Epidemiology and Economic Outcomes Associated with Timely versus Delayed Receipt of Appropriate Antibiotic Therapy among US Patients Hospitalized for Native Septic Arthritis: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Measures
2.2. Statistical Analysis
3. Results
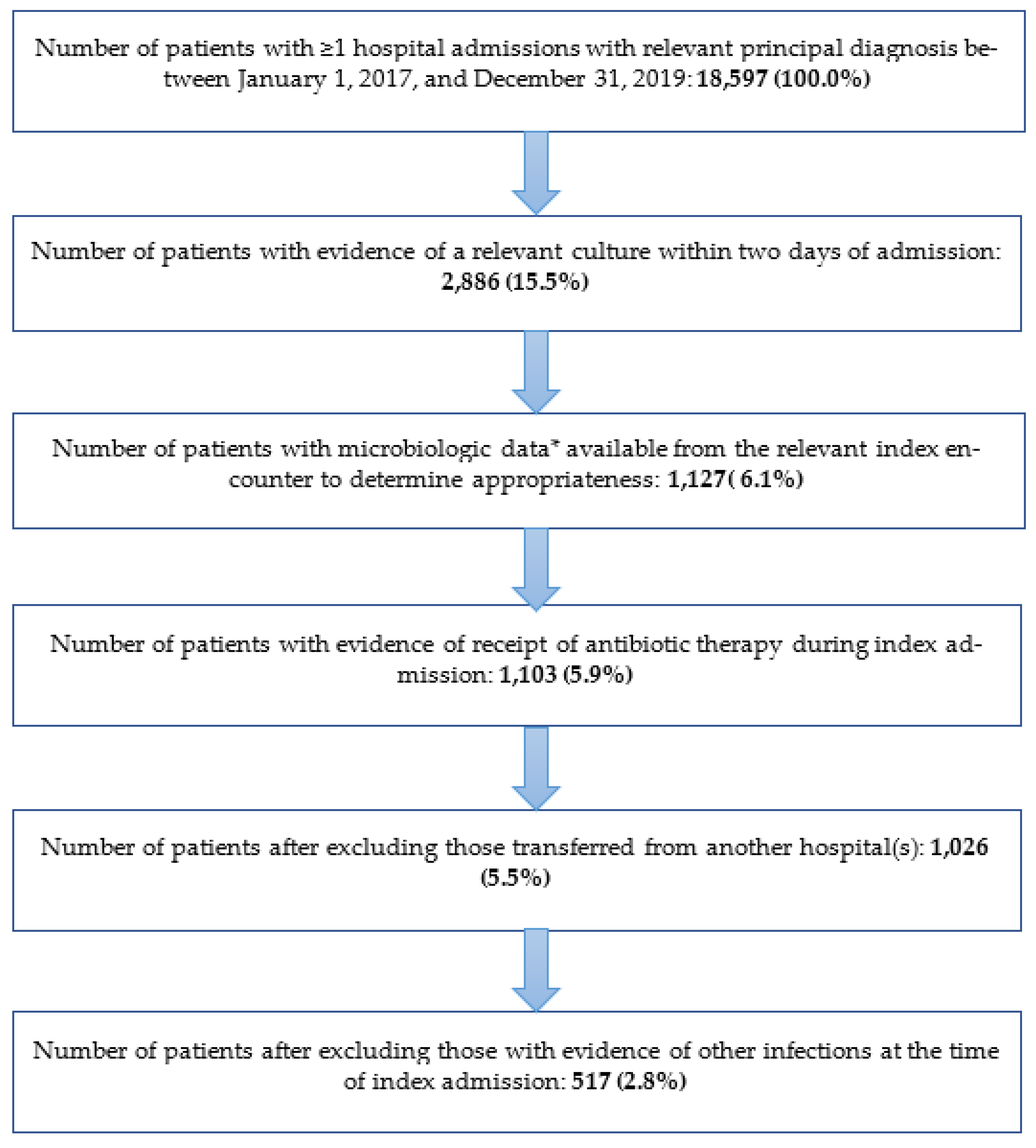
3.1. Study Population
3.2. Organisms and Antibiotics Identified
3.3. Patterns of Initial Therapy and Appropriateness Thereof
3.4. Patient and Hospital Characteristics
3.5. IPTW-Adjusted Utilization and Cost Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrand, J.; El Samad, Y.; Brunschweiler, B.; Grados, F.; Dehamchia-Rehailia, N.; Séjourne, A.; Schmit, J.-L.; Gabrion, A.; Fardellone, P.; Paccou, J. Morbimortality in adult patients with septic arthritis: A three-year hospital-based study. BMC Infect. Dis. 2016, 16, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobao, V.C.; Alfishawy, M.; Smith, C.; Byers, K.; Yassin, M.; Urish, K.L.; Shah, N.B. Risk Factors, Screening, and Treatment Challenges in Staphylococcus aureus Native Septic Arthritis. Open Forum Infect. Dis. 2020, 8, ofaa593. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S. Septic Arthritis in Emergency Departments in the US: A National Study of Health Care Utilization and Time Trends. Arthritis Care Res. 2018, 70, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Lodise, T.P.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Bonine, N.G. Antimicrobial Resistance or Delayed Appropriate Therapy-Does One Influence Outcomes More Than the Other Among Patients With Serious Infections Due to Carbapenem-Resistant Versus Carbapenem-Susceptible Enterobacteriaceae? Open Forum Infect. Dis. 2019, 6, ofz194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landewé, R.B.M.; Günther, K.P.; Lukas, C.; Braun, J.; Combe, B.; Conaghan, P.; Dreinhöfer, K.; Fritschy, D.; Getty, J.; van der Heide, H.J.; et al. EULAR/EFORT recommendations for the diagnosis and initial management of patients with acute or recent onset swelling of the knee. Ann. Rheum. Dis. 2009, 69, 12–19. [Google Scholar] [CrossRef]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Oster, G.; Berger, A.; Edelsberg, J.; Weber, D.J. Initial treatment failure in non-ICU community-acquired pneumonia: Risk factors and association with length of stay, total hospital charges, and mortality. J. Med. Econ. 2013, 16, 809–819. [Google Scholar] [CrossRef]
- Berger, A.; Oster, G.; Edelsberg, J.; Huang, X.; Weber, D.J. Initial Treatment Failure in Patients with Complicated Skin and Skin Structure Infections. Surg. Infect. 2013, 14, 304–312. [Google Scholar] [CrossRef]
- Edelsberg, J.; Berger, A.; Weber, D.J.; Mallick, R.; Kuznik, A.; Oster, G. Clinical and Economic Consequences of Failure of Initial Antibiotic Therapy for Hospitalized Patients With Complicated Skin and Skin-Structure Infections. Infect. Control. Hosp. Epidemiol. 2008, 29, 160–169. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Sabel, A.L.; Sarcone, E.E.; Price, C.S.; Mehler, P.S.; Burman, W.J. Skin and Soft-Tissue Infections Requiring Hospitalization at an Academic Medical Center: Opportunities for Antimicrobial Stewardship. Clin. Infect. Dis. 2010, 51, 895–903. [Google Scholar] [CrossRef]
- Surat, G.; Vogel, U.; Wiegering, A.; Germer, C.-T.; Lock, J. Defining the Scope of Antimicrobial Stewardship Interventions’ on the Prescription Quality of Antibiotics for Surgical Intra-Abdominal Infections. Antibiotics 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Tabak, Y.P.; Vankeepuram, L.; Ye, G.; Jeffers, K.; Gupta, V.; Murray, P.R. Blood Culture Turnaround Time in U.S. Acute Care Hospitals and Implications for Laboratory Process Optimization. J. Clin. Microbiol. 2018, 56, e00500-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madaras-Kelly, K.; Jones, M.; Remington, R.; Hill, N.; Huttner, B.; Samore, M. Development of an Antibiotic Spectrum Score Based on Veterans Affairs Culture and Susceptibility Data for the Purpose of Measuring Antibiotic De-Escalation: A Modified Delphi Approach. Infect. Control. Hosp. Epidemiol. 2014, 35, 1103–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premier. Premier Healthcare Database White Paper: Data That Informs and Performs. 2020. Available online: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf (accessed on 2 March 2020).
- Lodise, T.P.; Kanakamedala, H.; Hsu, W.; Cai, B. Impact of Incremental Delays in Appropriate Therapy on the Outcomes of Hospitalized Adult Patients with Gram-negative Bloodstream Infections: “Every day matters”. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Moehring, R.W.; Ashley, E.S.D.; E Davis, A.; Dyer, A.P.; Parish, A.; Ren, X.; Lokhnygina, Y.; A Hicks, L.; Srinivasan, A.; Anderson, D.J. Development of an Electronic Definition for De-escalation of Antibiotics in Hospitalized Patients. Clin. Infect. Dis. 2020, 73, e4507–e4514. [Google Scholar] [CrossRef]
- Lee, Hana for the Center for Drug Evaluation and Research; U.S. Food and Drug Administration. Causal Inference for Real-World Evidence: Propensity Score Methods and Case Study. Presented at the Regulatory-Industry Statistics Workshop on 22 September 2020. Available online: https://ww2.amstat.org/meetings/biop/2020/onlineprogram/handouts/SC4-Handouts.pdf (accessed on 14 June 2022).
- Lodise, T.P.; McKinnon, P.S.; Swiderski, L.; Rybak, M.J. Outcomes Analysis of Delayed Antibiotic Treatment for Hospital-Acquired Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2003, 36, 1418–1423. [Google Scholar] [CrossRef] [Green Version]
- van Duin, D.; Kaye, K.S.; Neuner, E.A.; Bonomo, R.A. Carbapenem-resistant Enterobacteriaceae: A review of treatment and outcomes. Diagn. Microbiol. Infect. Dis. 2013, 75, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, C.; Cabric, S.; Perka, C.; Trampuz, A.; Renz, N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2018, 90, 115–119. [Google Scholar] [CrossRef]
- Abdelrazik, E.; Oweda, M.; El-Hadidi, M. Benchmarking of Antimicrobial Resistance Gene Detection Tools in Assembled Bacterial Whole Genomes. In Proceedings of the 2021 3rd Novel Intelligent and Leading Emerging Sciences Conference (NILES), Giza, Egypt, 23–25 October 2021; pp. 273–278. [Google Scholar] [CrossRef]
- Tseng, W.-P.; Chen, Y.-C.; Yang, B.-J.; Chen, S.-Y.; Lin, J.-J.; Huang, Y.-H.; Fu, C.-M.; Chang, S.-C.; Chen, S.-Y. Predicting Multidrug-Resistant Gram-Negative Bacterial Colonization and Associated Infection on Hospital Admission. Infect. Control. Hosp. Epidemiol. 2017, 38, 1216–1225. [Google Scholar] [CrossRef]
- Timbrook, T.T.; Morton, J.B.; McConeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 64, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Hueth, K.D.; Thompson-Leduc, P.; Totev, T.I.; Milbers, K.; Timbrook, T.T.; Kirson, N.; Hasbun, R. Assessment of the Impact of a Meningitis/Encephalitis Panel on Hospital Length of Stay: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pizarraya, A.; Leone, M.; Garnacho-Montero, J.; Martin, C.; Martin-Loeches, I. Collaborative approach of individual participant data of prospective studies of de-escalation in non-immunosuppressed critically ill patients with sepsis. Expert Rev. Clin. Pharmacol. 2017, 10, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seddon, M.M.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Rac, H.; Haggard, E.; Mediwala, K.N.; Dash, S.; Al-Hasan, M.N. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin. Infect. Dis. 2018, 69, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Klinker, K.P.; Borgert, S.J.; Trikha, G.; Rand, K.H.; Ramphal, R. Time to Positivity of Blood Cultures Supports Antibiotic De-escalation at 48 Hours. Ann. Pharmacother. 2013, 48, 33–40. [Google Scholar] [CrossRef]
- Kinnear, C.L.; Patel, T.S.; Young, C.L.; Marshall, V.; Newton, D.W.; Read, A.F.; Woods, R.J. Impact of an Antimicrobial Stewardship Intervention on Within- and Between-Patient Daptomycin Resistance Evolution in Vancomycin-Resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2019, 63, e01800-18. [Google Scholar] [CrossRef] [Green Version]
- Lanata, M.M.; Diaz, A.; Hect, S.M.; Katragkou, A.; Gallup, N.; Buckingham, D.A.; Tansmore, J.L.; Sargel, C.; Watson, J.R.; Karsies, T. Empiric Vancomycin Reduction in a Pediatric Intensive Care Unit. Pediatrics 2021, 148, e2020009142. [Google Scholar] [CrossRef]
- Froehlich, M.; Ford, F.; Belal, M.; Creed, M.; Lobo, Z.; Psevdos, G. Marked Reduction of Vancomycin Utilization in a Veterans Hospital. Infect. Dis. Clin. Pr. 2020, 28, e7–e8. [Google Scholar] [CrossRef]
- Pliakos, E.E.; Andreatos, N.; Shehadeh, F.; Ziakas, P.D.; Mylonakis, E. The Cost-Effectiveness of Rapid Diagnostic Testing for the Diagnosis of Bloodstream Infections with or without Antimicrobial Stewardship. Clin. Microbiol. Rev. 2018, 31, e00095-17. [Google Scholar] [CrossRef] [Green Version]
- Lodise, T.P.; Zhao, Q.; Fahrbach, K.; Gillard, P.J.; Martin, A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: How long is too long? BMC Infect. Dis. 2018, 18, 625. [Google Scholar] [CrossRef]
| Pathogen | Study Sample (n = 517) |
|---|---|
| MSSA | 263 (50.9%) |
| MRSA | 65 (12.6%) |
| Streptococcus viridans group | 30 (5.8%) |
| Methicillin-susceptible S. epidermidis | 22 (4.3%) |
| Serratia marcescens | 17 (3.3%) |
| S. agalactiae | 17 (3.3%) |
| Pseudomonas aeruginosa | 14 (2.7%) |
| Methicillin-resistant S. epidermidis | 13 (2.5%) |
| Enterococcus faecalis | 10 (1.9%) |
| Escherichia coli | 8 (1.5%) |
| S. pneumoniae | 7 (1.4%) |
| Enterobacter cloacae complex | 6 (1.2%) |
| S. dysgalactiae | 6 (1.2%) |
| S. capitis | 5 (1.0%) |
| S. hominis | 5 (1.0%) |
| Other Staphylococcus sp. | 5 (1.0%) |
| Other strains of K. pneumoniae (non-ESBL) | 4 (0.8%) |
| S. gordonii | 4 (0.8%) |
| S. pyogenes | 4 (0.8%) |
| S. caprae | 3 (0.6%) |
| S. lugdunensis | 3 (0.6%) |
| K. oxytoca | 3 (0.6%) |
| Neisseria gonorrhoeae | 3 (0.6%) |
| Proteus mirabilis | 3 (0.6%) |
| Group C Streptococcus. sp. | 3 (0.6%) |
| Cutibacterium acnes | 3 (0.6%) |
| Citrobacter spp. | 2 (0.4%) |
| Morganella morganii | 2 (0.4%) |
| Vancomycin-resistant E. faecium | 2 (0.4%) |
| Haemophilus influenzae | 2 (0.4%) |
| Candida albicans | 2 (0.4%) |
| S. warneri | 1 (0.2%) |
| Other methicillin-resistant coagulase-negative Staphylococcus sp. | 1 (0.2%) |
| Achromobacter denitrificans | 1 (0.2%) |
| A. xylosoxidans | 1 (0.2%) |
| ESBL Klebsiella pneumoniae | 1 (0.2%) |
| N. sicca | 1 (0.2%) |
| Pasteurella multocida | 1 (0.2%) |
| Raoultella ornithinolytica | 1 (0.2%) |
| Providencia rettgeri | 1 (0.2%) |
| Peptoniphilus spp. | 1 (0.2%) |
| Other Enterococcus sp. | 1 (0.2%) |
| S. cristatus | 1 (0.2%) |
| Streptococcus sp. | 1 (0.2%) |
| Group B Streptococcus sp. | 1 (0.2%) |
| H. parainfluenzae | 1 (0.2%) |
| C. parapsilosis | 1 (0.2%) |
| S. zooepidemicus | 1 (0.2%) |
| Timely Appropriate Therapy (n = 438) | Delayed Appropriate Therapy (n = 26) | All Patients (n = 464) * | |
|---|---|---|---|
| Until day five/discharge | |||
| De-escalation | 159 (36.3%) | 4 (15.4%) | 163 (35.1%) |
| Escalation | 19 (4.3%) | 6 (23.1%) | 25 (5.4%) |
| No change | 135 (30.8%) | 6 (23.1%) | 141 (30.4%) |
| Unknown | 125 (28.5%) | 10 (38.5%) | 135 (29.1%) |
| Until last day of treatment/discharge | |||
| De-escalation | 240 (54.8%) | 9 (34.6%) | 249 (53.7%) |
| Escalation | 42 (9.6%) | 6 (23.1%) | 48 (10.3%) |
| No change | 154 (35.2%) | 9 (34.6%) | 163 (35.1%) |
| Unknown | 2 (0.5%) | 2 (7.7%) | 4 (0.9%) |
| Unweighted Study Sample (n = 517) | Weighted Study Sample (n = 438) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Timely (n = 491) | Delayed (n = 26) | Standardized Differences | p-Value | Timely (n = 412) | Delayed (n = 26) | Standardized Differences | p-Value |
| Demographics | ||||||||
| Mean (SD) age (years) | 53.7 (18.4) | 54.3 (18.9) | 0.03 | 0.88 | 54.7 (5.0) | 54.3 (18.9) | 0.03 | 0.92 |
| Male | 346 (70.5%) | 15 (57.7%) | 0.27 | 0.17 | 243 (59.0%) | 15 (57.7%) | 0.03 | 0.93 |
| Race/ethnicity | ||||||||
| White | 365 (74.3%) | 20 (76.9%) | 0.06 | 0.55 | 317 (76.9%) | 20 (76.9%) | 0.00 | 0.79 |
| Black | 52 (10.6%) | 3 (11.5%) | 0.03 | 41 (10.0%) | 3 (11.5%) | 0.05 | ||
| Hispanic | 26 (5.3%) | 1 (3.8%) | 0.07 | 18 (4.4%) | 1 (3.8%) | 0.03 | ||
| Asian | 10 (2.0%) | 1 (3.8%) | 0.11 | 6 (1.4%) | 1 (3.8%) | 0.16 | ||
| Other | 33 (6.7%) | 0 (0.0%) | NA | 26 (6.3%) | 0 (0.0%) | NA | ||
| Unknown/Missing | 5 (1.0%) | 1 (3.8%) | 0.18 | 4 (1.0%) | 1 (3.8%) | 0.18 | ||
| Payer type | ||||||||
| Medicare | 165 (33.6%) | 12 (46.2%) | 0.26 | 0.24 | 188 (45.7%) | 12 (46.2%) | 0.01 | 0.78 |
| Medicaid | 105 (21.4%) | 7 (26.9%) | 0.13 | 116 (28.1%) | 7 (26.9%) | 0.03 | ||
| Commercial | 36 (7.3%) | 0 (0.0%) | NA | 16 (3.8%) | 0 (0.0%) | NA | ||
| Other | 185 (37.7%) | 7 (26.9%) | 0.23 | 92 (22.4%) | 7 (26.9%) | 0.10 | ||
| Clinical Characteristics | ||||||||
| Comorbidities | ||||||||
| None identified | 148 (30.1%) | 7 (26.9%) | 0.07 | 0.73 | 75 (18.3%) | 7 (26.9%) | 0.21 | 0.73 |
| Asthma | 75 (15.3%) | 9 (34.6%) | 0.46 | 0.01 | 116 (28.2%) | 9 (34.6%) | 0.14 | 0.01 |
| Immunocompromised | ||||||||
| HIV/AIDS | 2 (0.4%) | 0 (0.0%) | NA | 1 | 3 (0.7%) | 0 (0.0%) | NA | 1 |
| Malignancies | 19 (3.9%) | 3 (11.5%) | 0.29 | 0.09 | 27 (6.5%) | 3 (11.5%) | 0.18 | 0.09 |
| Other | 31 (6.3%) | 3 (11.5%) | 0.18 | 0.24 | 50 (12.1%) | 3 (11.5%) | 0.02 | 0.24 |
| Total of above | 49 (10.0%) | 4 (15.4%) | 0.16 | 0.33 | 76 (18.3%) | 4 (15.4%) | 0.08 | 0.33 |
| Malnutrition/cachexia | 26 (5.3%) | 3 (11.5%) | 0.23 | 0.17 | 37 (8.9%) | 3 (11.5%) | 0.09 | 0.17 |
| Diabetes | ||||||||
| No complications | 113 (23.0%) | 4 (15.4%) | 0.20 | 0.47 | 115 (27.8%) | 4 (15.4%) | 0.31 | 0.47 |
| With complications | 59 (12.0%) | 5 (19.2%) | 0.20 | 0.28 | 71 (17.2%) | 5 (19.2%) | 0.05 | 0.28 |
| Total of above | 129 (26.3%) | 8 (30.8%) | 0.10 | 0.61 | 133 (32.2%) | 8 (30.8%) | 0.03 | 0.61 |
| Osteoarthritis | 140 (28.5%) | 5 (19.2%) | 0.22 | 0.3 | 110 (26.8%) | 5 (19.2%) | 0.18 | 0.30 |
| Obesity | 94 (19.1%) | 4 (15.4%) | 0.10 | 0.8 | 60 (14.5%) | 4 (15.4%) | 0.02 | 0.80 |
| Mean (SD) CCI Score | 1.2 (1.8) | 1.9 (1.8) | 0.40 | 0.05 | 2.0 (0.5) | 1.9 (1.8) | 0.02 | 0.93 |
| Markers of general frailty | ||||||||
| Corticosteroids | 190 (38.7%) | 10 (38.5%) | 0.01 | 0.98 | 153 (37.2%) | 10 (38.5%) | 0.03 | 0.98 |
| Vasoactives | 98 (20.0%) | 3 (11.5%) | 0.23 | 0.45 | 80 (19.4%) | 3 (11.5%) | 0.22 | 0.45 |
| Parenteral nutrition | 0 (0.0%) | 0 (0.0%) | NA | NA | 0 (0.0%) | 0 (0.0%) | NA | NA |
| Dialysis | 5 (1.0%) | 0 (0.0%) | NA | 1.00 | 4 (0.9%) | 0 (0.0%) | NA | 1.00 |
| Any of the above | 245 (49.9%) | 12 (46.2%) | 0.08 | 0.71 | 196 (47.5%) | 12 (46.2%) | 0.03 | 0.71 |
| Antibiotic use within 12 months of index | 98 (20.0%) | 8 (30.8%) | 0.25 | 0.18 | 131 (31.7%) | 8 (30.8%) | 0.02 | 0.18 |
| Antibiotic use within 6 months of index | 34 (6.9%) | 3 (11.5%) | 0.16 | 0.42 | 54 (13.0%) | 3 (11.5%) | 0.05 | 0.42 |
| Resource intensity >1 † | 183 (37.3%) | 6 (23.1%) | 0.31 | 0.14 | 5.52 (22.0%) | 6 (23.1%) | 0.03 | 0.14 |
| Prior hospitalization within 6 months | ||||||||
| All-cause | 78 (15.9%) | 5 (19.2%) | 0.09 | 0.65 | 81 (19.7%) | 5 (19.2%) | 0.01 | 0.65 |
| Infection-related | 40 (8.1%) | 3 (11.5%) | 0.11 | 0.47 | 49 (11.8%) | 3 (11.5%) | 0.01 | 0.47 |
| Hospital Characteristics | ||||||||
| Geographic region | ||||||||
| Midwest | 141 (28.7%) | 6 (23.1%) | 0.13 | 0.59 | 90 (21.9%) | 6 (23.1%) | 0.03 | 0.80 |
| Northeast | 123 (25.1%) | 5 (19.2%) | 0.14 | 124 (30.0%) | 5 (19.2%) | 0.25 | ||
| South | 198 (40.3%) | 14 (53.8%) | 0.27 | 176 (42.7%) | 14 (53.8%) | 0.23 | ||
| West | 29 (5.9%) | 1 (3.8%) | 0.10 | 22 (5.4%) | 1 (3.8%) | 0.07 | ||
| Number of beds | ||||||||
| <100 | 40 (8.1%) | 0 (0.0%) | NA | 0.26 | 16 (4.0%) | 0 (0.0%) | NA | 0.67 |
| 100–199 | 61 (12.4%) | 3 (11.5%) | 0.03 | 36 (8.9%) | 3 (11.5%) | 0.09 | ||
| 200–299 | 73 (14.9%) | 1 (3.8%) | 0.39 | 54 (13.1%) | 1 (3.8%) | 0.34 | ||
| 300–399 | 63 (12.8%) | 3 (11.5%) | 0.04 | 27 (6.5%) | 3 (11.5%) | 0.18 | ||
| 400–499 | 61 (12.4%) | 4 (15.4%) | 0.09 | 38 (9.1%) | 4 (15.4%) | 0.19 | ||
| ≥500 | 193 (39.3%) | 15 (57.7%) | 0.37 | 241 (58.4%) | 15 (57.7%) | 0.01 | ||
| Teaching hospital | 277 (56.4%) | 17 (65.4%) | 0.19 | 0.37 | 271 (65.7%) | 17 (65.4%) | 0.01 | 0.98 |
| Urban location | 444 (90.4%) | 25 (96.2%) | 0.23 | 0.33 | 384 (93.2%) | 25 (96.2%) | 0.13 | 0.64 |
| Outcomes | Timely Appropriate Therapy (n = 412) | Delayed Therapy (n = 26) | p-Value |
|---|---|---|---|
| Adjusted Mean (95% CI) | |||
| Duration of in-hospital antibiotic therapy, days | 7.3 (6.7–8.0) | 8.4 (7.7–9.2) | 0.02 |
| Total in-hospital antibiotic exposure days, days | 10.5 (9.7–11.5) | 11.6 (10.6–12.6) | 0.11 |
| LOS, days | 6.9 (6.3–7.6) | 8.3 (7.6–9.0) | 0.01 |
| In-hospital cost, $ | |||
| Antibiotics | $624 ($515–$756) | $1534 ($1286–$1829) | <0.01 |
| Other pharmacotherapies | $1068 ($932–$1,223) | $1639 ($1438–$1868) | <0.01 |
| Medical care | $5861 ($5458–$6294) | $6521 ($6085–$6988) | 0.03 |
| Room and board | $7551 ($6818–$8362) | $7975 ($7223–$8805) | 0.44 |
| Other costs | $659 ($535–$812) | $587 ($481–$716) | 0.44 |
| Total in-hospital cost | $15,490 ($14,242–$16,846) | $19,021 ($17,528–$20,641) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balada-Llasat, J.-M.; Stamas, N.; Vincent, T.; Timbrook, T.T.; Saiontz-Martinez, C.; Hemmert, R.B.; Berger, A. Epidemiology and Economic Outcomes Associated with Timely versus Delayed Receipt of Appropriate Antibiotic Therapy among US Patients Hospitalized for Native Septic Arthritis: A Retrospective Cohort Study. Antibiotics 2022, 11, 1732. https://doi.org/10.3390/antibiotics11121732
Balada-Llasat J-M, Stamas N, Vincent T, Timbrook TT, Saiontz-Martinez C, Hemmert RB, Berger A. Epidemiology and Economic Outcomes Associated with Timely versus Delayed Receipt of Appropriate Antibiotic Therapy among US Patients Hospitalized for Native Septic Arthritis: A Retrospective Cohort Study. Antibiotics. 2022; 11(12):1732. https://doi.org/10.3390/antibiotics11121732
Chicago/Turabian StyleBalada-Llasat, Joan-Miquel, Nicole Stamas, Tom Vincent, Tristan T. Timbrook, Cynthia Saiontz-Martinez, Rachael B. Hemmert, and Ariel Berger. 2022. "Epidemiology and Economic Outcomes Associated with Timely versus Delayed Receipt of Appropriate Antibiotic Therapy among US Patients Hospitalized for Native Septic Arthritis: A Retrospective Cohort Study" Antibiotics 11, no. 12: 1732. https://doi.org/10.3390/antibiotics11121732
APA StyleBalada-Llasat, J.-M., Stamas, N., Vincent, T., Timbrook, T. T., Saiontz-Martinez, C., Hemmert, R. B., & Berger, A. (2022). Epidemiology and Economic Outcomes Associated with Timely versus Delayed Receipt of Appropriate Antibiotic Therapy among US Patients Hospitalized for Native Septic Arthritis: A Retrospective Cohort Study. Antibiotics, 11(12), 1732. https://doi.org/10.3390/antibiotics11121732






