Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review
Abstract
:1. Introduction
2. Results
2.1. Context
2.2. Policy Actors
2.3. Content and Process
2.3.1. Dispensing Prescribed Medicines or Antimicrobials with a Prescription
2.3.2. Licensed Pharmacists in Retail Pharmacies
3. Discussion
4. Strengths and Limitations
5. Materials and Methods
5.1. Conceptual Framework
5.2. Literature Search
5.3. Data Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDA | China Drug Administration |
| CFDA | China Food and Drug Administration |
| FDA | Food and Drug Administration |
| LMIC | Low and middle-income country |
| NRCMS | New Rural Cooperative Medical Scheme |
| OTC | Over the counter |
| PA | Pharmaceutical Administration |
| UEBMI | Urban Employee Basic Medical Insurance |
| URBMI | Urban Resident Basic Medical Insurance |
Appendix A
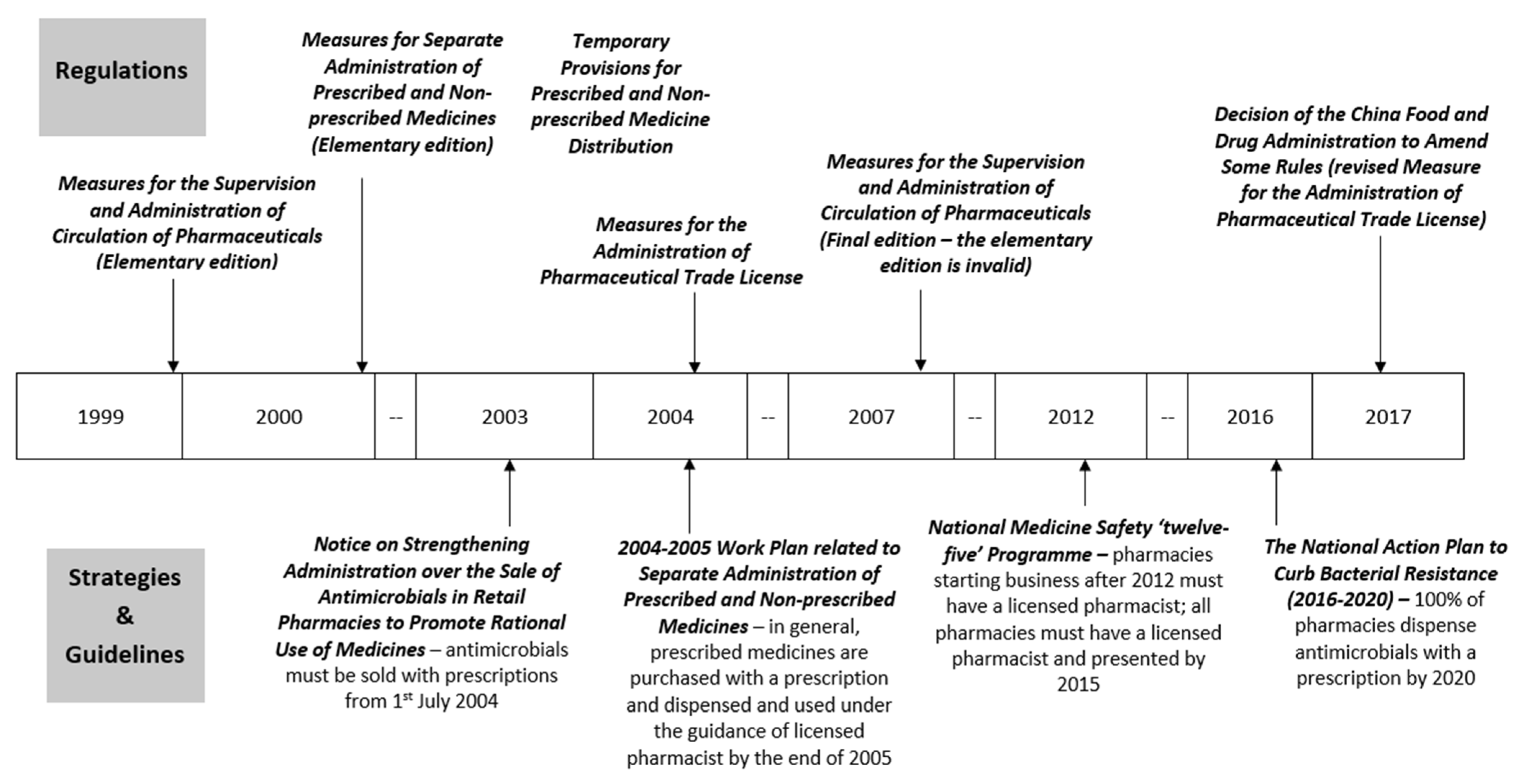
| Date | Policy (English & Mandarin) | Core Content | Key Points Related to Antibiotic Sales in Retail Pharmacies |
|---|---|---|---|
| Regulations | |||
| Introduce: 18 June 1999 Implement: 1 January 2000 | Measures for Separate Administration of Prescribed and Non-prescribed Medicines (Elementary edition) [40] 《处方药与非处方药分类管理办法(试行)》(国家药品监督管理局令第10号) | Overall guidance on activities related to prescribed and non-prescribed medicines among pharmaceutical entities, medical institutions, administrative departments, consumers, media. |
|
| Introduce: 28 December 1999 Implement: 1 January 2000 | Temporary Provisions for Prescribed and Non-prescribed Medicine Distribution [41] 《处方药与非处方药流通管理暂行规定》(国药管市〔1999〕454号) | Specific requirements on activities related to prescribed and non-prescribed medicines among pharmaceutical entities, medical institutions, and commercial business. |
|
| Introduce: 15 June 1999 Implement: 1 August 1999 | Measures for the Supervision and Administration of Circulation of Pharmaceuticals (Elementary edition) [Invalid] [42] 《药品流通监督管理办法(暂行)》(国家药品监督管理局令第7号) [失效] | Requirements on pharmaceutical related activities and punishments of violating these requirements. |
|
| Introduce: 31 January 2007 Implement: 1 May 2007 | Measures for the Supervision and Administration of Circulation of Pharmaceuticals [43] 《药品流通监督管理办法》(国家食品药品监督管理局令第26号) | ||
| Introduce: 4 February 2004 Implement: 1 April 2004 | Measures for the Administration of Pharmaceutical Trade License [Revised] [48] 《药品经营许可证管理办法》(国家食品药品监督管理局令第6号) [已修改] | Requirements on application, renewal and change of Pharmaceutical Trade License among pharmaceutical entities; Administration and inspection responsibilities of FDAs. |
|
| Introduce: 17 November 2017 Implement: 17 November 2017 | Decision of the China Food and Drug Administration to Amend Some Rules [49] 《国家食品药品监督管理总局关于修改部分规章的决定》(国家食品药品监督管理总局令第37号) | ||
| Strategies and Guidelines | |||
| 24 October 2003 | Notice on Strengthening Administration over the Sale of Antimicrobials in Retail Pharmacies to Promote Rational Use of Medicines [45] 关于加强零售药店抗菌药物销售监管促进合理用药的通知(国食药监安[2003]289号) | The FDAs and retail pharmacies’ roles in rational dispense of antimicrobials in retail pharmacies. |
|
| 11 June 2004 | Notice on Publishing ‘2004–2005 Work Plan related to Separate Administration of Prescribed and Non-prescribed Medicines’ by China Food and Drug Administration [44] 国家食品药品监督管理局关于印发《实施处方药与非处方药分类管理2004–2005年工作规划》的通知 (国食药监安[2004]262号) | The achievements and barriers related to management of prescribed and non-prescribed medicines; the goal, main principle, and the work plans for various stakeholders between 2004 and 2005. |
|
| 20 January 2012 | Notice on Publishing ‘National Medicine Safety ‘twelve-five’ Programme’ by State Council [51] 国务院关于印发国家药品安全“十二五”规划的通知 (国发[2012]5号) | A set of principles, objectives, and activities to improve national medicine quality and safety including one part related to promoting the pharmaceutical professional qualification system. |
|
| 5 August 2016 | The National Action Plan to Curb Bacterial Resistance (2016–2020) [37] 遏制细菌耐药国家行动计划(2016–2020年) | The main goals of this five-year plan and extensive approach to rational use of antimicrobials with measures on multiple stakeholders and components. |
|
References
- World Health Organisation (WHO). Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015.
- Neill, J.O. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance, HM Government, Wellcome Trust: London, UK, 2016.
- Hay, S.I.; Rao, P.C.; Dolecek, C.; Day, N.P.J.; Stergachis, A.; Lopez, A.D.; Murray, C.J.L. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018, 16, 78. [Google Scholar] [CrossRef] [Green Version]
- Bebell, L.M.; Muiru, A.N. Antibiotic Use and Emerging Resistance: How Can Resource-Limited Countries Turn the Tide? Glob. Heart 2014, 9, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Haring, D.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2197–2223. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Giske, C.G.; Wei, Z.Q.; Shen, P.; Heddini, A.; Li, L. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist. Updat 2011, 14, 236–250. [Google Scholar] [CrossRef]
- Das, P.; Horton, R. Antibiotics: Achieving the balance between access and excess. Lancet 2016, 387, 102–104. [Google Scholar] [CrossRef]
- Mendelson, M.; Røttingen, J.A.; Gopinathan, U.; Hamer, D.H.; Wertheim, H.; Basnyat, B.; Butler, C.; Tomson, G.; Balasegaram, M. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2016, 387, 188–198. [Google Scholar] [CrossRef]
- Heyman, G.; Cars, O.; Bejarano, M.; Peterson, S. Access, excess, and ethics—towards a sustainable distribution model for antibiotics. Ups. J. Med. Sci. 2014, 119, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.S. Walking the Line between Antimicrobial Access and Excess. UNICEF Connect. 2016. Available online: https://blogs.unicef.org/blog/walking-the-line-between-antimicrobial-access-and-excess/ (accessed on 12 May 2021).
- Mattos, K.P.H.; Visacri, M.B.; Quintanilha, J.C.F.; Lloret, G.R.; Cursino, M.A.; Levin, A.S.; Levy, C.E.; Moriel, P. Brazil’s resolutions to regulate the sale of antibiotics: Impact on consumption and Escherichia coli resistance rates. J. Glob. Antimicrob. Resist. 2017, 10, 195–199. [Google Scholar] [CrossRef]
- Moura, M.L.; Boszczowski, I.; Mortari, N.; Barrozo, L.V.; Neto, F.C.; Lobo, R.D.; Lima, A.C.P.; Levin, A.S. The impact of restricting over-the-counter sales of antimicrobial drugs: Preliminary analysis of national data. Medicine 2015, 94, e1605. [Google Scholar] [CrossRef] [PubMed]
- Santa-Ana-Tellez, Y.; Mantel-Teeuwisse, A.K.; Dreser, A.; Leufkens, H.G.; Wirtz, V.J. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS ONE 2012, 8, e75550. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, V.J.; Herrera-Patino, J.J.; Santa-Ana-Tellez, Y.; Dreser, A.; Elseviers, M.; Vander Stichele, R.H. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Trop. Med. Int. Health 2013, 18, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, G.; Kotwani, A.; Bhullar, L.; Joshi, J. Over-the-counter sales of antibiotics for human use in India: The challenges and opportunities for regulation. Med. Law Int. 2021, 21, 147–173. [Google Scholar] [CrossRef]
- Fang, Y. China should curb non-prescription use of antibiotics in the community. BMJ 2014, 348, g4233. [Google Scholar] [CrossRef] [Green Version]
- Mossialos, E.; Ge, Y.; Hu, J.; Wang, L. Pharmaceutical Policy in China: Challenges and Opportunities for Reform; World Health Organisation: Geneva, Swizerland, 2016.
- Yin, J.; Wang, Y.; Xu, X.; Liu, Y.; Yao, L.; Sun, Q. The progress of global antimicrobial resistance governance and its implication to China: A review. Antibiotics 2021, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Ngok, K.L.; Phillips, D. Social Policy in China: Development and Wellbeing; Policy Press: Bristol, UK, 2008. [Google Scholar]
- Liu, Q.; Wang, B.; Kong, Y.; Cheng, K.K. China’s primary health-care reform. Lancet 2011, 377, 2064–2066. [Google Scholar] [CrossRef]
- Li, H.; Sun, H. The historical evolution of China’s drug regulatory system. Value Health 2014, 17, A30–A31. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Santoro, M.A.; Meng, Q.; Liu, C.; Eggleston, K. Pharmaceutical policy in China. Health Aff. 2008, 27, 1042–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Yang, S.; Zhou, S.; Jiang, M.; Liu, J. Community pharmacy practice in China: Past, present and future. Int. J. Clin. Pharm. 2013, 35, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Certification Center for Licensed Pharmacist of NMPA. Report on National Licensed Pharmacist Registration of January 2016. Beijing: Certification Center for Licensed Pharmacist of NMPA. 2016. Available online: http://www.cqlp.org/cqlpadminmanage/upfiles/201602/20160202162755581.pdf (accessed on 14 May 2021).
- Yu, M.; Zhu, Y.; Song, X.; Yang, L.; Tao, T.; Zhao, Q.; Xu, B.; Zhao, G. Insights into residents’ behaviour of antibiotic purchasing from medical sale of retail pharmacies in rural China. Fudan Univ. J. Med. Sci. 2013, 40, 253–258. [Google Scholar]
- Ministry of Health (MoH), National Traditional Chinese Medicine Administrative Bureau & Health Department of Ministry of Logistics. Notice on Implementing Principles for Clinical Use of Antibiotics; MoH, National Traditional Chinese Medicine Administrative Bureau and Health Department of Ministry of Logistics: Beijing, China, 2004.
- MoH. Notice on Issuing ‘National Formulary (2010 Edition)’ by Ministry of Health; MoH: Beijing, China, 2010.
- Zhang, Y. The legislation of antibiotics. J. Fangyuan 2011, 23, 18–22. [Google Scholar]
- State Council. Implementation Plan of Main Areas of Health System Reform in the Near Future (2009–2011); State Council: Beijing, China, 2009.
- Xiao, Y.; Zhang, J.; Zheng, B.; Zhao, L.; Li, S.; Li, L. Changes in Chinese policies to promote the rational use of antibiotics. PLoS Med. 2013, 10, e1001556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MoH. Administrative Regulations for the Clinical Use of Antibiotics; MoH: Beijing, China, 2012.
- MoH (Department of Medical Affairs Administration). The 2011 Proposal of National Special Campaign for the Clinical Use of Antibiotics; MoH: Beijing, China, 2011.
- MoH (Department of Medical Affairs Administration). The 2012 Proposal of National Special Campaign for the Clinical Use of Antibiotics; MoH: Beijing, China, 2012.
- MoH (Department of Medical Affairs Administration). The 2013 Proposal of National Special Campaign for the Clinical Use of Antibiotics; MoH: Beijing, China, 2013.
- Xiao, Y.; Li, L. Legislation of clinical antibiotic use in China. Lancet Infect. Dis. 2013, 13, 189–191. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, L. The actions of China in containing antimicrobial resistance. AMR Control: Overcoming Global Antimicrobial Resistance; TIM PROBART & WAAAR: Woodbridge, UK, 2015; pp. 46–53. [Google Scholar]
- National Health and Family Planning Commission (NHFPC). The National Action Plan to Curb Bacterial Resistance (2016–2020); NHFPC: Beijing, China, 2016.
- WHO. People’s Republic of China Health System Review; WHO Regional Office for the Western Pacific: Manila, Philippines, 2015.
- China Drug Administration (CDA). Notice on the Introduction of List of First Branch of National over the Counter Medicines (Western Medicine and Chinese Patent Medicine) by Centre Drug Administration; CDA: Beijing, China, 1999.
- CDA. Measures for Separate Administration of Prescribed and Non-prescribed Medicines (Elementary Edition); CDA: Beijing, China, 1999.
- CDA. Temporary Provisions for Prescribed and Non-Prescribed Medicine Distribution; CDA: Beijing, China, 1999.
- CDA. Measures for the Supervision and Administration of Circulation of Pharmaceuticals (Elementary Edition) [Invalid]; CDA: Beijing, China, 1999.
- China Food and Drug Administration (CFDA). Measures for the Supervision and Administration of Circulation of Pharmaceuticals; CFDA: Beijing, China, 2007.
- CFDA. Notice on Publishing ‘2004–2005 Work Plan Related to Separate Administration of Prescribed and Non-prescribed Medicines’ by China Food and Drug Administration; CFDA: Beijing, China, 2004.
- CFDA. Notice on Strengthening Administration over the Sale of Antimicrobials in Retail Pharmacies to Promote Rational Use of Medicines; CFDA: Beijing, China, 2003.
- Ministry of Human Resources (MoHR); CDA. Temporary Regulations on Licensed Pharmacist Qualification; MoHRSS: Beijing, China; CDA: Beijing, China, 1999.
- CFDA. The Temporary Regulation Method on the Licensed Pharmacist Continuing Professional Development; CFDA: Beijing, China, 2003. [Google Scholar]
- CFDA. Measures for the Administration of Pharmaceutical Trade License [Revised]; CFDA: Beijing, China, 2004. [Google Scholar]
- CFDA. Decision of the China Food and Drug Administration to Amend Some Rules; CFDA: Beijing, China, 2017.
- CFDA. Good Supply Practice for Pharmaceutical Products (2016 Amendment); CFDA: Beijing, China, 2016.
- State Council. Notice on Publishing National Medicine Safety ‘Twelve-Five’ Programme by State Council; State Council: Beijing, China, 2012.
- Chen, J.; Wang, Y.; Chen, X.; Hesketh, T. Widespread illegal sales of antibiotics in Chinese pharmacies—A nationwide cross-sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 12. [Google Scholar] [CrossRef]
- Shi, L.; Chang, J.; Liu, X.; Zhai, P.; Hu, S.; Li, P.; Hayat, K.; Kabba, J.A.; Feng, Z.; Yang, C.; et al. Dispensing Antibiotics without a Prescription for Acute Cough Associated with Common Cold at Community Pharmacies in Shenyang, Northeastern China: A Cross-Sectional Study. Antibiotics 2020, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Ye, D.; Lv, B.; Jiang, M.; Zhu, S.; Yan, K.; Tian, Y.; Fang, Y. Sale of antibiotics without a prescription at community pharmacies in urban China: A multicentre cross-sectional survey. J. Antimicrob. Chemother. 2017, 72, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Certification Center for Licensed Pharmacist of NMPA. Report on National Licensed Pharmacist Registration of November 2015. Beijing: Certification Center for Licensed Pharmacist of NMPA. 2015. Available online: http://www.cqlp.org/info/link.aspx?id=2662&page=1 (accessed on 8 April 2021).
- Certification Center for Licensed Pharmacist of NMPA. Report on National Licensed Pharmacist Registration of March 2017. Beijing: Certification Center for Licensed Pharmacist of NMPA. 2017. Available online: http://www.cqlp.org/cqlpadminmanage/upfiles/201704/20170410164640578.pdf (accessed on 18 May 2021).
- Tao, J.; Zhang, T.; Xu, J.; Wu, C. Analysis of the current situation of antibiotics use in China a hospital-based perspective. Ther. Innov. Regul. Sci. 2013, 47, 23–31. [Google Scholar] [CrossRef]
- Zhang, T.; Graham, H.; White, P.C.L. Healthcare providers’ accounts of influences of antibiotic-related reforms on their behaviour with respect to the use of antibiotics for children: A qualitative study in China. Public Health Open Access 2018, 2, 000122. [Google Scholar] [CrossRef]
- Zhang, T.; Graham, H.; White, P.C.L. Healthcare providers’ accounts of parental influence on their behaviour with respect to the use of antibiotics for children: A qualitative study in China. J. Community Med. Public 2018, 5, 036. [Google Scholar]
- Certification Center for Licensed Pharmacist of NMPA. 2016 Final Report on National Licensed pharmacist Registration. Beijing: Certification Center for Licensed Pharmacist of NMPA. 2017. Available online: http://www.cqlp.org/info/link.aspx?id=3164&page=1 (accessed on 15 April 2021).
- Chalker, J.; Ratanawijitrasin, S.; Chuc, N.T.K.; Petzold, M.; Tomson, G. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand—A randomized controlled trial. Soc. Sci. Med. 2005, 60, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Chalker, J.; Chuc, N.T.K.; Falkenberg, T.; Tomson, G. Private pharmacies in Hanoi Vietnam: A randomised trial of a 2 year multi-component intervention on knowledge and stated practice regarding ARI, STD and antibiotic/steroid requests. Trop. Med. Int. Health 2002, 7, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Markovic-Pekovic, V.; Grubisa, N.; Burger, J.; Bojanic, L. Initiatives to reduce non-prescription sales and dispensing of antibiotics in the Republic of Srpska; findings and implications. J. Res. Pharm. Pract. 2017, 6, 120–125. [Google Scholar]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 9, 168–175. [Google Scholar] [CrossRef]
- Dai, D.; Jiang, B.; Han, C.; Shi, L.; Zhao, D. Investigation of medicines shortages in China. China Pharm. 2010, 9, 785–787. [Google Scholar]
- Shen, L.; Wen, Z. The bluebook of medicines distribution system: The development report of China medicines distribution industry. Soc. Sci. Acad. Press 2016, 2015, 137–153. [Google Scholar]
- Hou, J.L.; Ke, Y. Addressing the Shortage of Health Professionals in Rural China: Issues and Progress; Comment on ‘Have Health Human Resources Become More Equal between Rural and Urban Areas after the New Reform?’. Int. J. Health Policy Manag. 2015, 4, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.C.; Hsiao, W.C.; Chen, W.; Hu, S.; Ma, J.; Maynard, A. Early appraisal of China’s huge and complex health-care reforms. Lancet 2012, 379, 833–842. [Google Scholar] [CrossRef]
- Young, M.; Wolfheim, C.; Marsh, D.R.; Hammamy, D. World Health Organization/United Nations Children’s Fund joint statement on integrated community case management: An equity-focused strategy to improve access to essential treatment services for children. Am. J. Trop. Med. Hyg. 2012, 87, 6–10. [Google Scholar] [CrossRef]
- Yeboah-Antwi, K.; Pilingana, P.; Macleod, W.B.; Semrau, K.; Siazeele, K.; Kalesha, P.; Hamainza, B.; Seidenberg, P.; Mazimba, A.; Sabin, L.; et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: A cluster randomized controlled trial. PLoS Med. 2010, 7, e1000340. [Google Scholar] [CrossRef]
- Gyapong, M.G.; Garshong, B. Lessons Learned in Home Management of Malaria: Implementation Research in Four African Countries; WHO: Geneva, Switzerland, 2007.
- Mukanga, D.; Tiono, A.B.; Anyorigiya, T.; Källander, K.; Konaté, A.T.; Oduro, A.R.; Tibenderana, J.K.; Amenga-Etego, L.; Sirima, S.B.; Cousens, S.; et al. Integrated community case management of fever in children under five using rapid diagnostic tests and respiratory rate counting: A multi-country cluster randomized trial. J. Trop. Med. Hyg. 2012, 87, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Walt, G.; Gilson, L. Reforming the health sector in developing countries: The central role of policy analysis. Health Policy Plan 1994, 9, 353–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, F.; Lv, F.; Xu, P.; Zhang, D.; Meng, S.; Ju, L.; Jiang, H.; Ma, L.; Sun, J.; Wu, Z. Task shifting of HIV/AIDS case management to Community Health Service Centers in urban China: A qualitative policy analysis. BMC Health Serv. Res. 2015, 15, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depraetere, J.; Vandeviver, C.; Keygnaert, I.; Beken, T.V. The critical interpretive synthesis: An assessment of reporting practices. Int. J. Soc. Res. Methodol. 2021, 24, 669–689. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Lambert, H.; Zhao, L.; Liu, R.; Shen, X.; Wang, D.; Cabral, C. Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review. Antibiotics 2022, 11, 141. https://doi.org/10.3390/antibiotics11020141
Zhang T, Lambert H, Zhao L, Liu R, Shen X, Wang D, Cabral C. Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review. Antibiotics. 2022; 11(2):141. https://doi.org/10.3390/antibiotics11020141
Chicago/Turabian StyleZhang, Tingting, Helen Lambert, Linhai Zhao, Rong Liu, Xingrong Shen, Debin Wang, and Christie Cabral. 2022. "Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review" Antibiotics 11, no. 2: 141. https://doi.org/10.3390/antibiotics11020141
APA StyleZhang, T., Lambert, H., Zhao, L., Liu, R., Shen, X., Wang, D., & Cabral, C. (2022). Antibiotic Stewardship in Retail Pharmacies and the Access-Excess Challenge in China: A Policy Review. Antibiotics, 11(2), 141. https://doi.org/10.3390/antibiotics11020141






