Factors Associated with Antimicrobial Stewardship Practices on California Dairies: One Year Post Senate Bill 27
Abstract
:1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Logistic Regression Models
2.2.1. Predictors Concerning Familiarity of Dairies with the FDA “MIADs” Term
2.2.2. Predictors Concerning the Use of MIADs That Were Restricted from OTC Sales Beginning in January 2018
2.2.3. Predictors Concerning the Use of Preventive Alternatives to AMD on Dairy Farms
2.2.4. Predictors Concerning Changes in Management Practices to Prevent Spread or Outbreaks of Disease on Dairies
2.2.5. Predictors Concerning Change in a Dairy’s AMD Costs
2.2.6. Predictors Concerning Change in Reported Farm Animal Health
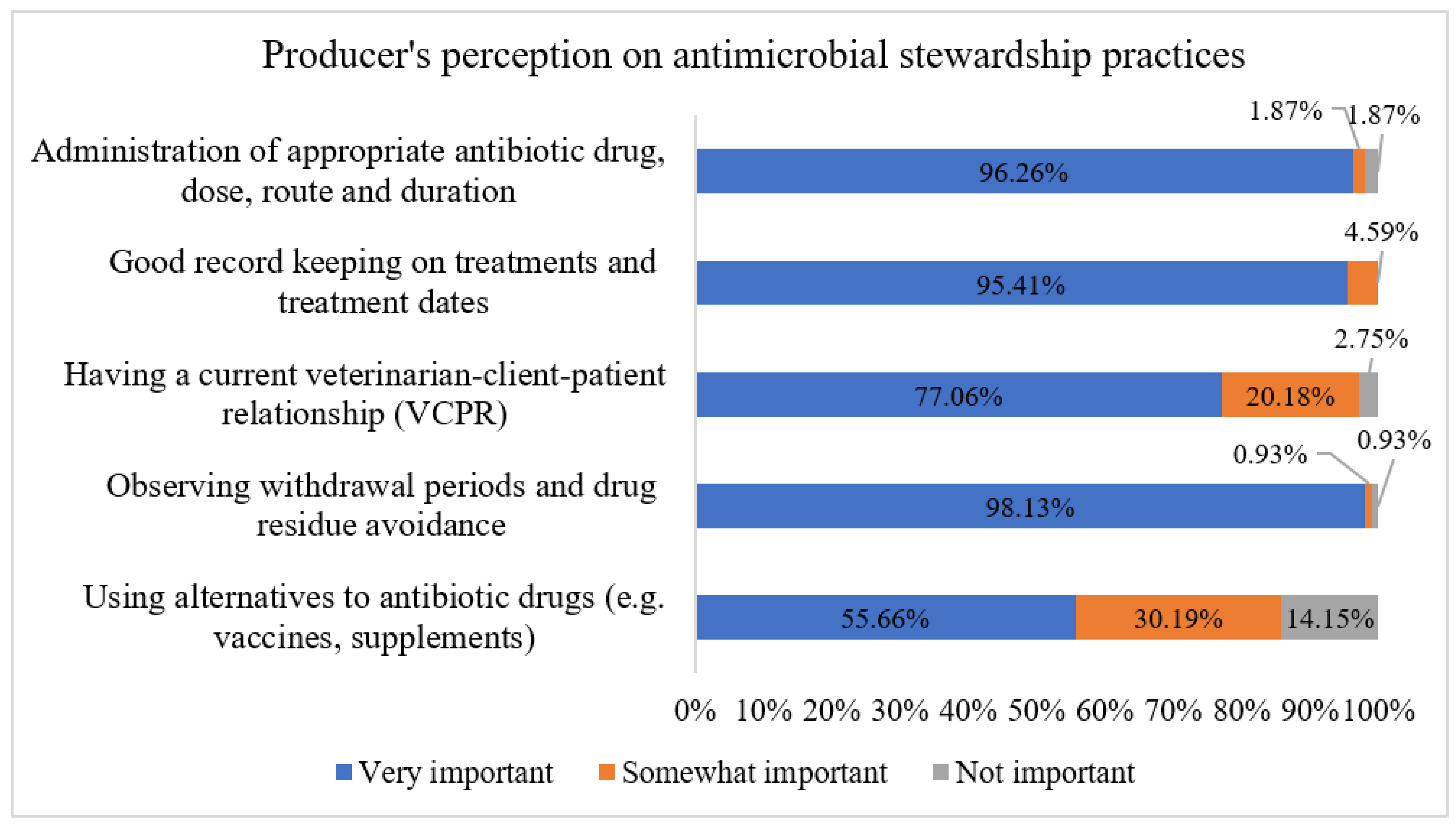
2.2.7. Predicting Factors Associated with Dairy Producers’ Perceptions Regarding the Importance of AMS Practices on Dairies Using Machine Learning Classification Models
3. Discussion
3.1. Predictors Concerning Familiarity of Dairies with the FDA “MIADs” Term
3.2. Predictors Concerning the Use of MIADs That Were Restricted from OTC Sales Beginning in January 2018
3.3. Predictors Concerning the Use of Preventive Alternatives to AMD on Dairy Farms
3.4. Predictors Concerning Changes in Management Practices to Prevent the Spread or Outbreak of Disease in Dairies
3.5. Predictors Concerning Change in Dairy’s AMD Costs
3.6. Predictors Concerning Farm’s Animal Health Compared to 2018
3.7. Comparing Survey Findings Immediately Post SP 27 (2018) and One Year Later (2019)
3.8. Factors Associated with Dairy Producers’ Perceptions Regarding the Importance of AMS Practices on Dairies
3.9. Study Limitations
4. Materials and Methods
4.1. Statistical Analyses
4.1.1. Descriptive Statistics
4.1.2. Logistic Regression Models
- (1)
- Familiarity of dairy producers with the FDA’s MIADs term. The familiarity of dairy producers with MIADs was identified if the survey respondent recognized the FDA classification of MIADs as important, highly important, or critically important drugs, and/or that MIADs are available for livestock only via prescription or veterinary feed directive pursuant to VCPR with a licensed veterinarian. Familiarity with MIADs was dichotomized into 2 levels: “familiar” and “not familiar.”
- (2)
- Changes made since January 2018 regarding the use of injectable, bolus, and/or intramammary dosage forms of OTC MIADs. This outcome was classified as “decreased OTC MIADs use” or “increased or no change in the use of OTC MIADs”.
- (3)
- Initiation or increased use of alternatives to AMD since January 2018. This third outcome was dichotomized as “yes” (use AMD alternatives) or “no” (do not use AMD alternatives).
- (4)
- Changes in management practices to prevent disease outbreak or spread since January 2018. This fourth outcome was dichotomized as “yes” (made changes in management practices in the form of improvement in vaccination programs, quarantined purchased and returned animals from offsite locations, improvements of farm biosecurity measures, or testing of pre-purchased animals for infectious diseases before joining the herd) and “no” (no changes in management practices).
- (5)
- Description of the farm’s AMD costs since January 2018. This fifth outcome modeled the changes in the farm AMD drug costs in 2019 and was dichotomized as “decreased AMD cost” or “increased/no change AMD cost”.
- (6)
- Description of the farm’s animal health conditions since January 2018. This sixth outcome modeled the changes in farm animal health and was dichotomized as “better animal health” or “worse/no change in animal health”.
4.1.3. Machine Learning Classification Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA (Food and Drug Administration), Center for Veterinary Medicine. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Food and Drug Administration; Center for Veterinary Medicine Website. 2019. Available online: https://www.fda.gov/media/144427/download (accessed on 7 July 2021).
- McManus, M.C. Mechanisms of bacterial resistance to antimicrobial agents. Am. J. Health-Syst. Pharm. 1997, 54, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-associated MRSA. The impact on humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [PubMed]
- CDC (U.S. Centers for Disease Control and Prevention). Biggest Threats and Data: 2019 AR Threats Report; Centers for Disease Control and Prevention Website. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 28 April 2020).
- WHO (World Health Organization). Global Action Plan on Antimicrobial Resistance. 2015. Available online: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 9 July 2020).
- FDA (Food and Drug Administration). Fact Sheet: Veterinary Feed Directive Final Rule and Next Steps. 2017. Available online: https://www.fda.gov/animal-veterinary/development-approval-process/fact-sheet-veterinary-feed-directive-final-rule-and-next-steps (accessed on 12 July 2021).
- FAC (Food and Agricultural Code); Sacramento, C.A. Livestock: Use of Antimicrobial Drugs [14400–14408]. 2015. Available online: https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?sectionNum=14401.&lawCode=FAC (accessed on 9 July 2021).
- Hogan, L.J., Jr. Senate Bill 471. Agriculture–Use of Antimicrobial Drugs—Limitations and Reporting Requirements. Chapter 679. 2019. Available online: https://legiscan.com/MD/text/SB471/2019 (accessed on 10 December 2020).
- Anderson, M.; Buckley, G.; Hayward, S. Oregon Senate Bill 920. 78th Oregon Legislative Assembly—2015 Regular Session. 2015. Available online: https://olis.oregonlegislature.gov/liz/2015R1/Downloads/MeasureDocument/SB920 (accessed on 26 January 2022).
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- EC (European Commission). New EU Rules on Veterinary Medicinal Products and Medicated Feed; European Commission Website. 2019. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ah_vet-med_feed_factsheet-2018_en.pdf (accessed on 9 July 2021).
- Speksnijder, D.C.; Mevius, D.J.; Bruschke, C.J.M.; Wagenaar, J.A. Reduction of veterinary antimicrobial use in The Netherlands. The Dutch success models. Zoonoses Public Health 2015, 62 (Suppl. 1), 79–87. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; de Knegt, L.V.; Andersen, V.D.; Wingstrand, A. Temporal relationship between decrease in antimicrobial prescription for Danish pigs and the “Yellow Card” legal intervention directed at reduction of antimicrobial use. Prev. Vet. Med. 2014, 117, 554–564. [Google Scholar] [CrossRef] [PubMed]
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Vet. J. 2020, 73, 2. [Google Scholar] [CrossRef] [Green Version]
- Ekong, P.S.; Abdelfattah, E.M.; Okello, E.; Williams, D.R.; Lehenbauer, T.W.; Karle, B.M.; Rowe, J.D.; Aly, S.S. 2018 Survey of factors associated with antimicrobial drug use and stewardship practices in adult cows on conventional California dairies: Immediate post-Senate Bill 27 impact. PeerJ 2021, 9, e11596. [Google Scholar] [CrossRef]
- Love, W.J.; Lehenbauer, T.W.; Karle, B.M.; Hulbert, L.E.; Anderson, R.J.; Van Eenennaam, A.L.; Farver, T.B.; Aly, S.S. Survey of management practices related to bovine respiratory disease in preweaned calves on California dairies. J. Dairy Sci. 2016, 99, 1483–1494. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.P.N.; Karle, B.M.; Heguy, J.M. Needs assessment for cooperative extension dairy programs in California. J. Dairy Sci. 2019, 102, 7597–7607. [Google Scholar] [CrossRef]
- Denis-Robichaud, J.; Cerri, R.L.A.; Jones-Bitton, A.; LeBlanc, S.J. Study of reproduction management on Canadian dairy farms. J. Dairy Sci. 2016, 99, 9339–9351. [Google Scholar] [CrossRef]
- Higham, L.E.; Deakin, A.; Tivey, E.; Porteus, V.; Ridgway, S.; Rayner, A.C. A survey of dairy cow farmers in the United Kingdom: Knowledge, attitudes and practices surrounding antimicrobial use and resistance. Vet. Rec. 2018, 183, 746. [Google Scholar] [CrossRef] [PubMed]
- CDFA (California Department of Food and Agriculture); Sacramento, C.A. California Agricultural Statistics Review 2017–2018. 2018. Available online: https://www.nass.usda.gov/Statistics_by_State/California/Publications/Annual_Statistical_Reviews/2019/2018cas-all.pdf (accessed on 12 July 2021).
- Abdelfattah, E.M.; Ekong, P.S.; Okello, E.; Williams, D.; Karle, B.; Rowe, J.; Marshall, E.; Lehenbauer, T.W.; Aly, S. 2019 Survey of Antimicrobial Drug Use and Stewardship Practices in Adult Cows on California Dairies: Post Senate Bill 27. Microorganisms 2021, 9, 1507. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.S.; Rossow, H.A.; Acetoze, G.; Lehenbauer, T.W.; Payne, M.; Meyer, D.; Maas, J.; Hoar, B. Survey of Beef Quality Assurance on California dairies. J. Dairy Sci. 2014, 97, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Ekong, P.S.; Abdelfattah, E.M.; Okello, E.; Williams, D.R.; Lehenbauer, T.W.; Karle, B.M.; Rowe, J.D.; Marshall, E.S.; Aly, S.S. 2018 Survey of antimicrobial drug use and stewardship practices in adult cows on California dairies: Post-Senate Bill 27. PeerJ 2021, 9, e11515. [Google Scholar] [CrossRef]
- Ekakoro, J.E.; Caldwell, M.; Strand, E.B.; Okafor, C.C. Perceptions of Tennessee cattle producers regarding the Veterinary Feed Directive. PLoS ONE 2019, 14, 1–19. [Google Scholar] [CrossRef]
- Padda, H.; Wemette, M.; Safi, A.G.; Beauvais, W.; Shapiro, M.A.; Moroni, P.; Ivanek, R. New York State dairy veterinarians’ perceptions of antibiotic use and resistance: A qualitative interview study. Prev. Vet. Med. 2021, 194, 105428. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Dürr, S.; Bodmer, M. Reducing Antimicrobial Use by Implementing Evidence-Based, Management-Related Prevention Strategies in Dairy Cows in Switzerland. Front. Vet. Sci. 2021, 7, 611682. [Google Scholar] [CrossRef]
- Vasquez, A.K.; Foditsch, C.; Dulièpre, S.-A.C.; Siler, J.D.; Just, D.R.; Warnick, L.D.; Nydam, D.V.; Sok, J. Understanding the effect of producers’ attitudes, perceived norms, and perceived behavioral control on intentions to use antimicrobials prudently on New York dairy farms. PLoS ONE 2019, 14, e0222442. [Google Scholar] [CrossRef] [Green Version]
- Sumner, C.L.; von Keyserlingk, M.A.G.; Weary, D.M. Perspectives of farmers and veterinarians concerning dairy cattle welfare. Anim. Front. 2018, 8, 8–13. [Google Scholar] [CrossRef]
- Jansen, J.; Renes, R.J.; Lam, T.J.G.M. Evaluation of two communication strategies to improve udder health management. J. Dairy Sci. 2010, 93, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Laanen, M.; Persoons, D.; Ribbens, S.; de Jong, E.; Callens, B.; Strubbe, M.; Maes, D.; Dewulf, J. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. Vet. J. 2013, 198, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Buchy, P.; Ascioglu, S.; Buisson, Y.; Datta, S.; Nissen, M.; Tambyah, P.A.; Vong, S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2020, 90, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, D.; Adaska, J.; Higginbotham, G.; Castillo, A.; Collar, C.; Sischo, W. Testing new dairy cattle for disease can boost herd health, cut costs. Calif. Agric. 2009, 63, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Buhman, M.; Dewel, G.; Griffen, D. Biosecurity Basics for Cattle Operations and Good Management Practices (GMP) for Controlling Infectious Diseases. Nebraska Cooperative Extension G00–1411-A. 2000. Available online: http://extensionpublications.unl.edu/assets/pdf/g1411.pdf (accessed on 5 July 2021).
- Ohlson, A.; Emanuelson, U.; Tråvén, M.; Alenius, S. The relationship between antibody status to bovine corona virus and bovine respiratory syncytial virus and disease incidence, reproduction, and herd characteristics in dairy herds. Acta Vet. Scand. 2010, 52, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ribbens, S.; Dewulfa, J.; Koenenb, F.; Mintiensc, K.; De Sadeleera, L.; de Kruifa, A.; Maes, D. A survey on biosecurity and management practices in Belgian pig herds. Prev. Vet. Med. 2008, 83, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Kurt, T.; Wong, N.; Fowler, H.; Gay, C.; Lillehoj, H.; Plummer, P.; Scott, H.M.; Hoelzer, K. Strategic Priorities for Research on Antibiotic Alternatives in Animal Agriculture—Results from an Expert Workshop. Front. Vet. Sci. 2019, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals-part 2: New approaches and potential solutions. Vet. Res. 2018, 49, 141. [Google Scholar] [CrossRef] [Green Version]
- Buckley, B.S.; Henschke, N.; Bergman, H.; Skidmore, B.; Klemm, E.J.; Villanueva, G. Impact of vaccination on antibiotic usage: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.; Ricci, A.; Auce, Z.; Beechinor, J.G.; Bergendahl, H.; Breathnach, R.; Bureš, J.; Da Silva, D.; Pedro, J.; Hederová, J. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, 4666. [Google Scholar]
- Lam, T.J.G.M.; Jansen, J.; Wessels, R.J. The RESET Mindset Model applied on decreasing antibiotic usage in dairy cattle in the Netherlands. Ir. Vet. J. 2017, 70, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ollivett, T.L. Thoracic ultrasound to monitor lung health and assist decision making in preweaned dairy calves. AABP Proc. 2018, 51, 185–187. [Google Scholar]
- Lago, A.; Godden, S.M.; Bey, R.; Ruegg, P.L.; Leslie, K. The selective treatment of clinical mastitis based on on-farm culture results: I. Effects on antibiotic use, milk withholding time, and short-term clinical and bacteriological outcomes. J. Dairy Sci. 2011, 94, 4441–4456. [Google Scholar] [CrossRef] [PubMed]
- Macrae, A.; Esslemont, R. The prevalence and cost of important endemic diseases and fertility in dairy herds in the UK. In Bovine Medicine, 3rd ed.; Crockfort, P.D., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Donadeu, F.X.; Howes, N.L.; Esteves, C.L.; Howes, M.P.; Byrne, T.J.; Macrae, A.I. Farmer and Veterinary Practices and Opinions Related to the Diagnosis of Mastitis and Metabolic Disease in UK Dairy Cows. Front. Vet. Sci. 2020, 7, 127. [Google Scholar] [CrossRef]
- Ramirez, C.R.; Dumas, S.E.; Schlist, K.L.; French, D.D.; Bichi, E.; Rivero, B.R.C.; Aldridge, B.M.; Lowe, J.F. Evidence in support of veterinary involvement in antimicrobial stewardship training programs in the dairy industry: A preliminary study. Bov. Pract. 2017, 51, 200–204. [Google Scholar]
- Habing, G.; Djordjevic, C.; Schuenemann, G.M.; Lakritz, J. Understanding antimicrobial stewardship: Disease severity treatment thresholds and antimicrobial alternatives among organic and conventional calf producers. Prev. Vet. Med. 2016, 130, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Bystritsky, R.; Beltran, A.; Young, A.; Wong, A.; Hu, X.; Doernberg, S. Machine learning for the prediction of antimicrobial stewardship intervention in hospitalized patients receiving broad-spectrum agents. Infect. Control. Hosp. Epidemiol. 2020, 41, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, L.Y.; Gilkerson, J.R.; Billman-Jacobe, H.; Stevenson, M.A.; Thursky, K.; Bailey, K.E.; Browning, G.F. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J. Vet. Intern. Med. 2018, 32, 1092–1099. [Google Scholar] [CrossRef]
- Jones, P.J.; Marierb, E.A.; Trantera, R.B.; Wub, G.; Watsonb, E.; Tealeb, C.J. Factors affecting dairy farmers’ attitudes towards antimicrobial medicine usage in cattle in England and Wales. Prev. Vet. Med. 2015, 121, 30–40. [Google Scholar] [CrossRef]
- Friedman, D.B.; Kanwat, C.P.; Headrick, M.L.; Patterson, N.J.; Neely, J.C.; Smith, L.U. Importance of Prudent Antibiotic Use on Dairy Farms in South Carolina: A Pilot Project on Farmers’ Knowledge, Attitudes and Practices. Zoonoses Public Health 2007, 54, 366–375. [Google Scholar] [CrossRef]
- USDA. Health and Management Practices on U.S. Dairy Operations; USDA–APHIS–VS–CEAH–NAHMS; National Animal Health Monitoring System: Fort Collins, CO, USA, 2014. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/monitoring-and-surveillance/nahms/NAHMS_Dairy_Studies (accessed on 30 October 2020).
- Aly, S.S.; Anderson, R.J.; Adaska, J.M.; Jiang, J.; Gardner, I.A. Association between Mycobacterium avium subspecies paratuberculosis infection and milk production in two California dairies. J. Dairy Sci. 2010, 93, 1030–1040. [Google Scholar] [CrossRef]
- Slob, N.; Catal, C.; Kassahun, A. Application of machine learning to improve dairy farm management: A systematic literature review. Prev. Vet. Med. 2021, 187, 105237. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hyde, R.M.; Down, P.M.; Bradley, A.J.; Breen, J.E.; Hudson, C.; Leach, K.A.; Green, M.J. Automated prediction of mastitis infection patterns in dairy herds using machine learning. Sci. Rep. 2020, 10, 4289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
| Variables | Coefficient | SE | Odds Ratio | 95% CI | p-Value 2 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Region 1 | ||||||
| GSCA | Referent | |||||
| NCA + NSJV | −0.49 | 0.51 | 0.61 | 0.22 | 1.63 | 0.32 |
| Herd size, milking cows | ||||||
| <1304 | Referent | |||||
| ≥1304 | −1.10 | 0.63 | 0.33 | 0.09 | 1.17 | 0.09 |
| Breed | ||||||
| Holstein | Referent | |||||
| Jersey | −0.82 | 1.41 | 0.44 | 0.02 | 7.01 | 0.56 |
| Crossbreed | 1.75 | 1.25 | 5.74 | 0.49 | 66.25 | 0.16 |
| Mix/Other | −0.37 | 0.59 | 0.69 | 0.21 | 2.22 | 0.54 |
| Which AMD treatment information do you track or record? | ||||||
| No milk or meat withdrawal interval | Referent | |||||
| Included milk and meat withdrawal interval | 2.36 | 0.69 | 10.57 | 2.73 | 40.94 | <0.01 |
| Included milk or meat withdrawal interval | −0.34 | 0.99 | 0.71 | 0.10 | 5.01 | 0.74 |
| Do you have a veterinarian–client–patient relationship (VCPR)? | ||||||
| No | Referent | |||||
| Yes | 2.73 | 1.24 | 15.29 | 1.34 | 173.53 | 0.03 |
| Variables | Coefficient | SE | Odds Ratio | 95% CI | p-Value 2 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Region 1 | ||||||
| GSCA | Referent | |||||
| NCA + NSJV | −0.79 | 0.64 | 0.45 | 0.13 | 1.61 | 0.22 |
| Herd size | ||||||
| <1304 | Referent | |||||
| ≥1304 | −0.75 | 0.77 | 0.47 | 0.10 | 2.13 | 0.32 |
| Breed 3 | ||||||
| Holstein | Referent | |||||
| Others (Jersey, crossbreed, and mix) | 0.68 | 0.68 | 1.97 | 0.51 | 7.61 | 0.32 |
| Participate in any dairy quality assurance programs? | ||||||
| No | Referent | |||||
| Yes | 1.26 | 0.65 | 3.55 | 0.98 | 12.80 | 0.05 |
| Changes made by farm regarding MIADs previously available OTC since 2018 | ||||||
| Increased/no changes | Referent | |||||
| Decreased | 1.79 | 0.74 | 5.99 | 1.38 | 25.84 | 0.01 |
| Variables | Coefficient | SE | Odds Ratio | 95% CI | p-Value 2 | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Region 1 | ||||||
| GSCA | Referent | |||||
| NCA + NSJV | −0.30 | 0.51 | 0.76 | 0.28 | 2.10 | 0.59 |
| Herd size | ||||||
| <1304 | Referent | |||||
| ≥1304 | −0.33 | 0.53 | 0.72 | 0.25 | 2.10 | 0.54 |
| Breed 3 | ||||||
| Holstein | Referent | |||||
| Other (Jersey, crossbreed, and mix) | −0.02 | 0.55 | 0.97 | 0.32 | 2.90 | 0.96 |
| Who decides AMD to treat sick cows? | ||||||
| Dairy personnel only | Referent | |||||
| Veterinarian involved | −1.20 | 0.51 | 0.31 | 0.12 | 0.84 | 0.02 |
| Have you used on-farm diagnostic techniques to guide AMD treatment? | ||||||
| No | Referent | |||||
| Yes | 1.51 | 0.52 | 4.53 | 1.61 | 12.71 | <0.05 |
| Have you made changes in management to prevent disease outbreak/spread? | ||||||
| No | Referent | |||||
| Yes | 1.10 | 0.52 | 2.91 | 1.10 | 8.10 | 0.04 |
| Farm’s AMD costs | ||||||
| Increased/no change | Referent | |||||
| Decreased | 1.30 | 0.54 | 3.68 | 1.30 | 10.78 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelfattah, E.M.; Ekong, P.S.; Okello, E.; Williams, D.R.; Karle, B.M.; Lehenbauer, T.W.; Aly, S.S. Factors Associated with Antimicrobial Stewardship Practices on California Dairies: One Year Post Senate Bill 27. Antibiotics 2022, 11, 165. https://doi.org/10.3390/antibiotics11020165
Abdelfattah EM, Ekong PS, Okello E, Williams DR, Karle BM, Lehenbauer TW, Aly SS. Factors Associated with Antimicrobial Stewardship Practices on California Dairies: One Year Post Senate Bill 27. Antibiotics. 2022; 11(2):165. https://doi.org/10.3390/antibiotics11020165
Chicago/Turabian StyleAbdelfattah, Essam M., Pius S. Ekong, Emmanuel Okello, Deniece R. Williams, Betsy M. Karle, Terry W. Lehenbauer, and Sharif S. Aly. 2022. "Factors Associated with Antimicrobial Stewardship Practices on California Dairies: One Year Post Senate Bill 27" Antibiotics 11, no. 2: 165. https://doi.org/10.3390/antibiotics11020165
APA StyleAbdelfattah, E. M., Ekong, P. S., Okello, E., Williams, D. R., Karle, B. M., Lehenbauer, T. W., & Aly, S. S. (2022). Factors Associated with Antimicrobial Stewardship Practices on California Dairies: One Year Post Senate Bill 27. Antibiotics, 11(2), 165. https://doi.org/10.3390/antibiotics11020165








