Antibiotic Resistance of Enterococcus spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021
Abstract
:1. Introduction
2. Results
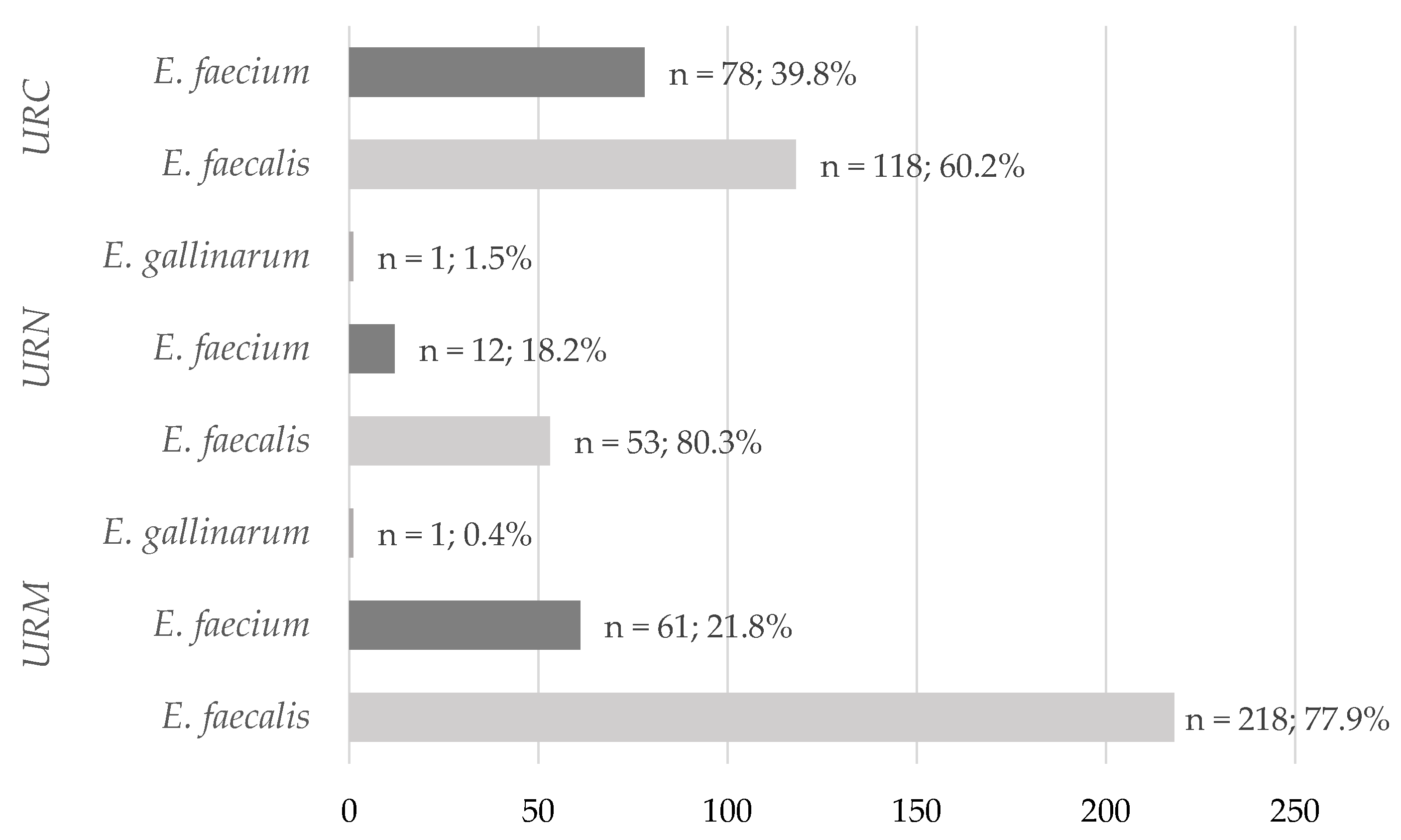
2.1. Species Prevalence Analysis
2.2. Antimicrobial Susceptibility Testing
2.3. Occurrence of Resistance Mechanisms and Resistance Patterns
3. Discussion
4. Materials and Methods
4.1. Identification of Enterococcal Uropathogenes
4.2. Antimicrobial Susceptibility Testing
4.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC Suffering from a Urinary Tract Infection? Available online: https://www.cdc.gov/antibiotic-use/uti.html (accessed on 15 September 2021).
- Przegląd Urologiczny-Zakażenie Układu Moczowego. Available online: http://www.przeglad-urologiczny.pl/artykul.php?1093 (accessed on 15 September 2021).
- Nicolle, L.E. Urinary Tract Infection. Crit. Care Clin. 2013, 29, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Salvatore, S.; Cattoni, E.; Siesto, G.; Serati, M.; Sorice, P.; Torella, M. Urinary Tract Infections in Women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Bronk, M.; Kochowska-Bronk, M.; Śledzińska, A.; Samet, A. Bakterie z Rodzaju Enterococcus Jako Ważny Czynnik Etiologicznym Zakażeń Układu Moczowego u Pacjentów Ambulatoryjnych. Forum Med. Rodz. 2010, 4, 189–193. [Google Scholar]
- Kot, B.; Grużewska, A.; Szweda, P.; Wicha, J.; Parulska, U. Antibiotic Resistance of Uropathogens Isolated from Patients Hospitalized in District Hospital in Central Poland in 2020. Antibiotics 2021, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.F.; Kavanagh, K.; Sobel, J.D.; Kauffman, C.A.; Newman, C.A. Candida Urinary Tract Infection: Pathogenesis. Clin. Infect. Dis. 2011, 52, S437–S451. [Google Scholar] [CrossRef] [Green Version]
- Żukowska, A.; Hryniewicz, W. Rekomendacje Diagnostyki, Terapii i Profilaktyki Antybiotykowej Zakażeń w Szpitalu—2020, 2nd ed.; Narodowy Instytut Leków: Warszawa, Poland, 2020; pp. 29–36. [Google Scholar]
- EAU Guidelines on Urological Infections. Available online: https://uroweb.org/guidelines/urological-infections (accessed on 18 November 2022).
- Kang, C.-I.; Kim, J.; Park, D.W.; Kim, B.-N.; Ha, U.-S.; Lee, S.-J.; Yeo, J.K.; Min, S.K.; Lee, H.; Wie, S.-H. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect. Chemother. 2018, 50, 67–100. [Google Scholar] [CrossRef]
- Hryniewicz, W.; Ozorowski, T.; Żukowska, A. Szpitalna Lista Antybiotyków—2020, 2nd ed.; Narodowy Instytut Leków: Warszawa, Poland, 2020; p. 29. [Google Scholar]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and Acquired Resistance Mechanisms in Enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [Green Version]
- Shortliffe, L.M.D.; McCue, J.D. Urinary Tract Infection at the Age Extremes: Pediatrics and Geriatrics. Am. J. Med. 2002, 113 (Suppl. 1A), 55S–66S. [Google Scholar] [CrossRef]
- Barros, M.; Martinelli, R.; Rocha, H. Enterococcal Urinary Tract Infections in a University Hospital: Clinical Studies. Braz. J. Infect. Dis. 2009, 13, 294–296. [Google Scholar] [CrossRef] [Green Version]
- Joyanes, P.; Pascual, A.; Martínez-Martínez, L.; Hevia, A.; Perea, E.J. In Vitro Adherence of Enterococcus Faecalis and Enterococcus Faecium to Urinary Catheters. EJCMID 2000, 19, 124–127. [Google Scholar] [CrossRef]
- Donelli, G.; Guaglianone, E. Emerging Role of Enterococcus Spp in Catheter-Related Infections: Biofilm Formation and Novel Mechanisms of Antibiotic Resistance. J. Vasc. Access 2004, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.; Fedi, A.C.; Reiter, K.C.; Caierão, J.; d’Azevedo, P.A. Correlation between Biofilm Formation and GelE, Esp, and Agg Genes in Enterococcus Spp. Clinical Isolates. Virulence 2014, 5, 634–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Rosa, R.; Creti, R.; Venditti, M.; D’Amelio, R.; Arciola, C.R.; Montanaro, L.; Baldassarri, L. Relationship between Biofilm Formation, the Enterococcal Surface Protein (Esp) and Gelatinase in Clinical Isolates of Enterococcus Faecalis and Enterococcus Faecium. FEMS Microbiol. Lett. 2006, 256, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, M.; Tošić, T.; Jovanović, S.; Stošović, R.; Stevanović, G.; Velebit, B.; Zervos, M.J. Presence of the Esp Gene in Enterococcus Faecium Derived from Oropharyngeal Microbiota of Haematology Patients. Arch. Oral. Biol 2018, 88, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mahmoudi, M.; Motahar, M.S.; Soltani, S.; Pourmand, M.R. Virulence Determinants and Antimicrobial Resistance Patterns of Vancomycin-Resistant Enterococcus Faecium Isolated from Different Sources in Southwest Iran. Iran. J. Public Health 2018, 47, 264–272. [Google Scholar]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach, K.; Al-Ahmad, A. Subinhibitory Antibiotic Concentrations Enhance Biofilm Formation of Clinical Enterococcus Faecalis Isolates. Antibiotics 2021, 10, 874. [Google Scholar] [CrossRef]
- Antimicrobial Resistance and Healthcare-Associated Infections—Annual Epidemiological Report 2014 [2012 data]. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-and-healthcare-associated-infections-annual (accessed on 31 December 2021).
- Healthcare-Associated Infections in Intensive Care Units—Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-annual-epidemiological-1 (accessed on 31 December 2021).
- Toner, L.; Papa, N.; Aliyu, S.H.; Dev, H.; Lawrentschuk, N.; Al-Hayek, S. Vancomycin Resistant Enterococci in Urine Cultures: Antibiotic Susceptibility Trends over a Decade at a Tertiary Hospital in the United Kingdom. Investig. Clin. Urol. 2016, 57, 129–134. [Google Scholar] [CrossRef]
- Suh, W.; Kim, B.N.; Kang, H.M.; Yang, E.A.; Rhim, J.-W.; Lee, K.-Y. Febrile Urinary Tract Infection in Children: Changes in Epidemiology, Etiology, and Antibiotic Resistance Patterns over a Decade. Clin. Exp. Pediatr. 2020, 64, 293–300. [Google Scholar] [CrossRef]
- Ganesh, R.; Shrestha, D.; Bhattachan, B.; Rai, G. Epidemiology of Urinary Tract Infection and Antimicrobial Resistance in a Pediatric Hospital in Nepal. BMC Infect. Dis. 2019, 19, 420. [Google Scholar] [CrossRef]
- Bitsori, M.; Maraki, S.; Raissaki, M.; Bakantaki, A.; Galanakis, E. Community-Acquired Enterococcal Urinary Tract Infections. Pediatr. Nephrol. 2005, 20, 1583–1586. [Google Scholar] [CrossRef]
- Bardoloi, V.; Yogeesha Babu, K.V. Comparative Study of Isolates from Community-Acquired and Catheter-Associated Urinary Tract Infections with Reference to Biofilm-Producing Property, Antibiotic Sensitivity and Multi-Drug Resistance. J. Med. Microbiol. 2017, 66, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Sorlózano-Puerto, A.; Gómez-Luque, J.M.; de Dios Luna-Del-Castillo, J.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Etiological and Resistance Profile of Bacteria Involved in Urinary Tract Infections in Young Children. BioMed. Res. Int. 2017, 2017, e4909452. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Gumede, L.Y.E.; van der Hoven, L.A.; de Gita, G.N.; de Kock, E.J.E.; de Lange, T.; Maseko, V.; Kekana, V.; Smuts, F.P.; Perovic, O. Antimicrobial Susceptibility of Organisms Causing Community-Acquired Urinary Tract Infections in Gauteng Province, South Africa. S. Afr. Med. J. 2013, 103, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, R.; Timilsina, S.; Jha, P.; Bhattarai, N.R.; Poudyal, N.; Gurung, R.; Khanal, B.; Bhattachary, S.K. Study of Antimicrobial Susceptibility Pattern of Gram Positive Organisms Causing UTI in a Tertiary Care Hospital in Eastern Region of Nepal. Health Renaiss. 2013, 11, 119–124. [Google Scholar] [CrossRef]
- Cornia, P.B.; Takahashi, T.A.; Lipsky, B.A. The Microbiology of Bacteriuria in Men: A 5-Year Study at a Veterans’ Affairs Hospital. Diagn. Microbiol. Infect. Dis. 2006, 56, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Z.; Huang, L.; Liu, Z.; Shi, Y.; Zhang, M.; Li, H.; Zeng, L.; Ni, J.; Zhu, Y.; et al. Safety of Quinolones in Children: A Systematic Review and Meta-Analysis. Pediatr. Drugs 2022, 24, 447–464. [Google Scholar] [CrossRef]
- Ferede, Z.T.; Tullu, K.D.; Derese, S.G.; Yeshanew, A.G. Prevalence and Antimicrobial Susceptibility Pattern of Enterococcus Species Isolated from Different Clinical Samples at Black Lion Specialized Teaching Hospital, Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 793. [Google Scholar] [CrossRef]
- Kawalec, M.; Pietras, Z.; Daniłowicz, E.; Jakubczak, A.; Gniadkowski, M.; Hryniewicz, W.; Willems, R.J.L. Clonal Structure of Enterococcus Faecalis Isolated from Polish Hospitals: Characterization of Epidemic Clones. J. Clin. Microbiol. 2007, 45, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Piekarska, K.; Ławrynowicz-Paciorek, M.; Magdziak, A.; Bareja, E.; Rudecka, A.; Wyrebiak, A.; Pietrzyk, M.; Kochman, M. Drug sensitivity of Enterococcus sp. strains isolated from clinical material or from healthy persons. Med. Dosw. Mikrobiol. 2002, 54, 305–315. [Google Scholar]
- Rudy, M.; Nowakowska, M.; Wiechuła, B.; Zientara, M.; Radosz-Komoniewska, H. [Antibiotic susceptibility analysis of Enterococcus spp. isolated from urine]. Przegl. Lek. 2004, 61, 473–476. [Google Scholar]
- KIM, Y.B.; SEO, H.J.; SEO, K.W.; JEON, H.Y.; KIM, D.K.; KIM, S.W.; LIM, S.-K.; LEE, Y.J. Characteristics of High-Level Ciprofloxacin-Resistant Enterococcus Faecalis and Enterococcus Faecium from Retail Chicken Meat in Korea. J. Food Prot. 2018, 81, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Espíndola, L.C.P.; do Nascimento, M.V.M.R.; do Souto, R.M.; Colombo, A.P.V. Antimicrobial Susceptibility and Virulence of Enterococcus Spp. Isolated from Periodontitis-Associated Subgingival Biofilm. J. Periodontol. 2021, 92, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Aldegheri, M.; Ligozzi, M.; Lopez, H.; Sucari, A.; Satta, G. Overproduction of a Low-Affinity Penicillin-Binding Protein and High-Level Ampicillin Resistance in Enterococcus Faecium. Antimicrob. Agents Chemother. 1994, 38, 1980–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protonotariou, E.; Dimitroulia, E.; Pournaras, S.; Pitiriga, V.; Sofianou, D.; Tsakris, A. Trends in Antimicrobial Resistance of Clinical Isolates of Enterococcus Faecalis and Enterococcus Faecium in Greece between 2002 and 2007. J. Hosp. Infect. 2010, 75, 225–227. [Google Scholar] [CrossRef]
- Courvalin, P. Vancomycin Resistance in Gram-Positive Cocci. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S25–S34. [Google Scholar] [CrossRef]
- Monticelli, J.; Knezevich, A.; Luzzati, R.; Di Bella, S. Clinical Management of Non-Faecium Non-Faecalis Vancomycin-Resistant Enterococci Infection. Focus on Enterococcus Gallinarum and Enterococcus Casseliflavus/Flavescens. J. Infect. Chemother. 2018, 24, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Zhanel, G.G.; Laing, N.M.; Nichol, K.A.; Palatnick, L.P.; Noreddin, A.; Hisanaga, T.; Johnson, J.L.; Hoban, D.J.; the NAVRESS Group. Antibiotic Activity against Urinary Tract Infection (UTI) Isolates of Vancomycin-Resistant Enterococci (VRE): Results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J. Antimicrob. Chemother. 2003, 52, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Remschmidt, C.; Schröder, C.; Behnke, M.; Gastmeier, P.; Geffers, C.; Kramer, T.S. Continuous Increase of Vancomycin Resistance in Enterococci Causing Nosocomial Infections in Germany— 10 Years of Surveillance. Antimicrob Resist Infect Control 2018, 7, 54. [Google Scholar] [CrossRef]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The Rise in Vancomycin-Resistant Enterococcus Faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control 2019, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The Ongoing Challenge of Vancomycin-Resistant Enterococcus Faecium and Enterococcus Faecalis in Europe: An Epidemiological Analysis of Bloodstream Infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Krawczyk, B.; Samet, A.; Bronk, M.; Hellmann, A.; Kur, J. Emerging Linezolid-Resistant, Vancomycin Resistant Enterococcus Faecium from a Patient of a Haematological Unit in Poland. Pol. J. Microbiol. 2004, 53, 193–196. [Google Scholar] [PubMed]
- Wardenburg, K.E.; Potter, R.F.; D’Souza, A.W.; Hussain, T.; Wallace, M.A.; Andleeb, S.; Burnham, C.-A.D.; Dantas, G. Phenotypic and Genotypic Characterization of Linezolid-Resistant Enterococcus Faecium from the USA and Pakistan. J. Antimicrob. Chemother. 2019, 74, 3445–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burleson, B.S.; Ritchie, D.J.; Micek, S.T.; Dunne, W.M. Enterococcus Faecalis Resistant to Linezolid: Case Series and Review of the Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.A.; van Wamel, W.J.B.; Posthuma, G.; Bonten, M.J.M.; Willems, R.J.L. Five Genes Encoding Surface-Exposed LPXTG Proteins Are Enriched in Hospital-Adapted Enterococcus Faecium Clonal Complex 17 Isolates. J. Bacteriol. 2007, 189, 8321–8332. [Google Scholar] [CrossRef]
- Klare, I.; Konstabel, C.; Mueller-Bertling, S.; Werner, G.; Strommenger, B.; Kettlitz, C.; Borgmann, S.; Schulte, B.; Jonas, D.; Serr, A.; et al. Spread of Ampicillin/Vancomycin-Resistant Enterococcus Faecium of the Epidemic-Virulent Clonal Complex-17 Carrying the Genes Esp and Hyl in German Hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 815–825. [Google Scholar] [CrossRef]
- Top, J.; Willems, R.; Bonten, M. Emergence of CC17 Enterococcus Faecium: From Commensal to Hospital-Adapted Pathogen. FEMS Immunol. Med. Microbiol. 2008, 52, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Yan, L.; Yang, S.; Yang, D. Antimicrobial-Resistant Evolution and Global Spread of Enterococcus Faecium Clonal Complex (CC) 17: Progressive Change from Gut Colonization to Hospital-Adapted Pathogen. China CDC Wkly 2022, 4, 17–21. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Disk Diffusion Method for Antimicrobial Susceptibility Testing, Version 9.0; 2021. Available online: http://www.eucast.org (accessed on 1 November 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 11.0; 2021. Available online: http://www.eucast.org (accessed on 1 November 2022).
| Year | Enterococcal Species | n | % | Re-Isolates, n (%) | |
|---|---|---|---|---|---|
| 2016 (n = 36) | Occurrence of Uropathogens | E. faecalis | 26 | 72.2 | 4 (11.1) |
| E. faecium | 10 | 27.8 | |||
| 2017 (n = 47) | E. faecalis | 26 | 55.3 | 11 (23.4) | |
| E. faecium | 20 | 42.6 | |||
| E. gallinarum | 1 | 2.1 | |||
| 2018 (n = 58) | E. faecalis | 37 | 63.8 | 13 (22.4) | |
| E. faecium | 21 | 36.2 | |||
| 2019 (n = 100) | E. faecalis | 62 | 62.0 | 24 (24.0) | |
| E. faecium | 38 | 38.0 | |||
| 2020 (n = 167) | E. faecalis | 134 | 80.2 | 44 (26.3) | |
| E. faecium | 32 | 19.2 | |||
| E. gallinarum | 1 | 0.6 | |||
| 2021 (n = 134) | E. faecalis | 104 | 77.6 | 22 (16.4) | |
| E. faecium | 30 | 22.4 |
| Uropathogens | Hospital Clinics | |||
|---|---|---|---|---|
| Nephrology (n = 260) | Urology (n = 282) | |||
| Women (n = 120) | Men (n = 140) | Women (n = 91) | Men (n = 191) | |
| Occurrence of Uropathogens, n (%) | ||||
| E. faecalis | 64 (53.3) | 90 (64.3) | 75 (82.4) | 160 (83.8) |
| E. faecium | 56 (46.7) | 49 (35.0) | 16 (17.6) | 30 (15.7) |
| E. gallinarum | 0 | 1 (0.7) | 0 | 1 (0.5) |
| Number of All Isolates | AMP | IPM | NOR | VAN | TEC | LZD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| 2016 | E.faecalis | 26 | 0 | (0) | 0 | (0) | 16 | (61.5) | 0 | (0) | 0 | (0) | 0 | (0) |
| E. faecium | 10 | 10 | (100.0) | 10 | (100.0) | 10 | (100.0) | 2 | (2.0) | 2 | (2.0) | 0 | (0) | |
| 2017 | E.faecalis | 26 | 0 | (0) | 0 | (0) | 16 | (61.5) | 1 | (3.9) | 0 | (0) | 0 | (0) |
| E. faecium | 20 | 19 | (95.0) | 19 | (95.0) | 20 | (100.0) | 12 | (60.0) | 11 | (55.0) | 0 | (0) | |
| 2018 | E.faecalis | 37 | 0 | (0) | 0 | (0) | 19 | (51.4) | 0 | (0) | 0 | (0) | 0 | (0) |
| E. faecium | 21 | 21 | (100.0) | 21 | (100.0) | 21 | (100.0) | 14 | (66.7) | 14 | (66.7) | 0 | (0) | |
| 2019 | E.faecalis | 62 | 0 | (0) | 0 | (0) | 34 | (54.8) | 0 | (0) | 0 | (0) | 0 | (0) |
| E. faecium | 38 | 38 | (100.0) | 38 | (100.0) | 37 | (100.0) | 23 | (60.5) | 23 | (60.5) | 0 | (0) | |
| 2020 | E.faecalis | 134 | 0 | (0) | 0 | (0) | 64 | (47.8) | 1 | (0.8) | 0 | (0) | 1 | (0.8) |
| E. faecium | 32 | 32 | (100.0) | 32 | (100.0) | 32 | (100.0) | 14 | (43.8) | 11 | (34.4) | 0 | (0) | |
| 2021 | E.faecalis | 104 | 2 | (1.9) | 2 | (1.9) | 57 | (54.8) | 3 | (2.9) | 0 | (0) | 0 | (0) |
| E. faecium | 30 | 30 | (100.0) | 30 | (100.0) | 30 | (100.0) | 18 | (60.0) | 11 | (36.7) | 0 | (0) | |
| Total | E.faecalis | 389 (A) | 2 | (0.5) | 2 | (0.5) | 200 | (51.4) | 5 | (1.3) | 0 | (0) | 1 | (0.3) |
| E. faecium | 151 (B) | 150 | (99.3) | 150 | (99.3) | 150 | (99.3) | 83 | (55.0) | 72 | (47.7) | 0 | (0) | |
| p-Value Comparison of A and B | <0.00001 | <0.00001 | <0.00001 | <0.00001 | <0.00001 | 0.533 | ||||||||
| Antimicrobial Resistant Mechanism | E. faecalis (n = 389) | E. faecium (n = 151) | p = Value |
|---|---|---|---|
| Isolates, n (%) | |||
| VRE | 5 (−1.3) | 11 (7.3) | 0.0002 |
| GRE | 0 | 72 (42.7) | <0.00001 |
| LRE | 1 (0.3) | 0 | 0.533 |
| Uropatogenes | Combination of Antibiotics | No. of Isolates | |
|---|---|---|---|
| n | % | ||
| E. faecalis (n = 389) | AMP/AMC, IPM, NOR | 1 | 0.3 |
| AMP/AMC, IPM, NOR, VAN | 1 | 0.3 | |
| E. faecium (n = 151) | AMP/AMC, IPM, NOR | 68 | 45.0 |
| AMP/AMC, IPM, NOR, VAN | 11 | 7.3 | |
| AMP/AMC, IPM, NOR, VAN, TEC | 72 | 47.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraszewska, Z.; Skowron, K.; Kwiecińska-Piróg, J.; Grudlewska-Buda, K.; Przekwas, J.; Wiktorczyk-Kapischke, N.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Antibiotic Resistance of Enterococcus spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021. Antibiotics 2022, 11, 1749. https://doi.org/10.3390/antibiotics11121749
Kraszewska Z, Skowron K, Kwiecińska-Piróg J, Grudlewska-Buda K, Przekwas J, Wiktorczyk-Kapischke N, Wałecka-Zacharska E, Gospodarek-Komkowska E. Antibiotic Resistance of Enterococcus spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021. Antibiotics. 2022; 11(12):1749. https://doi.org/10.3390/antibiotics11121749
Chicago/Turabian StyleKraszewska, Zuzanna, Krzysztof Skowron, Joanna Kwiecińska-Piróg, Katarzyna Grudlewska-Buda, Jana Przekwas, Natalia Wiktorczyk-Kapischke, Ewa Wałecka-Zacharska, and Eugenia Gospodarek-Komkowska. 2022. "Antibiotic Resistance of Enterococcus spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021" Antibiotics 11, no. 12: 1749. https://doi.org/10.3390/antibiotics11121749
APA StyleKraszewska, Z., Skowron, K., Kwiecińska-Piróg, J., Grudlewska-Buda, K., Przekwas, J., Wiktorczyk-Kapischke, N., Wałecka-Zacharska, E., & Gospodarek-Komkowska, E. (2022). Antibiotic Resistance of Enterococcus spp. Isolated from the Urine of Patients Hospitalized in the University Hospital in North-Central Poland, 2016–2021. Antibiotics, 11(12), 1749. https://doi.org/10.3390/antibiotics11121749







