Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus
Abstract
:1. Introduction
2. Antifungal and Antiaflatoxigenic Activities of Plant-Derived Natural Compounds against A. flavus
3. Antifungal Mechanisms of Plant-Derived Natural Compounds and EOs against A. flavus
3.1. Acting on the Cell Wall of A. flavus
3.2. Acting on the Cell Membrane of A. flavus
3.3. Acting on the Mitochondria of A. flavus
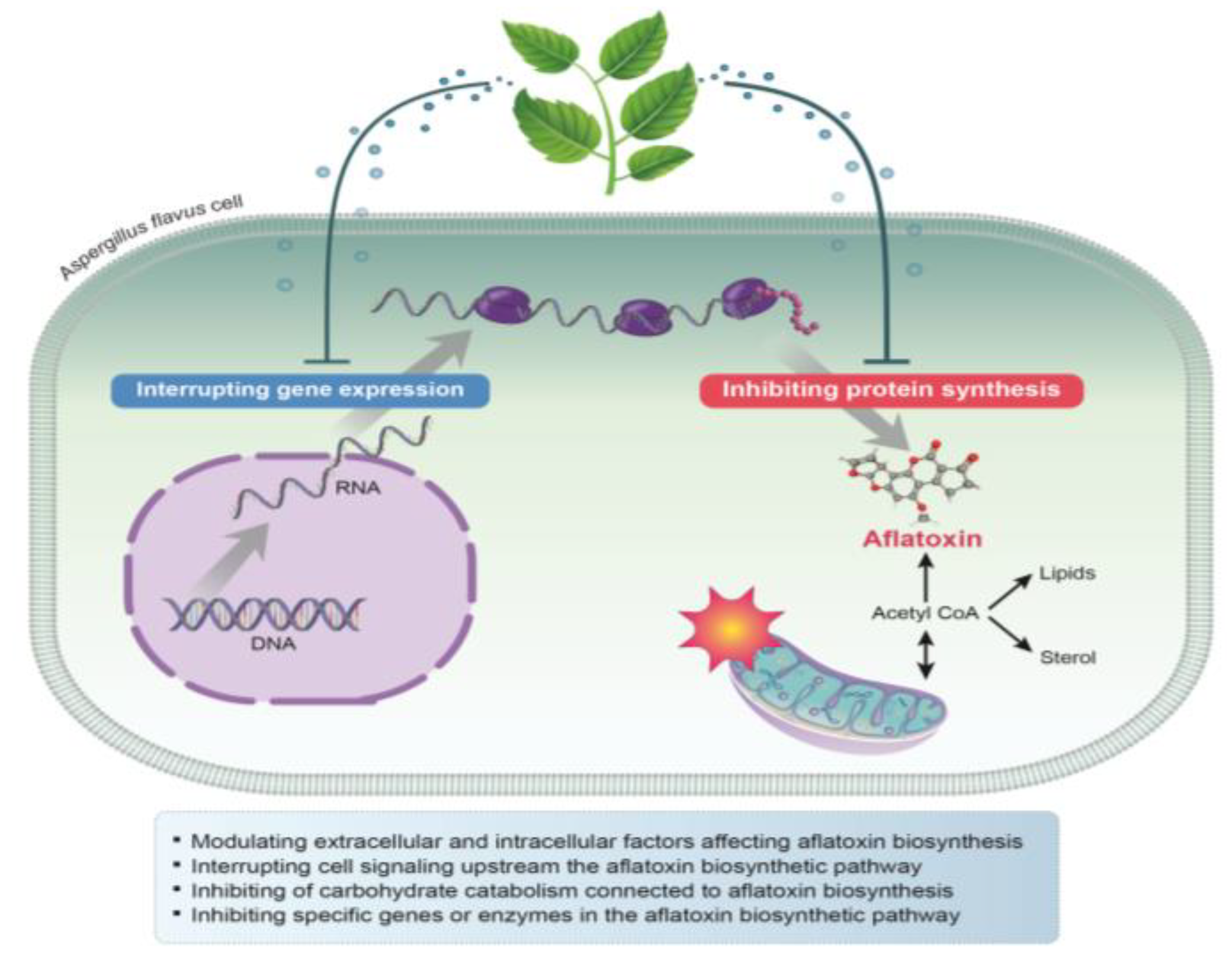
4. Antiaflatoxigenic Mechanisms of Plant-Derived Natural Compounds and EOs against A. flavus
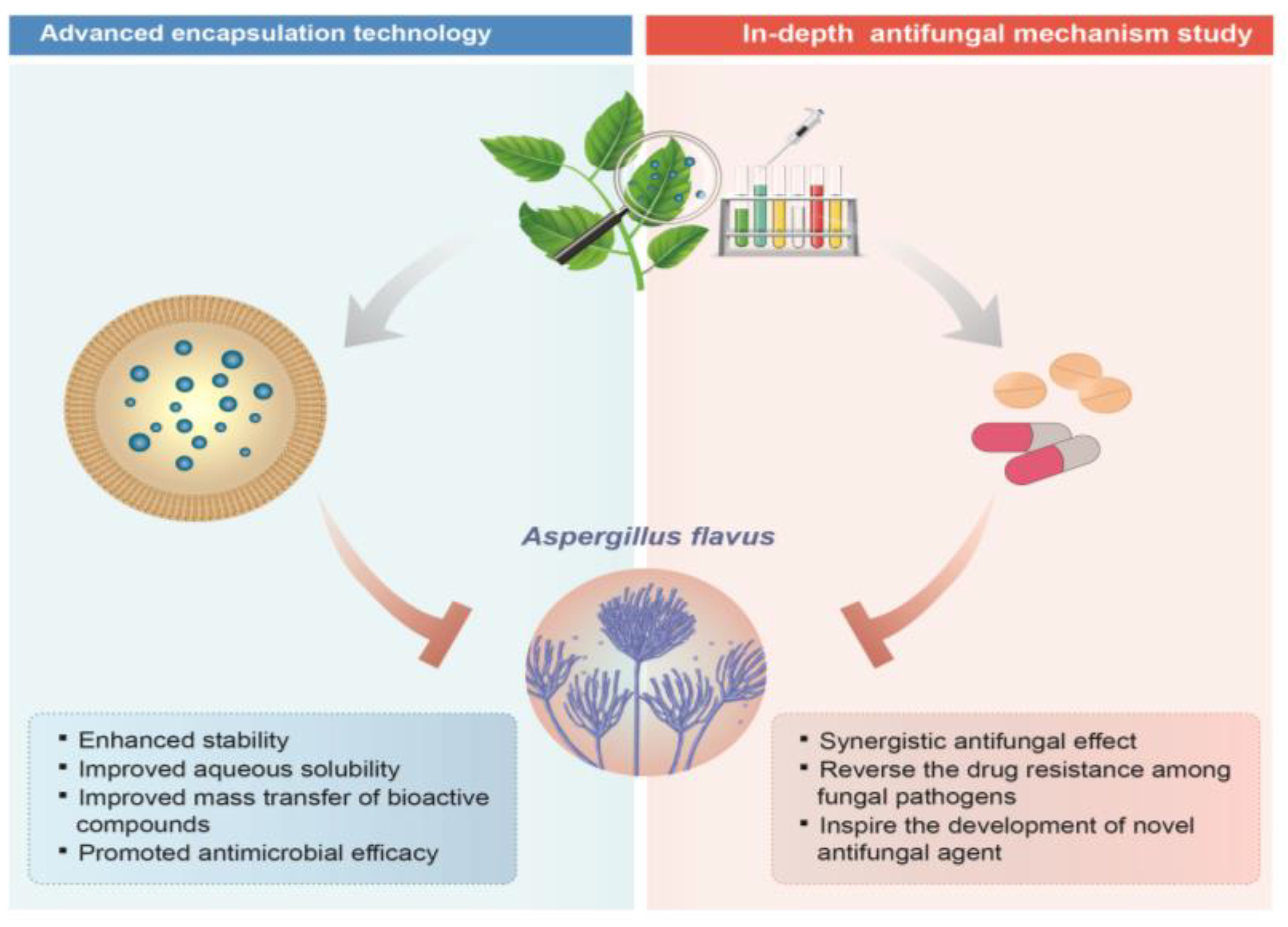
5. Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Fountain, J.; Scully, B.; Ni, X.; Kemerait, R.; Lee, D.; Chen, Z.-Y.; Guo, B. Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Baranyi, N.; Kocsubé, S.; Varga, J. Aflatoxins: Climate change and biodegradation. Curr. Opin. Food Sci. 2015, 5, 60–66. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: A prospective study. Clin. Infect. Dis 2021, 73, e3606–e3614. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef]
- Bluma, R.V.; Etcheverry, M.G. Application of essential oils in maize grain: Impact on Aspergillus section Flavi growth parameters and aflatoxin accumulation. Food Microbiol. 2008, 25, 324–334. [Google Scholar] [CrossRef]
- Lv, X.; Pan, L.; Wang, J.; Lu, L.; Yan, W.; Zhu, Y.; Xu, Y.; Guo, M.; Zhuang, S. Effects of triazole fungicides on androgenic disruption and CYP3A4 enzyme activity. Environ. Pollut. 2017, 222, 504–512. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical profiling, antioxidant, antimicrobial and cholinesterase inhibitory effects of essential oils isolated from the leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, S.; Khan, N.M.; Khan, H.; Khan, S.; Ahmad, S.; Rehman, N.; Aziz, R. Antimicrobial and antioxidant role of the aerial parts of Aconitum violaceum. J. Mex. Chem. Soc. 2021, 65, 84–93. [Google Scholar] [CrossRef]
- Ali, F.; Jan, A.K.; Khan, N.M.; Ali, R.; Mukhtiar, M.; Khan, S.; Khan, S.A.; Aziz, R. Selective biological activities and phytochemical profiling of two wild plant species, Teucrium polium and Capsicum annum from Sheringal, Pakistan. Chiang Mai J. Sci. 2018, 45, 881–887. [Google Scholar]
- Khan, H.; Ali, F.; Khan, N.M.; Shah, A.; Rahman, S.U. GC-MS Analysis of fixed oil from Nelumbo nucifera Gaertn seeds: Evaluation of antimicrobial, antileishmanial and urease inhibitory activities. J. Chem. Soc. Pak. 2016, 38, 1168–1173. [Google Scholar]
- Hu, Z.Y.; Yuan, K.; Zhou, Q.; Lu, C.; Du, L.H.; Liu, F. Mechanism of antifungal activity of Perilla frutescens essential oil against Aspergillus flavus by transcriptomic analysis. Food Control 2021, 123, 107703. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Carvajal-Moreno, M.; Correa, B.; Rojo-Callejas, F. Cellular, physiological and molecular approaches to investigate the antifungal and anti-aflatoxigenic effects of thyme essential oil on Aspergillus flavus. Food Chem. 2020, 315, 126096. [Google Scholar] [CrossRef]
- Prakash, B.; Dubey, N.K. Evaluation of chemically characterised essential oils of Coleus aromaticus, Hyptis suaveolens and Ageratum conyzoides against storage fungi and aflatoxin contamination of food commodities. Int. J. Food Sci. Technol. 2011, 46, 754–760. [Google Scholar] [CrossRef]
- Esper, R.H.; Goncalez, E.; Marques, M.O.; Felicio, R.C.; Felicio, J.D. Potential of essential oils for protection of grains contaminated by aflatoxin produced by Aspergillus flavus. Front. Microbiol. 2014, 5, 269. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dwivedy, A.K.; Dubey, N. Trachyspermum ammi L. essential oil as plant based preservative in food system. Ind. Crops Prod. 2015, 69, 104–109. [Google Scholar] [CrossRef]
- Soliman, K.M.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Prakash, B.; Mishra, P.K.; Kedia, A.; Dubey, N. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT Food Sci. Technol. 2014, 56, 240–247. [Google Scholar] [CrossRef]
- Venkatesh, H.N.; Sudharshana, T.N.; Abhishek, R.U.; Thippeswamy, S.; Manjunath, K.; Mohana, D.C. Antifungal and antimycotoxigenic properties of chemically characterised essential oil of Boswellia serrata Roxb. ex Colebr. Int. J. Food Prop. 2017, 20, 1856–1868. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božik, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapour phase against bread spoilage toxicogenic aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Jakšić, S.; Mojović, L.; Djukić-Vuković, A.; Mladenović, D.; Pejin, J. Effects of caraway and juniper essential oils on aflatoxigenic fungi growth and aflatoxins secretion in polenta. J. Food Process. Preserv. 2019, 43, e14224. [Google Scholar] [CrossRef]
- Kazemi, M. Effect of Carum copticum essential oil on growth and aflatoxin formation by Aspergillus strains. Nat. Prod. Res. 2015, 29, 1065–1068. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Palni, U.T.; Tripathi, N. Application of Chenopodium ambrosioides Linn. essential oil as botanical fungicide for the management of fungal deterioration in pulses. Biol. Agric. Hortic. 2013, 29, 197–208. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Yadav, S.; Singh, S.C.; Dubey, N.K. Safety profile assessment and efficacy of chemically characterized Cinnamomum glaucescens essential oil against storage fungi, insect, aflatoxin secretion and as antioxidant. Food Chem. Toxicol. 2013, 53, 160–167. [Google Scholar] [CrossRef]
- Gómez, J.V.; Tarazona, A.; Mateo-Castro, R.; Gimeno-Adelantado, J.V.; Jiménez, M.; Mateo, E.M. Selected plant essential oils and their main active components, a promising approach to inhibit aflatoxigenic fungi and aflatoxin production in food. Food Addit. Contam. Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1581–1595. [Google Scholar] [CrossRef]
- Khorasani, S.; Azizi, M.H.; Barzegar, M.; Hamidi-Esfahani, Z.; Kalbasi-Ashtari, A. Inhibitory effects of cinnamon, clove and celak extracts on growth of Aspergillus flavus and its aflatoxins after spraying on pistachio nuts before cold storage. J. Food Saf. 2017, 37, e12383. [Google Scholar] [CrossRef]
- Kiran, S.; Kujur, A.; Prakash, B. Assessment of preservative potential of Cinnamomum zeylanicum Blume essential oil against food borne molds, aflatoxin B1 synthesis, its functional properties and mode of action. Innov. Food Sci. Emerg. Technol. 2016, 37, 184–191. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, R.; Kumar, A.; Prakash, B.; Singh, S.; Dubey, N.K. Effect of Citrus reticulata and Cymbopogon citratus essential oils on Aspergillus flavus growth and aflatoxin production on Asparagus racemosus. Mycopathologia 2010, 170, 195–202. [Google Scholar] [CrossRef]
- Pandey, A.K.; Palni, U.T.; Tripathi, N.N. Evaluation of Clausena pentaphylla (Roxb.) DC oil as a fungitoxicant against storage mycoflora of pigeon pea seeds. J. Sci. Food Agric. 2013, 93, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Boukaew, S.; Prasertsan, P.; Sattayasamitsathit, S. Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Ind. Crops Prod. 2017, 97, 558–566. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Jamalian, J.; Heshmati, H.; Haghighi-Manesh, S. The inhibitory potential of Zataria multiflora and Syzygium aromaticum essential oil on growth and aflatoxin production by Aspergillus flavus in culture media and Iranian white cheese. Food Sci. Nutr. 2018, 6, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22, 1992–1999. [Google Scholar] [CrossRef]
- Kedia, A.; Dwivedy, A.K.; Pandey, A.K.; Kumar, R.R.; Regmi, P.; Dubey, N.K. Efficacy of chemically characterized Foeniculum vulgare Mill seed essential oil in protection of raw tobacco leaves during storage against fungal and aflatoxin contamination. J. Appl. Microbiol. 2015, 119, 991–998. [Google Scholar] [CrossRef]
- Jantapan, K.; Poapolathep, A.; Imsilp, K.; Poapolathep, S.; Tanhan, P.; Kumagai, S.; Jermnak, U. Inhibitory Effects of Thai essential oils on potentially aflatoxigenic Aspergillus parasiticus and Aspergillus flavus. Biocontrol. Sci. 2017, 22, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dubey, N.K.; Srivastava, S. Antifungal evaluation of Ocimum sanctum essential oil against fungal deterioration of raw materials of Rauvolfia serpentina during storage. Ind. Crops Prod. 2013, 45, 30–35. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sonker, N.; Singh, P. Efficacy of some essential oils against Aspergillus flavus with special reference to Lippia alba oil an inhibitor of fungal proliferation and aflatoxin B1 production in green gram seeds during storage. J. Food Sci. 2016, 81, M928–M934. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Kedia, A.; Dwivedy, A.K.; Jha, D.K.; Dubey, N.K. Efficacy of Mentha spicata essential oil in suppression of Aspergillus flavus and aflatoxin contamination in chickpea with particular emphasis to mode of antifungal action. Protoplasma 2016, 253, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Songsamoe, S.; Matan, N.; Matan, N. Antifungal activity of Michelia alba oil in the vapor phase and the synergistic effect of major essential oil components against Aspergillus flavus on brown rice. Food Control 2017, 77, 150–157. [Google Scholar] [CrossRef]
- Gibriel, Y.; Hamza, A.; Gibriel, A.; Mohsen, S. In Vivo effect of mint (Mentha viridis) essential oil on growth and aflatoxin production by Aspergillus flavus isolated from stored corn. J. Food Saf. 2011, 31, 445–451. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Chemical composition of Ocimum basilicum L. essential oil and its efficacy as a preservative against fungal and aflatoxin contamination of dry fruits. Int. J. Food Sci. Technol. 2011, 46, 1840–1846. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dwivedy, A.K.; Dubey, N.K. Assessment of chemically characterised Rosmarinus officinalis L. essential oil and its major compounds as plant-based preservative in food system based on their efficacy against food-borne moulds and aflatoxin secretion and as antioxidant. Int. J. Food Sci. Technol. 2015, 50, 1792–1798. [Google Scholar] [CrossRef]
- Tian, F.; Lee, S.Y.; Chun, H.S. Comparison of the antifungal and antiaflatoxigenic potential of liquid and vapor phase of Thymus vulgaris essential oil against Aspergillus flavus. J. Food Prot. 2019, 82, 2044–2048. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Mishra, P.K.; Dubey, N.K. Safety assessment of Zanthoxylum alatum Roxb. essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of Piper nigrum L. fruits. Int. J. Food Microbiol. 2012, 153, 183–191. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Feng, Z.; Miao, X.; Peng, X.; Wang, Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Ind. Crops Prod. 2014, 60, 151–159. [Google Scholar] [CrossRef]
- Madegowda, B.H.; Rameshwaran, P.; Nagaraju, N.P.; Murthy, P.S. In-vitro Mycological activity of essential oil from Zingiber zerumbet rhizomes. J. Essent. Oil Res. 2016, 28, 81–88. [Google Scholar] [CrossRef]
- Moghadam, H.D.; Sani, A.M.; Sangatash, M.M. Antifungal activity of essential oil of Ziziphora clinopodioides and the inhibition of aflatoxin B1 production in maize grain. Toxicol. Ind. Health 2016, 32, 493–499. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, W.; Yang, X.; Xie, L.; Wen, J.; Yang, M. GC-MS combined with chemometric techniques for the quality control and original discrimination of Curcumae longae rhizome: Analysis of essential oils. J. Sep. Sci. 2014, 37, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Amador, R.M.; Fregapane, G.; Salvador, M.D. Influence of cultivar and technological conditions on the volatile profile of virgin pistachio oils. Food Chem. 2020, 311, 125957. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Plant-based natural flavonoids show strong inhibition of aflatoxin production and related gene expressions correlated with chemical structure. Food Microbiol. 2023, 109, 104141. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Passone, M.A.; Girardi, N.S.; Etcheverry, M. Antifungal and antiaflatoxigenic activity by vapor contact of three essential oils, and effects of environmental factors on their efficacy. LWT Food Sci. Technol. 2013, 53, 434–444. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.J. Development and evaluation of a model predicting the survival of Escherichia coli O157:H7 NCTC 12900 in homemade eggplant salad at various temperatures, pHs, and oregano essential oil concentrations. Appl. Environ. Microbiol. 2000, 66, 1646–1653. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Sarrazin, S.L.; Oliveira, R.B.; Barata, L.E.; Mourao, R.H. Chemical composition and antimicrobial activity of the essential oil of Lippia grandis Schauer (Verbenaceae) from the western Amazon. Food Chem. 2012, 134, 1474–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Dang Vu, K.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Goni, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Lamberts, L.; De Bie, E.; Vandeputte, G.E.; Veraverbeke, W.S.; Derycke, V.; De Man, W.; Delcour, J.A. Effect of milling on colour and nutritional properties of rice. Food Chem. 2007, 100, 1496–1503. [Google Scholar] [CrossRef]
- Tang, J.D.; Ciaramitaro, T.; Tomaso-Peterson, M.; Diehl, S.V. Activity of two strobilurin fungicides against three species of decay fungi in agar plate tests. In Proceedings of the International Research Group on Wood Protection, Section 3, Wood Protecting Chemicals: Paper Prepared for the IRG48 Scientific Conference on Wood Protection, Ghent, Belgium, 4–8 June 2017; pp. 2–13. [Google Scholar]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Park, S.B.; Chun, H.S. Effect of plant-based compounds on the antifungal and antiaflatoxigenic efficiency of strobilurins against Aspergillus flavus. J. Hazard. Mater. 2021, 415, 125663. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 2011, 90, 1083–1094. [Google Scholar] [CrossRef]
- Belofsky, G.; Kolaczkowski, M.; Adams, E.; Schreiber, J.; Eisenberg, V.; Coleman, C.M.; Zou, Y.; Ferreira, D. Fungal ABC transporter-associated activity of isoflavonoids from the root extract of Dalea formosa. J. Nat. Prod. 2013, 76, 915–925. [Google Scholar] [CrossRef]
- Fukuda, I.; Ashida, H. Modulation of drug-metabolizing enzymes and transporters by polyphenols as an anticarcinogenic effect. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1127–1135. [Google Scholar] [CrossRef]
- Ziberna, L.; Fornasaro, S.; Čvorović, J.; Tramer, F.; Passamonti, S. Bioavailability of flavonoids: The role of cell membrane transporters. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 489–511. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Zhu, X.M.; Xie, Y.L. Antifungal efficacy of paeonol on Aspergillus flavus and its mode of action on cell walls and cell membranes. LWT-Food Sci. Technol. 2021, 149, 111985. [Google Scholar] [CrossRef]
- Belewa, V.; Baijnath, H.; Frost, C.; Somai, B.M. Tulbaghia violacea Harv. plant extract affects cell wall synthesis in Aspergillus flavus. J. Appl. Microbiol. 2017, 122, 921–931. [Google Scholar] [CrossRef] [PubMed]
- da Silva Bomfim, N.; Nakassugi, L.P.; Faggion Pinheiro Oliveira, J.; Kohiyama, C.Y.; Mossini, S.A.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Alves Abreu Filho, B.; Machinski, M., Jr. Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Marei, G.I.K.; Rasoul, M.A.A.; Abdelgaleil, S.A. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Bang, K.H.; Lee, D.W.; Park, H.M.; Rhee, Y.H. Inhibition of fungal cell wall synthesizing enzymes by trans-cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Pauli, A. Anticandidal low molecular compounds from higher plants with special reference to compounds from essential oils. Med. Res. Rev. 2006, 26, 223–268. [Google Scholar] [CrossRef] [PubMed]
- Dwivedy, A.K.; Kumar, M.; Upadhyay, N.; Prakash, B.; Dubey, N.K. Plant essential oils against food borne fungi and mycotoxins. Curr. Opin. Food Sci. 2016, 11, 16–21. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freires Ide, A.; Murata, R.M.; Furletti, V.F.; Sartoratto, A.; Alencar, S.M.; Figueira, G.M.; de Oliveira Rodrigues, J.A.; Duarte, M.C.; Rosalen, P.L. Coriandrum sativum L. (Coriander) essential oil: Antifungal activity and mode of action on Candida spp., and molecular targets affected in human whole-genome expression. PLoS ONE 2014, 9, e99086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Huang, B.; Luo, X.; Zeng, H.; Ban, X.; He, J.; Wang, Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012, 130, 520–527. [Google Scholar] [CrossRef]
- Kohiyama, C.Y.; Ribeiro, M.M.Y.; Mossini, S.A.G.; Bando, E.; da Silva Bomfim, N.; Nerilo, S.B.; Rocha, G.H.O.; Grespan, R.; Mikcha, J.M.G.; Machinski Jr, M. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015, 173, 1006–1010. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [Green Version]
- OuYang, Q.; Tao, N.; Jing, G. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genom. 2016, 17, 599. [Google Scholar] [CrossRef] [Green Version]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Dagley, M.J.; Gentle, I.E.; Beilharz, T.H.; Pettolino, F.A.; Djordjevic, J.T.; Lo, T.L.; Uwamahoro, N.; Rupasinghe, T.; Tull, D.L.; McConville, M. Cell wall integrity is linked to mitochondria and phospholipid homeostasis in Candida albicans through the activity of the post-transcriptional regulator Ccr4-Pop2. Mol. Microbiol. 2011, 79, 968–989. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y. ( E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef]
- Ma, W.B.; Zhao, L.L.; Johnson, E.T.; Xie, Y.L.; Zhang, M.M. Natural food flavour (E)-2-hexenal, a potential antifungal agent, induces mitochondria-mediated apoptosis in Aspergillus flavus conidia via a ROS-dependent pathway. Int. J. Food Microbiol. 2022, 370, 109633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Transcriptomic analysis shows the antifungal mechanism of honokiol against Aspergillus flavus. Int. J. Food Microbiol. 2022, 384, 109972. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Rao, A.; Zhang, Y.; Muend, S.; Rao, R. Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob. Agents Chemother. 2010, 54, 5062–5069. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.; Hrimpeng, K.; Lopes, G.; Vaz, S.; Goncalves, M.J.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of Ferulago capillaris essential oil against Candida, Cryptococcus, Aspergillus and dermatophyte species. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1311–1320. [Google Scholar] [CrossRef]
- Loi, M.; Paciolla, C.; Logrieco, A.F.; Mule, G. Plant bioactive compounds in pre- and postharvest management for aflatoxins reduction. Front. Microbiol. 2020, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.K.; Dwivedy, A.K.; Singh, V.K.; Das, S.; Singh, A.; Dubey, N.K. Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci. Pollut. Res. 2019, 26, 25414–25431. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osiewacz, H.D. Mitochondrial quality control in aging and lifespan control of the fungal aging model Podospora anserina. Biochem. Soc. Trans. 2011, 39, 1488–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnár, A.P.; Nemeth, Z.; Fekete, E.; Flipphi, M.; Keller, N.P.; Karaffa, L. Analysis of the relationship between alternative respiration and sterigmatocystin formation in Aspergillus nidulans. Toxins 2018, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluma, R.; Amaiden, M.R.; Daghero, J.; Etcheverry, M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J. Appl. Microbiol. 2008, 105, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Scarpari, M.; Punelli, M.; Scala, V.; Zaccaria, M.; Nobili, C.; Ludovici, M.; Camera, E.; Fabbri, A.A.; Reverberi, M.; Fanelli, C. Lipids in Aspergillus flavus-maize interaction. Front. Microbiol. 2014, 5, 74. [Google Scholar] [CrossRef]
- Zaccaria, M.; Ludovici, M.; Sanzani, S.M.; Ippolito, A.; Cigliano, R.A.; Sanseverino, W.; Scarpari, M.; Scala, V.; Fanelli, C.; Reverberi, M. Menadione-induced oxidative stress re-shapes the oxylipin profile of Aspergillus flavus and its lifestyle. Toxins 2015, 7, 4315–4329. [Google Scholar] [CrossRef]
- Roze, L.V.; Laivenieks, M.; Hong, S.Y.; Wee, J.; Wong, S.S.; Vanos, B.; Awad, D.; Ehrlich, K.C.; Linz, J.E. Aflatoxin biosynthesis is a novel source of reactive oxygen species-a potential redox signal to initiate resistance to oxidative stress? Toxins 2015, 7, 1411–1430. [Google Scholar] [CrossRef] [Green Version]
- Umesha, S.; Manukumar, H.M.; Chandrasekhar, B.; Shivakumara, P.; Shiva Kumar, J.; Raghava, S.; Avinash, P.; Shirin, M.; Bharathi, T.R.; Rajini, S.B.; et al. Aflatoxins and food pathogens: Impact of biologically active aflatoxins and their control strategies. J. Sci. Food Agric. 2017, 97, 1698–1707. [Google Scholar] [CrossRef]
- Kim, J.H.; Campbell, B.C.; Yu, J.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Bhatnagar, D.; Cleveland, T.E. Examination of fungal stress response genes using Saccharomyces cerevisiae as a model system: Targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Appl. Microbiol. Biotechnol. 2005, 67, 807–815. [Google Scholar] [CrossRef]
- Jahanshiri, Z.; Shams-Ghahfarokhi, M.; Allameh, A.; Razzaghi-Abyaneh, M. Inhibitory effect of eugenol on aflatoxin B1 production in Aspergillus parasiticus by downregulating the expression of major genes in the toxin biosynthetic pathway. World J. Microbiol. Biotechnol. 2015, 31, 1071–1078. [Google Scholar] [CrossRef]
- Moon, Y.S.; Lee, H.S.; Lee, S.E. Inhibitory effects of three monoterpenes from ginger essential oil on growth and aflatoxin production of Aspergillus flavus and their gene regulation in aflatoxin biosynthesis. Appl. Biol. Chem. 2018, 61, 243–250. [Google Scholar] [CrossRef]
- Lv, C.; Wang, P.; Ma, L.; Zheng, M.; Liu, Y.; Xing, F. Large-scale comparative analysis of eugenol-induced/repressed genes expression in Aspergillus flavus using RNA-seq. Front. Microbiol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, B.; Shukla, R.; Singh, P.; Mishra, P.K.; Dubey, N.K.; Kharwar, R.N. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res. Int. 2011, 44, 385–390. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Efficacy of Acorus calamus L. essential oil as a safe plant-based antioxidant, aflatoxin B1 suppressor and broad spectrum antimicrobial against food-infesting fungi. Int. J. Food Sci. Technol. 2013, 48, 128–135. [Google Scholar] [CrossRef]
- Singh, H.P.; Mittal, S.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem. 2009, 114, 642–645. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Canovas, J.; Barrajon-Catalan, E.; Micol, V.; Carcel, J.A.; Garcia-Perez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Giacometti, J.; Kovacevic, D.B.; Putnik, P.; Gabric, D.; Bilusic, T.; Kresic, G.; Stulic, V.; Barba, F.J.; Chemat, F.; Barbosa-Canovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedy, A.K.; Singh, V.K.; Upadhyay, N.; Chaudhari, A.K.; Das, S.; Dubey, N.K. Essential oils based formulations as safe preservatives for stored plant masticatories against fungal and mycotoxin contamination: A review. Biocatal. Agric. Biotechnol. 2019, 17, 313–317. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Ait Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Moretti, M.D.; Sanna-Passino, G.; Demontis, S.; Bazzoni, E. Essential oil formulations useful as a new tool for insect pest control. AAPS PharmSciTech 2002, 3, E13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, A.; Deepika; Dubey, N.K. Fabrication, characterization and practical efficacy of Myristica fragrans essential oil nanoemulsion delivery system against postharvest biodeterioration. Ecotoxicol. Environ. Saf. 2020, 189, 110000. [Google Scholar] [CrossRef] [PubMed]
- Gundewadi, G.; Sarkar, D.J.; Rudra, S.G.; Singh, D. Preparation of basil oil nanoemulsion using Sapindus mukorossi pericarp extract: Physico-chemical properties and antifungal activity against food spoilage pathogens. Ind. Crops Prod. 2018, 125, 95–104. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Antimicrobial activity of nanoemulsions of cinnamon, rosemary, and oregano essential oils on fresh celery. LWT 2019, 112, 108247. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]




| Name of the Plant | Major Components | Food Systems | Antifungal Activity | Antiaflatoxigenic Activity | References |
|---|---|---|---|---|---|
| Ageratum conyzoides | β-Caryophyllene; germacrene-D; dimetoxy ageratocromene | Wheat; corn; soybean | 80.8% inhibition at 1 μL/mL (vapor) in wheat grain (12 months); 79.5% and 100% inhibition in corn and soybeans, respectively, with 5 μL EO (disk diffusion assay, 5 days) | 93.7% reduction with 50 μL in 60 g corn (direct contact) (10 days); >75% reduction with 50 μL in 60 g in soybeans (direct contact) (10 days) | [16,17] |
| Ajowan (Trachyspermum ammi L.) | p-Cymene; thymol | Wheat; chickpea | 46.2% and 65.2% inhibition at 0.8 μL/mL (vapor) in wheat and chickpea, respectively (12 months) | 100% inhibition at 0.8 μL/mL (vapor) in wheat and chickpea (12 months) | [18] |
| Anise (Pimpinella anisum L.) | Anethol | Corn; wheat | MIC: 1000–3000 μg/g (dependent on aw) in corn (11 days); 100% inhibition at 1% (v/w) in wheat (14 days) | 100% inhibition at 1000 → 3000 μg/g (dependent on aw) in corn (11 days); 100% inhibition at 1% (v/w) in wheat (8 weeks) | [6,19] |
| Boldo (Pëumus boldus Mol) | α-Terpinolene; α-terperpine; p-cimene | Corn | MIC: 500–2000 μg/g (dependent on aw) (11 days) | 100% inhibition at 500–2000 μg/g (dependent on aw) (11 days) | [6] |
| Boswellia carterii Birdw | Phenylethyl alcohol; benzyl acetate | Pepper fruits (Piper nigrum L.) | 65.4% inhibition at 1.75 μL/mL (vapor) (6 months) | No record | [20] |
| Boswellia serrata | 3-Carene; β-ocimene | Corn | No record | 95.6% inhibition at 10 μL/g (10 days) | [21] |
| Cananga odorata | β-Caryophyllene | Chickpea | 77.4% inhibition at 2 μL/mL (vapor) (6 months) | No record | [22] |
| Caraway (Carum carvi) | Limonene; carvone | Bread; polenta | 90% inhibition at 636.47 μL/L (vapor) in bread (14 days) | 100% inhibition in bread requires > 500 μL/L of EO (vapor) (14 days); 100% inhibition at 4.5 μg/g in polenta (14 days) | [23,24] |
| Carum copticum | p-Cymene; γ-terpinene; thymol | Cherry tomato | 58.0% inhibition at 100 μL/mL (vapor) (30 days) | No record | [25] |
| Chenopodium ambrosioides Linn. | α-Terpinene; p-cymene; Ascaridole | Pigeon pea | 100% inhibition at 0.29 μL/mL (vapor) (6 months) | No record | [26] |
| Cicuta virosa L. var. latisecta Celak | p-Cymene; γ-terpinene; cuminaldehyde | Cherry tomato | 89.9% inhibition at 200 μg/mL (vapor) (9 days) | No record | [27] |
| Cinnamomum glaucescens | 1,8-Cineole; 2-propenoic acid | Chickpea | 71.1% inhibition at 4.5 μL/mL (vapor) (12 months) | No record | [28] |
| Cinnamon (Cinnamomum verum) | Cinnamaldehyde; eugenol | Maize extract medium; bread | 90% inhibition at 820 → 1000 mg/L (depending on temperature and water activity) in maize extract medium (12 days); 90% inhibition at 558.44 μL/L (vapor) in bread (14 days) | 500–1000 mg/L (depending on temperature and water activity) in maize extract medium (12 days); 100% inhibition at 500 μL/L (vapor) in bread (14 days) | [23,29] |
| Cinnamon (Cinnamomum zeglanicum) | Cinnamaldehyde; eugenol; phenol, 2-methoxy-4-(2-propenyl)phenol | Pistachio; finger millet (Eleusine coracana); wheat | 60–83% inhibition at 0.5 μL/mL (vapor) in finger millet (up to 6 months); 100% inhibition at 2% (v/w) in wheat (14 days) | 100% inhibition with 25 mL of 9% (vol/vol) EO solution in 500 g pistachio (3 months); 100% inhibition at 2% (v/w) in wheat (8 weeks) | [19,30,31] |
| Citrus reticulata | Limonene; geranial; neral | Tuberous roots of Asparagus racemosus | 68.6% inhibition at 500 ppm (v/v) (12 months) | 61.76% inhibition at 500 ppm (v/v) (12 months) | [32] |
| Clausena pentaphylla | Sabinene; α-terpinolene; methyl eugenol | Pigeon pea seed | 100% inhibition at 0.29 μL/mL (vapor) (6 months) | No record | [33] |
| Clove (Caryophyllus aromaticus) | eugenol | Pistachio | No record | 100% inhibition with 25 mL of 9% (vol/vol) EO in 500 g pistachio (3 months). | [30] |
| Clove (Syzygium aromaticum L.) | Benzenemethanol; eugenol; eugeyl acetate; β-caryophyllene | Corn; Iranian white cheese; bread; tomato paste | 100% inhibition at 500–3000 μg/g (dependent on water activity) in corn (11 days); 100% inhibition at 10 μL/L (vapor) in corn (5 days); 100% inhibition at 150 ppm in Iranian white cheese (up to 40 days); 90% inhibition at 674.49 μL/L (vapor) in bread (14 days); 48% inhibition at 500 ppm in tomato paste (2 months) | 100% inhibition at 1000–2000 μg/g (dependent on water activity) in corn (11 days); 100% inhibition at 10 μL/L (vapor) in corn (5 days); 100% inhibition at 50–150 ppm in Iranian white cheese (up to 40 days); 100% inhibition at 500 μL/L (vapor) in bread (14 days); | [6,23,34,35,36] |
| Coleus aromaticus | Thymol; γ-terpinene; p-cymene | Wheat | 87.37% inhibition at 0.1 μL/mL (vapor) (12 months); | No record | [16] |
| Commiphora myrrha | α–Elemene; curzerene; furanoeudesma-1,3-diene | Chickpea | 55.4% inhibition at 3 μL/mL (vapor) (6 months) | No record | [22] |
| Coriander (Coriandrum sativum L.) | Linalool; λ-terpinene | Chickpea | 65.5% inhibition at 2.5 μL/mL (vapor) (6 months) | No record | [22] |
| Cymbopogon citratus | Geranial; neral; myrcene | Tuberous root of Asparagus racemosus | 78.4% inhibition at 500 ppm (v/v) (12 months) | 100% inhibition at 500 ppm (v/v) on (12 months) | [32] |
| Dill (Anethum graveolens L.) | Carvone; limonene; apiol | Cherry tomato | 88.9% inhibition at 120 μg/mL (vapor) (9 days) | No record | [37] |
| Fennel (Foeniculum vulgare) | Estragole; anethole | Tobacco leave | 51.20–55.35% inhibition at 1.25 μL/mL (vapor) (6 months); | 100% inhibition at 1.25 μL/mL (vapor) (6 months); | [38] |
| Fingerroot (Boesenbergia rotunda) | Nerol; L-camphor | In-shell peanut | No record | 98.3% and 18.0% inhibition at 16% (v/v, in mineral oil) when applied via direct exposure and vapor exposure, respectively (10 days). | [39] |
| Hedychium spicatum | 1,8-Cineole | Chickpea | 72.0% inhibition at 2.5 μL/mL (vapor) | No record | [22] |
| Holy basil (Ocimum sanctum) | Eugenol; β-caryophyllene | Apocynaceae (Rauvolfia serpentina L., medicinal plant) | 74.0% inhibition at 1 μL/mL (vapor) (6 months) | No record | [40] |
| Hyptis suaveolens | β -Caryophyllene; caryophyllene oxide; sabinene | Wheat | 83.3% inhibition at 1.2 μL/mL (vapor) (12 months) | No record | [16] |
| Juniper (Juniperus communis L.) | α-Pinene | Polenta | No record | 100% inhibition at 50 μg/g (14 days) | [24] |
| Lemongrass (Cymbopogon citrati [DC] Stapf.) | Neral; geranial | Bread | 90% inhibition at 134.12 μL/L (vapor) (14 days) | 100% inhibition at 125 μL/L (vapor) (14 days) | [23] |
| Lippia alba | Myrcene; neral; geranial | Green gram seed | 92.5% inhibition at 80 μL/0.25 L (vapor) (6 months) | 100% inhibition at 80 μL/0.25 L (vapor) (6 months) | [41] |
| Litsea cubeba | D-Limonene; (Z)-limonene oxide; (E)-limonene oxide | Licorice | 100% inhibition at 5 μL/g (vapor) (20 days); | 100% inhibition at 5 μL/g (vapor) (20 days); | [42] |
| Marjoram (Origanum majorana L.) | Terpinen-4-ol; cis-sabinene hydrate; p-cymene | Chickpea | 67.9% inhibition at 3 μL/mL (vapor) (6 months) | No record | [22] |
| Mentha spicata L. | Carvone; limonene | Chickpea | 52.2% inhibition at 1 μL/mL (vapor) (12 months); | 100% inhibition at 1 μL/mL (vapor) (12 months); | [43] |
| Michelia alba | Linalool | Brown rice | 100% inhibition at 300 μL/L (vapor) (12 weeks) | No record | [44] |
| Mint (Mentha viridis) | Menthone; carvone | Wheat; corn | 100% inhibition at 200 mL/100 g in corn (21 days); 92% inhibition at 2% (v/w) in wheat (14 days) | 100% inhibition at 300 mL/100 g in corn (21 days); >99% inhibition at 2% (v/w) in wheat (8 weeks) | [19,45] |
| Mountain thyme (Hedeoma multiflora Benth) | α-Terpinolene; p-cymene; carvacrol | Corn | 100% inhibition at 500–2000 μg/g (dependent on water activity) (11 days); | 100% inhibition at 1000 μg/g (11 days); | [6] |
| Ocimum basilicum L. | Methyl eugenol | Dry fruits (cashew nut, almond, grapes, chironji, groundnut, date palm, and coconut) | 53.8–65.5% inhibition at 1 μg/mL (vapor) (6 months) | No record | [46] |
| Oregano (Origanum vulgare L.) | Carvacrol; linalool; 4-terpineol | Maize extract medium; bread; corn; soybean | 90% inhibition at 820 → 1000 mg/L (depending on temperature and water activity) in maize extract medium (12 days); 90% inhibition at 319.85 μL/L (vapor) in bread (14 days); 100% inhibition with 5 μL EO in corn and soybean (disk diffusion assay, 5 days) | 100% inhibition at >1000 mg/L in maize extract medium (12 days); 100% inhibition at 125 μL/L (vapor) in bread (14 days); >90% and 88.16% inhibition with 200 μL EO in 60 g corn and soybean, respectively (direct contact) (10 days) | [17,23,29] |
| Pine (Pinus pinaster) | β-Caryophyllene; β-selinene | In-shell peanut | No record | 98.1% and 12.9% inhibition at 16% (v/v, in mineral oil) when applied via direct exposure and vapor exposure, respectively (10 days) | [39] |
| Poleo (Lippia turbinate var. integrifolia (griseb)) | Peperitenone oxide; limonene | Corn | 100% inhibition at 500–2000 μg/g (depending on water active) (11 days) | 100% inhibition at 500–2000 μg/g (depending on water active) (11 days) | [6] |
| Rosewood (Aniba rosaeodora) | Linalool | In-shell peanut | No record | 98.5% and 17.2% inhibition at 16% (v/v, in mineral oil) when applied via direct exposure and vapor exposure, respectively (10 days) | [39] |
| Rosmarinus officinalis L. | α-Pinene; 1, 8-cineole; camphor | Black pepper (Piper nigrum) | 73.5% inhibition at 1.5 μL/mL (vapor) (6 months); | No record | [47] |
| Styrax tonkinensis | Benzoic acid; 6-phenyl-tetrahydro-naphthaline | In-shell peanut | No record | 95.8% and 20.2% inhibition at 16% (v/v, in mineral oil) when applied via direct exposure and vapor exposure, respectively (10 days). | [39] |
| Summer savory (Satureja hortensis) | γ-terpinene; carvacrol; thymol | Tomato paste | 59% inhibition at 500 ppm (2 months) | No record | [36] |
| Thyme (Thymus vulgaris L.) | Carvacrol; a-terpinolene; thymol; p-cymene; β-phellandrene; linalool | Bread; tomato paste; wheat; brown rice; white rice | 90% inhibition at 474.2 μL/L (vapor) in bread (14 days); 87% inhibition at 500 ppm in tomato paste (2 months); 100% inhibition at 1% (v/w) in wheat (14 days) | 100% inhibition at 250 μL/L (vapor) in bread (14 days); 100% inhibition at 1% (v/w) on wheat (8 weeks); 72.7% inhibition at 10 μg/mL (vapor) in brown rice; 18.0% inhibition at 10 μg/mL (vapor) in white rice | [19,23,36,48] |
| Thyme (Zataria multiflora) | Thymol; carvacrol | Iranian white cheese | 89.0% inhibition at 600 ppm (up to 40 days) | 92.9% inhibition at 600 ppm (up to 40 days) | [35] |
| Thymus daenensis Celak | Thymol; carvacrol | Pistachio | No record | 100% inhibition with 25 ml of 9% (vol/vol) EO solution in 500 g pistachio (3 months) | [30] |
| Turmeric (Curcuma longa L.) | Tumerone; ar-turmerone; β-sesquiphellandrene; zingiberene; cycloisolongifolene | Corn | ~90% inhibition at 4 μg/mL (5 days) | ~93% inhibition at 4 μg/mL (5 days) | [49] |
| Vatica (Vatica diospyroides Symington) | Benzyl acetate | Corn | 100% inhibition at 50 μL/L (vapor) (5 days) | 100% inhibition at 50 μL/L (vapor) (5 days) | [34] |
| Ylang ylang (Cananga odorata) | Linalool; benzyl acetate; tetradecane; germacrene D | In-shell peanut | No record | 96.4% and 25.1% inhibition at 16% (v/v, in mineral oil) when applied via direct exposure and vapor exposure, respectively (10 days). | [39] |
| Zanthoxylum alatum | Linalool; methyl cinnamate | Black pepper (Piper nigrum) | 87.6% inhibition at 2.5 μL/mL (vapor) (6 months) | No record | [50] |
| Zanthoxylum molle Rehd | Limonene; terpinen-4-ol; 2-undecanone | Cherry tomato | 91.7% inhibition at 0.2 μg/mL (vapor) (9 days) | No record | [51] |
| Zingiber zerumbet | α-Caryophyllene; zerumbone | Corn | 100% inhibition at 200 ppm (15 days) | 100% inhibition at 100 ppm (direct contact) (15 days) | [52] |
| Ziziphora clinopodioides | Pulegone; piperitenone; p-menth-3-en-8-ol | Corn | No record | 99.8% inhibition at 6250 μg/mL (29 days) | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Zheng, Y.; Chun, H.S. Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics 2022, 11, 1727. https://doi.org/10.3390/antibiotics11121727
Tian F, Woo SY, Lee SY, Park SB, Zheng Y, Chun HS. Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics. 2022; 11(12):1727. https://doi.org/10.3390/antibiotics11121727
Chicago/Turabian StyleTian, Fei, So Young Woo, Sang Yoo Lee, Su Been Park, Yaxin Zheng, and Hyang Sook Chun. 2022. "Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus" Antibiotics 11, no. 12: 1727. https://doi.org/10.3390/antibiotics11121727
APA StyleTian, F., Woo, S. Y., Lee, S. Y., Park, S. B., Zheng, Y., & Chun, H. S. (2022). Antifungal Activity of Essential Oil and Plant-Derived Natural Compounds against Aspergillus flavus. Antibiotics, 11(12), 1727. https://doi.org/10.3390/antibiotics11121727





