Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity on Planktonic Bacteria
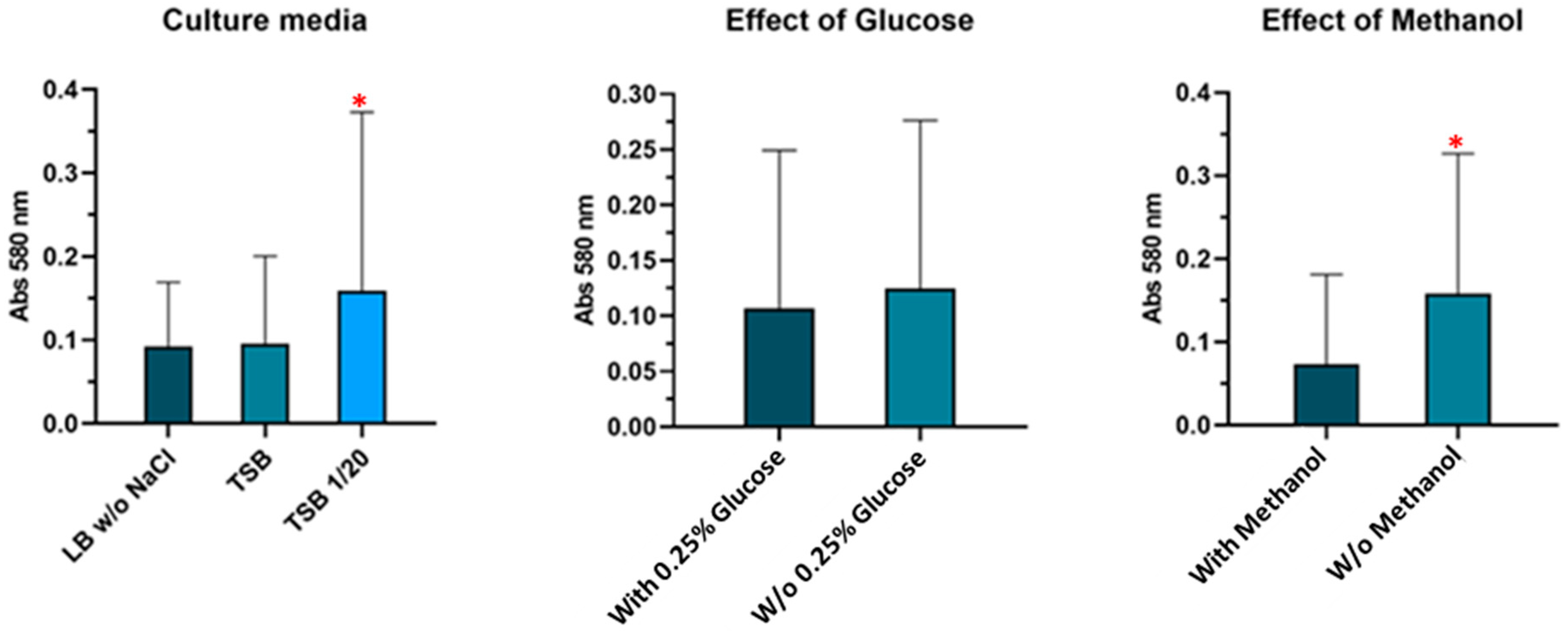
2.2. Assessment of Biofilm Formation
2.3. Antibiofilm Activity
2.4. Analysis of Antimicrobial Synergy
2.5. In Vitro Cytotoxicity Assessment
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Bacterial Strains
4.3. Minimal Inhibitory Concentration Determination
4.4. Minimal Bactericidal Concentration Determination
4.5. Biofilm Formation
4.6. Antibiofilm Activity
4.7. Antimicrobial Synergy Study
4.8. In Vitro Cytotoxicity Assay
4.9. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. AMR One Health Network Meeting. 2017. Available online: https://ec.europa.eu/health/sites/default/files/antimicrobial_resistance/docs/ev_20210325_co02_en.pdf (accessed on 15 June 2017).
- World Health Organization. Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. 2020. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 15 April 2021).
- Wallinga, D.; Smit, L.A.M.; Davis, M.F.; Casey, J.A.; Nachman, K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Health Rep. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Collignon, P.J.; McEwen, S.A. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Brunel, J.-M.; Dubus, J.-C.; Reynaud-Gaubert, M.; Rolain, J.-M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef]
- Gharaibeh, M.H.; Shatnawi, S.Q. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: A review. Vet. World 2019, 12, 1735–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.; Sharp, J. Rethinking one health: Emergent human, animal and environmental assemblages. Soc. Sci. Med. 2020, 258, 113093. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 20 January 2022).
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob Chemother 2018, 73, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The composition of Ehrlich’s Salvarsan: Resolution of a century-old debate. Angew Chem. Int. Ed. 2005, 44, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs RD 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Herrera, R.P.; Gimeno, M.C. Anticancer properties of gold complexes with biologically relevant ligands. Pure Appl. Chem. 2019, 91, 247–269. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Higby, G.J. Gold in medicine. Gold Bull 1982, 15, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratia, C.; Soengas, R.G.; Soto, S.M. Gold-Derived Molecules as New Antimicrobial Agents. Front. Microbiol. 2022, 13, 846959. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Leitão, J.H.; Silva, R.A.; Belo, D.; Santos, I.C.; Guerreiro, J.F.; Martins, M.; Fontinha, D.; Prudêncio, M.; Almeida, M.; et al. On the path to gold: Monoanionic Au bisdithiolate complexes with antimicrobial and antitumor activities. J. Inorg. Biochem. 2020, 202, 110904. [Google Scholar] [CrossRef]
- Abás, E.; Aguirre-Ramírez, D.; Laguna, M.; Grasa, L. Selective Anticancer and Antimicrobial Metallodrugs Based on Gold(III) Dithiocarbamate Complexes. Biomedicines 2021, 9, 1775. [Google Scholar] [CrossRef]
- Sánchez, E.B.; Iglesias, M.J.; el Hajjouji, H.; Roces, L.; García-Granda, S.; Villuendas, P.; Urriolabeitia, E.P.; Ortiz, F.L. Cycloaurated Phosphinothioic Amide Complex as a Precursor of Gold(I) Nanoparticles: Efficient Catalysts for A3 Synthesis of Propargylamines under Solvent-Free Conditions. Organometallics 2017, 36, 1962–1973. [Google Scholar] [CrossRef]
- Ratia, C.; Cepas, V.; Soengas, R.; Navarro, Y.; Andrés, M.V.-D.; Iglesias, M.J.; Lozano, F.; López-Ortiz, F.; Soto, S.M. A CS-Cyclometallated Gold(III) Complex as a Novel Antibacterial Candidate Against Drug-Resistant Bacteria. Front. Microbiol. 2022, 13, 815622. [Google Scholar] [CrossRef]
- Soto, S.; Ratia, C.; Cepas, V.; López, Y.; López-Ortiz, F.; Iglesias, M.J.; Soengas, R.G. A Gold(III) Complex, a Conjugate of the Gold(III) Complex, a Pharmaceutical Composition Comprising the Gold(III) Complex and Uses and a Process for Preparing the Gold(III) Complex. WO Patent Application 2019211222 A1, 7 November 2019. [Google Scholar]
- Mobarki, N.; Almerabi, B.; Hattan, A. Antibiotic Resistance Crisis. Int. J. Med. Dev. Ctries. 2019, 3, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, B.; Greko, C. Antibiotic resistance-consequences for animal health, welfare, and food production. Upsala J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Radulović, N.S.; Stojanović, N.M.; Glišić, B.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Mitić, K.V.; Nikolić, M.G.; Djuran, M.I. Water-soluble gold(III) complexes with N-donor ligands as potential immunomodulatory and antibiofilm agents. Polyhedron 2018, 141, 164–180. [Google Scholar] [CrossRef]
- Ben Vezina, B.; Rosa, M.N.; Canu, A.; Tola, S. Genomic surveillance reveals antibiotic resistance gene transmission via phage recombinases within sheep mastitis-associated Streptococcus uberis. BMC Vet. Res. 2022, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Role of Biofilms in Antimicrobial Resistance. ASAIO J. 2000, 46, S47–S52. [Google Scholar] [CrossRef]
- Abdullahi, U.F.; Igwenagu, E.; Mu’Azu, A.; Aliyu, S.; Umar, M.I. Intrigues of biofilm: A perspective in veterinary medicine. Vet. World 2016, 9, 12–18. [Google Scholar] [CrossRef]
- Bowler, P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef]
- Mizdal, C.R.; Stefanello, S.T.; Flores, V.D.C.; Agertt, V.A.; Bonez, P.C.; Rossi, G.G.; da Silva, T.C.; Soares, F.A.A.; Marques, L.D.L.; de Campos, M.M.A. The antibacterial and anti-biofilm activity of gold-complexed sulfonamides against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2018, 123, 440–448. [Google Scholar] [CrossRef]
- Kumar, H.; Chen, B.H.; Kuca, K.; Nepovimova, E.; Kaushal, A.; Nagraik, R.; Bhatia, S.K.; Dhanjal, D.S.; Kumar, V.; Kumar, A.; et al. Understanding of colistin usage in food animals and available detection techniques: A review. Animals 2020, 10, 1892. [Google Scholar] [CrossRef]
- Spapen, H.; Jacobs, R.; Van Gorp, V.; Troubleyn, J.; Honoré, P.M. Renal and neurological side effects of colistin in critically ill patients. Ann. Intensiv. Care 2011, 1, 14. [Google Scholar] [CrossRef]
- Malone, M.; Goeres, D.M.; Gosbell, I.; Vickery, K.; Jensen, S.; Stoodley, P. Approaches to biofilm-associated infections: The need for standardized and relevant biofilm methods for clinical applications. Expert Rev. Anti-Infect. Ther. 2017, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. 2019. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 17 November 2021).
- Petrosillo, N.; Ioannidou, E.; Falagas, M. Colistin monotherapy vs. combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008, 14, 816–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan-Krohn, T.; Pironti, A.; Kirby, J.E. Synergistic Activity of Colistin-Containing Combinations against Colistin-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00873-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muenraya, P.; Sawatdee, S.; Srichana, T.; Atipairin, A. Silver nanoparticles conjugated with colistin enhanced the anti-microbial activity against Gram-negative bacteria. Molecules 2022, 27, 5780. [Google Scholar] [CrossRef] [PubMed]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic efficacy of colistin and silver nanoparticles impregnated human amniotic membrane in a burn wound infected rat model. Nat. Sci. Rep. 2022, 12, 6414. [Google Scholar]
- Samanta, T.; Roymahapatra, G.; Porto, W.F.; Seth, S.; Ghorai, S.; Saha, S.; Sengupta, J.; Franco, O.L.; Dinda, J.; Mandal, S.M. N, N′-Olefin Functionalized Bis-Imidazolium Gold(I) Salt Is an Efficient Candidate to Control Keratitis-Associated Eye Infection. PLoS ONE 2013, 8, e58346. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Naz, S.; Khan, F.; Aziz, M.; Asadullah Khan, M.N.; Khan, S.I. Antibacterial fatty acids destabilize hydro-phobic and multicellular aggregates of biofilm in S. aureus. J. Antibiot. 2017, 70, 115–121. [Google Scholar] [CrossRef]
- Maia, P.I.D.S.; Deflon, V.M.; Abram, U. Gold(III) complexes in medicinal chemistry. Futur. Med. Chem. 2014, 6, 1515–1536. [Google Scholar] [CrossRef]
- Messori, L.; Abbate, F.; Marcon, G.; Orioli, P.; Fontani, M.; Mini, E.; Mazzei, T.; Carotti, S.; O’Connell, T.; Zanello, P. Gold(III) Complexes as Potential Antitumor Agents: Solution Chemistry and Cytotoxic Properties of Some Selected Gold(III) Compounds. J. Med. Chem. 2000, 43, 3541–3548. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization, and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 8260496. [Google Scholar] [CrossRef]
- Fan, D.; Yang, C.-T.; Ranford, J.D.; Vittal, J.J.; Lee, P.F. Synthesis, characterization, and biological activities of 2-phenylpyridine gold(iii) complexes with thiolate ligands. Dalton Trans. 2003, 3376–3381. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Henderson, W.; Nicholson, B.K. Cycloaurated triphenylphosphine-sulfide and -selenide. Dalton Trans. 2010, 39, 1855–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 30th Edition Vol. CLSI Supplement M100 (Wayne, PA, USA: Clinical and Laboratory Standard Institute). 2020. Available online: https://clsi.org/standards/prod-ucts/microbiology/documents/m100/ (accessed on 18 November 2020).
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, J.E.; Rankin, S.C. Isolation and Identification of Aerobic and Anaerobic Bacteria. In Canine and Feline Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2013; pp. 17–28. [Google Scholar] [CrossRef]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Neiger, M.R.; González, J.F.; Gonzalez-Escobedo, G.; Kuck, H.; White, P.; Gunn, J.S. Pathoadaptive Alteration of Salmonella Biofilm Formation in Response to the Gallbladder Environment. J. Bacteriol. 2019, 201, e00774-18. [Google Scholar] [CrossRef] [Green Version]
- González, J.F.; Alberts, H.; Lee, J.; Doolittle, L.; Gunn, J.S. Biofilm formation protects Salmonella from the antibiotic ciprof-loxacin in vitro and in vivo in the mouse model of chronic carriage. Sci. Rep. 2018, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Saiman, L. Clinical utility of synergy testing for multidrug-resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis: ‘the motion for’. Paediatr. Respir. Rev. 2007, 8, 249–255. [Google Scholar] [CrossRef]



| Species | Reference | 1 | 2a | 2b | 2c | 2d | 2e | 2f | 3 | CIP | CST | AMP | SXT | VAN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella rissen | E3G2V1C7R | 64 | 16 | 8 | 8 | 16 | 32 | 8 | 8 | 0.25 | 0.25 | 128 | 4 | - |
| Salmonella panama | E3G3V2C1 | 64 | 16 | 8 | 8 | 16 | 64 | 8 | 16 | 0.1 | 0.5 | 128 | 8 | - |
| Salmonella anatum | E3G2V1C34R | 64 | 16 | 8 | 8 | 16 | 64 | 8 | 16 | 0.25 | 0.25 | 128 | 16 | - |
| E. coli | GN1044 | 64 | 16 | 8 | 8 | 16 | 32 | 8 | 8 | 0.015 | 32 | 128 | 16 | - |
| E. coli | GN1035 | 64 | 16 | 8 | 8 | 16 | 64 | 4 | 8 | 0.5 | 8 | 128 | 16 | - |
| E. coli | 2462 ME2-1 | 64 | 16 | 16 | 8 | 16 | 32 | 8 | 8 | 1 | 0.125 | 128 | 16 | - |
| E. coli | 2461 ME2-2 | 64 | 16 | 8 | 8 | 16 | 32 | 8 | 8 | 1 | 0.125 | 128 | 16 | - |
| E. coli | GN444 | 64 | 16 | 16 | 8 | 16 | 32 | 4 | 16 | 1 | 0.125 | 128 | 16 | - |
| E. coli | 173 | 64 | 16 | 16 | 16 | 16 | 32 | 8 | 8 | 1 | 0.125 | 128 | 16 | - |
| Pseudomonas aeruginosa | 3162PE1 | 64 | 32 | 16 | 16 | 32 | 64 | 8 | 16 | 0.125 | 0.5 | 64 | - | - |
| Staphylococcus haemolyticus | 620DD1 | 16 | 0.03 | 0.5 | 1 | 0.125 | 0.5 | 0.25 | 0.125 | - | - | 1 | - | 2 |
| Staphylococcus xylosus | 930DE1 | 16 | 0.25 | 0.5 | 1 | 0.25 | 1 | 0.5 | 0.25 | - | - | 0.125 | - | 4 |
| Staphylococcus chromogenes | 401-PD1 | 8 | 0.25 | 0.125 | 0.5 | 0.125 | 0.25 | 0.25 | 0.06 | - | - | 2 | - | 2 |
| Staphylococcus chromogenes | 21AD-1 | 8 | 0.125 | 0.125 | 0.5 | 0.06 | 0.5 | 0.5 | 0.125 | - | - | 2 | - | 2 |
| Staphylococcus chromogenes | 7439PE-1 | 8 | 0.06 | 0.25 | 0.125 | 0.06 | 0.5 | 0.125 | 0.06 | - | - | 8 | - | 2 |
| Streptococcus uberis | 380 ME-3 | 32 | 1 | 1 | 2 | 2 | 8 | 1 | 0.5 | - | - | 64 | - | 1 |
| Species | Reference | 1 | 2a | 2b | 2c | 2d | 2e | 2f | 3 | CST | VAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella rissen | E3G2V1C7R | 64 | 64 | 16 | 16 | 16 | 64 | 32 | 128 | 0.25 | - |
| Salmonella panama | E3G3V2C1 | 128 | 64 | 32 | 32 | 16 | 64 | 16 | 64 | 0.5 | - |
| Salmonella anatum | E3G2V1C34R | 64 | 64 | 32 | 32 | 16 | 64 | 16 | 64 | 0.25 | - |
| E. coli | GN1044 | 64 | 32 | 16 | 16 | 16 | 64 | 16 | 64 | 16 | - |
| E. coli | GN1035 | 64 | 64 | 32 | 32 | 32 | 64 | 32 | 64 | 16 | - |
| E. coli | 2462 ME2-1 | 64 | 64 | 16 | 16 | 16 | 64 | 32 | 64 | 0.25 | - |
| E. coli | 2461 ME2-2 | 64 | 16 | 8 | 8 | 16 | 64 | 16 | 32 | 0.125 | - |
| E. coli | GN444 | 64 | 16 | 16 | 8 | 16 | 64 | 8 | 32 | 0.25 | - |
| E. coli | 173 | 64 | 16 | 16 | 16 | 16 | 64 | 32 | 32 | 0.125 | - |
| Pseudomonas aeruginosa | 3162PE1 | 128 | 32 | 32 | 32 | 64 | 64 | 32 | 32 | 1 | - |
| Staphylococcus haemolyticus | 620DD1 | 16 | 0.125 | 0.5 | 1 | 0.25 | 1 | 0.5 | 0.25 | - | 2 |
| Staphylococcus xylosus | 930DE1 | 16 | 0.5 | 0.5 | 1 | 0.5 | 1 | 2 | 0.5 | - | 4 |
| Staphylococcus chromogenes | 401-PD1 | 16 | 0.5 | 0.125 | 1 | 0.125 | 1 | 0.5 | 0.125 | - | 2 |
| Staphylococcus chromogenes | 21AD-1 | 16 | 0.25 | 0.125 | 2 | 0.25 | 0.5 | 0.5 | 0.125 | - | 2 |
| Staphylococcus chromogenes | 7439PE-1 | 8 | 0.06 | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.06 | - | 2 |
| Streptococcus uberis | 380 ME-3 | 128 | 16 | 81 | 8 | 16 | 16 | 8 | 16 | - | 2 |
| Species | Reference | 1 | 2a | 2b | 2c | 2d | 2e | 2f | 3 | CST | VAN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella panama | E3G3V2C1 | 16 | 64 | 8 | 8 | 8 | >64 | 8 | 16 | 4 | - |
| Salmonella anatum | E3G2V1C34R | 16 | >64 | 16 | 16 | 16 | >64 | 32 | 32 | 4 | - |
| E. coli | 2462 ME2-1 | 16 | 32 | 8 | 4 | 16 | >64 | 4 | 16 | 2 | - |
| E. coli | 2461 ME2-2 | 16 | 32 | 8 | 4 | 16 | >64 | 4 | 8 | 2 | - |
| E. coli | GN444 | 16 | 32 | 8 | 8 | 16 | >64 | 4 | 8 | 4 | - |
| Staphylococcus haemolyticus | 620DD1 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | - | 16 |
| Staphylococcus chromogenes | 401-PD1 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | - | 16 |
| Staphylococcus chromogenes | 21AD-1 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | - | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratia, C.; Sueiro, S.; Soengas, R.G.; Iglesias, M.J.; López-Ortiz, F.; Soto, S.M. Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance. Antibiotics 2022, 11, 1728. https://doi.org/10.3390/antibiotics11121728
Ratia C, Sueiro S, Soengas RG, Iglesias MJ, López-Ortiz F, Soto SM. Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance. Antibiotics. 2022; 11(12):1728. https://doi.org/10.3390/antibiotics11121728
Chicago/Turabian StyleRatia, Carlos, Sara Sueiro, Raquel G. Soengas, María José Iglesias, Fernando López-Ortiz, and Sara María Soto. 2022. "Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance" Antibiotics 11, no. 12: 1728. https://doi.org/10.3390/antibiotics11121728







