StyleBianco, Gabriele, Paolo Gaibani, Sara Comini, Matteo Boattini, Giuliana Banche, Cristina Costa, Rossana Cavallo, and Patrice Nordmann. 2022. "Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms" Antibiotics 11, no. 12: 1681. https://doi.org/10.3390/antibiotics11121681
APA StyleBianco, G., Gaibani, P., Comini, S., Boattini, M., Banche, G., Costa, C., Cavallo, R., & Nordmann, P. (2022). Synergistic Effect of Clinically Available Beta-Lactamase Inhibitors Combined with Cefiderocol against Carbapenemase-Producing Gram-Negative Organisms. Antibiotics, 11(12), 1681. https://doi.org/10.3390/antibiotics11121681












