Abstract
Databases such as PubMed, Scopus and Google Scholar were searched. Data extraction and assessment of study protocol was done by two independent reviewers and the results were reviewed by a third. OpenMeta analyst and comprehensive meta-analysis (CMA) were used for the meta-analysis. The random effect model was used, publication bias and between-study heterogeneity was assessed. Seventeen studies were added to the final meta-analysis. Studies were sampled from 2000–2018 and of the 8684 isolates tested, 2824 were VRE. The pooled prevalence of VRE among poultry in Malaysia was estimated at 24.0% (95% CI; 16.7–33.1%; I2 = 98.14%; p < 0.001). Between-study variability was high (t2 = 0.788; heterogeneity I2 = 98.14% with heterogeneity chi-square (Q) = 858.379, degrees of freedom (df) = 16, and p < 0.001). The funnel plot showed bias which was confirmed by Egger’s test and estimates from the leave-one-out forest plot did not affect the pooled prevalence. Pooled prevalence of VRE in chickens and ducks were 29.2% (CI = 18.8–42.5%) and 11.2%, CI = 9.0–14.0%) respectively. Enterococcus faecalis was reported most with more studies being reported in Peninsular Malaysia Central region and used antibiotic disc diffusion as detection method. Increased surveillance of VRE in poultry in Malaysia is required.
1. Introduction
Enterococcus has emerged as a significant nosocomial and community-acquired pathogen as a result of its ability to develop resistance to antimicrobials, particularly vancomycin. Vancomycin is the final treatment option, particularly for Enterococcus [1,2]. Human antimicrobial use, as well as their use as growth promoters in the livestock industry, were thought to have resulted in the emergence of enterococcal-resistant strains. A good example is the use of avoparcin as a feed additive to promote livestock growth [3].
The National Pharmaceuticals Regulatory Agency (NPRA) and the Department of Veterinary Services (DVS) in Malaysia have prohibited the use of avoparcin and vancomycin to reduce the spread or prevalence of vancomycin-resistant Enterococcus (VRE). DVS has been monitoring veterinary drug residues, including antibiotics, in animal feed since 2013, in accordance with EEC Directive 1990 [4]. This will invariably entail the monitoring of two antibiotic groups: group A, which includes banned substances such as avoparcin, chloramphenicol and vancomycin, and group B, which includes drugs with MRLs such as tetracycline. The most frequently used antibiotic classes in Malaysia are aminoglycosides, Beta-Lactams, microlides, tetracyclines, polymyxins, quinolones, sulfonamides and amphenicols [4]. What has brought VRE to the forefront in Malaysia is not only its critical public health concern but its potential economic impact on the livestock sector [3]. Antibiotic resistance poses great threat to food safety and public health when the resistant bacteria spread from food animals to poultry farmers, farmworkers and veterinarians through the food chain. As a result, antimicrobial resistance in poultry is a significant public health risk that warrants a discreet yet robust response. VRE has been reported in Malaysia amongst health workers, animals, hospital patients and farmworkers [3]. The epidemiology and transmission of resistant bacteria between humans and animals has increased, and their zoonotic potential cannot be underestimated [5].
In order to assess the risks and distribution of vancomycin-resistant Enterococcus (VRE) in poultry in Malaysia, a meta-analysis and a systematic review were carried out. This could help provide basic information for vigilance and the conceptualization of suitable and tailored policies in Malaysia to control antimicrobial resistance in poultry.
2. Results
2.1. Search Results and Eligible Studies
A total of 300 studies were identified through searching of databases and 150 duplicates were removed. The 150 articles left had their titles and abstracts screened, and 130 articles were excluded having found not to meet any of the inclusion criteria. Twenty full-text articles were assessed for eligibility with seven excluded for lack of sufficient information and the non-use of vancomycin for the antimicrobial susceptibility test (Figure 1). A total of 13 full-text studies were used for qualitative analysis (Figure 1). To have a near accurate estimate of VRE in poultry in Malaysia, studies reporting the prevalence in more than one type of poultry bird, environment or poultry product were analyzed as different studies. Ten studies reported the prevalence in a single poultry bird, poultry environment or poultry product while three studies reported the prevalence in more than one [6,7,8] (Table 1).
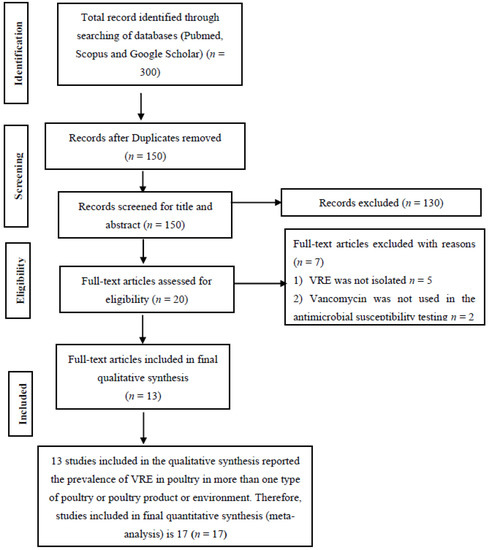
Figure 1.
PRISMA flow diagram for the selection of eligible articles included in the study.

Table 1.
Characteristics of included studies reporting the prevalence of vancomycin-resistant enterococcus in poultry in Malaysia.
2.2. The Pooled Prevalence of VRE in Poultry in Malaysia
The pooled prevalence of VRE in poultry in Malaysia was estimated at 24.0% (95% CI; 16.7–33.1%; I2 = 98.14%; p < 0.001) (Figure 2). Random-effects meta-analyses were carried out using the total sample size and number of positives (effect size, standard error of effect size) to estimate the prevalence of VRE in poultry in Malaysia. Between-study variability was high (t2 = 0.788; heterogeneity I2 = 98.14% with heterogeneity chi-square (Q) = 858.379, degrees of freedom (df) = 16, and p < 0.001). No individual study affected the heterogeneity and pooled prevalence of VRE in poultry in Malaysia as seen in the leave-one-out forest plot that was generated in the sensitivity analysis (Figure 3). More so, publication bias was observed as shown in the asymmetrical funnel plot (Figure 4). In addition to the funnel plots, the Trim-and-Fill method was then applied to include the “missing” studies from the analysis. The asymmetric studies were trimmed to locate the unbiased effect and fills the plot by re-inserting the trimmed studies as well as their imputed counterparts. Accordingly, four studies were missed and fell at the right side of the pooled estimate (Figure 5). In the Trim-and-Fill method, the adjusted estimate of VRE in poultry in Malaysia was 33.12% (95% CI; 24.3–43.3%). The Egger’s regression t = 1.777 (intercept = −4.0972; 95% CI; −0.75–2.57; p = 0.096) and Begg’s rank test (p = 0.30310) did not suggest significant publication bias.
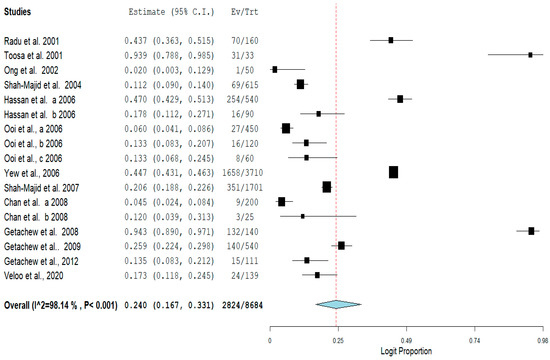
Figure 2.
Forest plot showing the pooled prevalence of VRE in poultry in Malaysia.
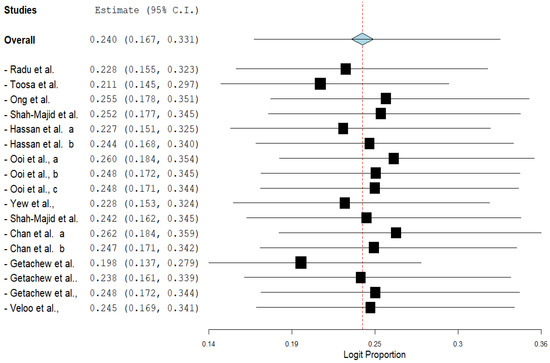
Figure 3.
Leave-one-out forest plot of VRE in poultry in Malaysia.
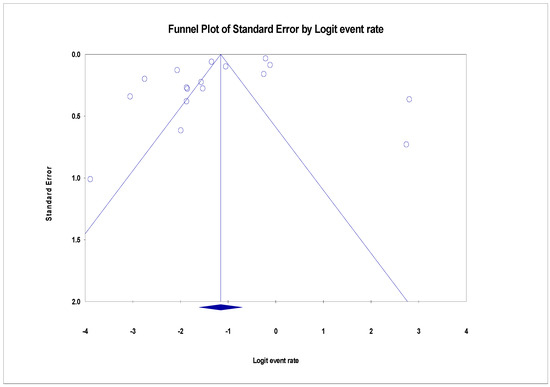
Figure 4.
Funnel plot showing publication bias in studies reporting the prevalence of VRE in in poultry in Malaysia. Studies on the right side are fewer than those on the left and thus asymmetrical. The funnel plot is used for the visualization of bias.
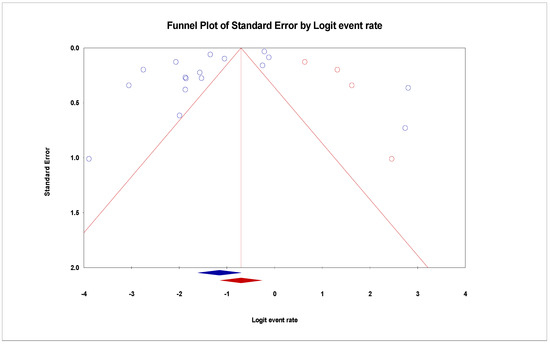
Figure 5.
Funnel plot showing 4 added studies (in Red) in the Trim-and-Fill method reporting the prevalence of VRE in in poultry in Malaysia. This method simply looks for missing studies that will eventually eliminate bias. In this case, 4 studies will have to be added to the right side for the plot to be symmetrical.
2.3. Subgroup Meta-Analysis
To identify the possible sources of heterogeneity among studies, as substantial heterogeneity was observed, subgroup analysis was carried out using the year of study of the included studies, the regions where the studies were reported, the sources of VRE isolates and the method used in detecting VRE.
The result of subgroup meta-analysis by study year revealed overall large variability in studies reporting the prevalence of VRE (the Higgins I2 statistic = 98.14% with heterogeneity chi-square (Q) = 858.379, degrees of freedom = 16, and p < 0.001). However, most studies (n = 6) were reported in 2006 with only a single study reported as recently as 2018 and published in 2020 (Table 2). Studies carried out in 2005 (n = 2) and 2004 (n = 2) had a moderate (I2 = 56.15%) and highest (I2 = 99.49%) heterogeneity respectively (Table 2). The forest plot for subgroup meta-analysis by study year is also shown in Figure 6.

Table 2.
Subgroup analysis for comparison of VRE in poultry in Malaysia across study year.
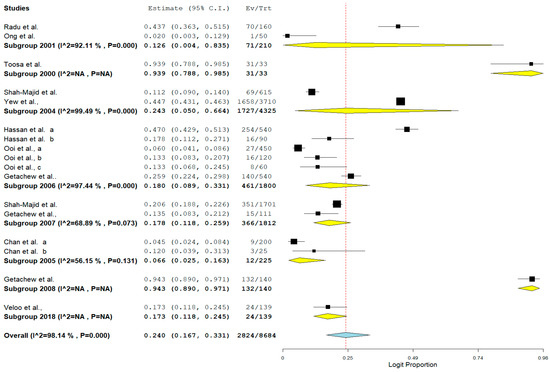
Figure 6.
Forest plot showing the subgroup meta-analysis by study year of VRE in poultry in Malaysia.
The result of subgroup meta-analysis by study region showed that majority of the studies (n = 14) that reported the prevalence of VRE in poultry in Malaysia were from the Central region of Peninsular Malaysia with a prevalence of 29% and a high heterogeneity (I2 = 98.36%). The East coast region of the Malaysian Peninsular only had 2 studies with a prevalence of 6.6% and a moderate heterogeneity (I2 = 56.15%) (Table 3). In addition, the forest plot of subgroup meta-analysis by study region is shown in Figure 7.

Table 3.
Subgroup analysis for comparison of VRE in poultry in Malaysia across study region.
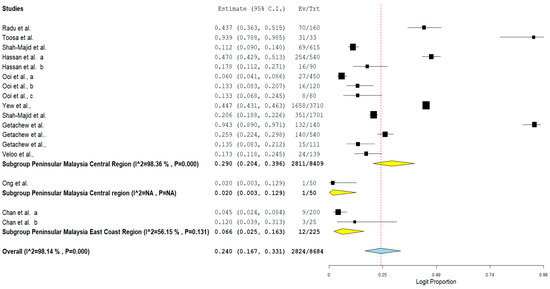
Figure 7.
Forest plot showing the subgroup meta-analysis by study region of VRE in poultry in Malaysia.
Furthermore, the result of subgroup meta-analysis according to isolate sources revealed that most of the studies (n = 8) had their VRE isolated from chicken with a prevalence of 29.2% and a high heterogeneity (I2 = 98.61%) (Table 4). This was followed by 5 studies reporting the isolation of VRE from poultry environment with a prevalence of 12.5% and also a high heterogeneity (I2 = 81.07%) (Table 4). The forest plot is also shown in Figure 8.

Table 4.
Subgroup analysis for comparison of VRE in poultry in Malaysia across isolate sources.
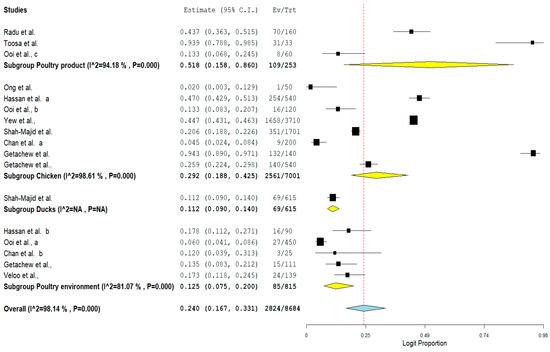
Figure 8.
Forest plot showing the subgroup meta-analysis by Isolate sources of VRE in poultry in Malaysia.
Lastly, the result of subgroup meta-analysis according to the detection method showed that 5 studies each utilized disc diffusion and agar dilution with a prevalence of 45.6% and 23.1%, respectively (Table 5). Only one study utilized polymerase chain reaction (PCR) as a method of detection with a prevalence of 43.7% and CI of 36.3–51.5%. (Table 5). The forest plot is shown in Figure 9.

Table 5.
Subgroup analysis for comparison of VRE in poultry in Malaysia across detection methods.
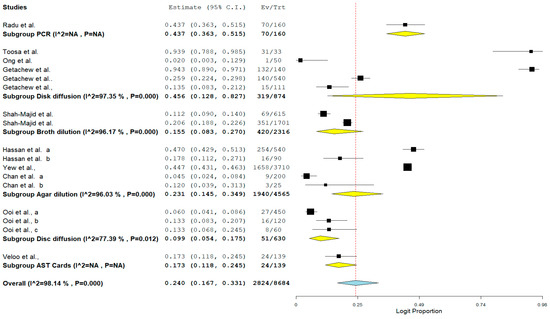
Figure 9.
Forest plot showing the subgroup meta-analysis by detection method of VRE in poultry in Malaysia.
2.4. Meta-Regression
A separate meta-analysis was performed for each variable included and these are: the study year; the study region; the isolate source; and the detection method. A multivariate meta-regression analysis was utilized when the p-values of the variables in a single meta-regression is <0.25. In the final meta-analysis, all the variables listed were included. In the multivariate meta-regression, no analysis was recorded for studies carried out in 2007, 2008 and 2018. Most of the variables analyzed using multivariate meta-regression contributed to the heterogeneity observed in this study with a p-value of <0.05. Exceptions to these were studies whose isolates were from poultry products (p = 0.0873) and chicken (p = 0.520), study that utilized the use of AST cards as its detection methods (p = 0.427) and a study carried out in the year 2000 (p = 0.994) (Table 6).

Table 6.
Final multivariable meta-regression model of VRE in poultry in Malaysia.
2.5. Species Distribution of Enterococcus in Poultry in Malaysia
Nine species of Enterococcus were isolated in 11 studies reporting the prevalence of VRE in poultry in Malaysia, while 2 studies [13,18] did not report the species of Enterococcus isolated. Enterococcus faecalis was the most isolated (n = 563) and this was followed by E. faecium with 201 isolates (Table 7).

Table 7.
Enterococcus species distribution in poultry in Malaysia.
3. Discussion
This is the first study to use meta-analysis and a systematic review to determine the prevalence of VRE in poultry in Malaysia, to the best of our knowledge. The pooled prevalence in this study is based on a thorough analysis of data from scientific publications on the prevalence of VRE in poultry in Malaysia published between 2001 and 2020. A meta-analysis was performed on 17 studies. The literature reviewed was heterogeneous, as expected, because the review included VRE reports from various regions, different study years, a variety of isolate sources and different methods for VRE detection. As a result, a random effect size model was used. This study’s high heterogeneity could be attributed to small-study effects and publication bias, because smaller studies sometimes show unusual, and often larger, treatment effects when compared to larger ones.
A small study with a larger-than-average impact is more likely to meet the statistical significance criterion, which may lead to an overestimation of true therapeutic effects. The assessment of publication bias is critical in meta-analysis. This is due to the fact that not all research findings are published, particularly those that are deemed unfavorable to a developed protocol or product, or those that would elicit only a minor amount of interest. Thus, studies that report relatively significant treatment effects are more likely to be submitted and/or approved for publication than studies that report more modest treatment effects. Our meta-analysis revealed a high level of variability, implying that the observed variability was compensated for by factors other than chance. The majority of the variables examined in these studies resulted in the observed heterogeneity. Similarly, studies with isolates from poultry products and chicken, studies with AST cards as detection methods and a study conducted in the year 2000 were not indicators of study heterogeneity.
There were no previous studies on meta-analysis to compare the prevalence of VRE in poultry in Malaysia, as this is the first study to analyze the prevalence of VRE in poultry in Malaysia. However, Wada et al. [1] reported a VRE pooled prevalence of 25% in Malaysia. In this current review, studies reporting the highest prevalence of VRE in poultry in Malaysia were mostly carried out in the Central region of the country. Most universities and research facilities such as the Department of Veterinary Services located in Putrajaya and National Pharmaceuticals Regulatory Agency (NPRA) which specializes in the surveillance of pathogens and regulations of drugs are located in the Central region of the country and this could be the reason why most of the studies were reported from that region. The density of poultry production in these regions could also be a factor. It is important that other regions are actively involved in the surveillance of Enterococcus and other pathogens, as this will help project the true estimate of the prevalence of VRE in poultry in Malaysia. Further, more up-to-date research is required as the most recent study reporting the prevalence of VRE in poultry was carried out in 2018 and published in 2020. It is either because the occurrence of resistant Enterococcus has reduced as a result of the ban on avoparcin or there is simply not enough surveillance going on to ascertain an actual estimate. Avoparcin and vancomycin were banned in Malaysia since 2013 by the National Pharmaceuticals Regulatory Agency (NPRA) and the Department of Veterinary Services (DVS) [4].
It is not surprising that most of the studies reported the isolation of VRE from chickens. Malaysians were expected to consume 48.7 kg of poultry meat per person in 2021, according to projections. This places Malaysia among the world’s top consumers of poultry meat [4]. In addition, the disc diffusion and agar dilution were the two most utilized VRE detection methods in poultry in Malaysia. Eight studies utilized the disc diffusion method, which has been reported to be straightforward and functional, with a well-standardized design, and it has the ability to provide categorical data that are simply understood by all practitioners, as well as the ability to choose from a variety of discs to test [19]. The lack of mechanization or automation of the disc test is one of its drawbacks. Only one study utilized PCR in the detection of VRE. For the detection and characterization of distinct VRE species, PCR is a quicker and more sensitive approach [20]. Finally, E. faecalis was the most isolated species of VRE in poultry in Malaysia from our analysis. The most frequent species capable of producing illness and creating an antibiotic resistance concern are E. faecalis and E. faecium, with E. faecalis accounting for the bulk of infections [21] E. faecalis is now recognized as a severe source of both hospital- and community-acquired urinary tract infections (UTIs), which can result in serious, life-threatening consequences such as bacteremia [22].
The use of antibiotics in animal husbandry varies by location and country. Antibiotics are sold in significantly larger quantities in low- and middle-income nations than they are in high-income countries [23]. As a region of fast-developing and integrated economies, Southeast Asia (SEA) is considered a hotspot location for antimicrobial resistance (AMR) [24]. Low- and middle-income nations utilized more antibiotics classified as medically important when compared to high-income countries [23]. The presence of veterinary drug traces in food samples confirmed this, indicating that violation rates in underdeveloped nations were higher than in developed nations [25].
SEA countries have had fast growth in the aquaculture and poultry production sectors in recent decades, accounting for a relatively large proportion of the worldwide veterinary antibiotic market [24]. Antibiotic management and therapy has become one of the most effective ways for these countries to avoid uncontrolled epidemic infections that could threaten their economies [24].
4. Materials and Methods
4.1. Study Design and Protocol
For this study, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocol (PRISMA-P 2015) guidelines [26] was used as the checklist (Supplementary File S1).
4.2. Literature Review
Abstracts from PROSPERO database and database of abstracts of reviews of effects (DARE) (http://www.library.UCSF.edu (accessed on 5 November 2021) were searched to ensure that no other meta-analysis on the prevalence of VRE in poultry in Malaysia exists or is ongoing. This was then followed by searching PubMed, Scopus and Google Scholar for published studies about the prevalence of VRE in poultry in Malaysia. These databases were searched using the search strategy; (“vancomycin resistant enterococci”[MeSH Terms] OR (“vancomycin resistant”[All Fields] AND “enterococci”[All Fields]) OR “vancomycin resistant enterococci”[All Fields] OR (“vancomycin”[All Fields] AND “resistant”[All Fields] AND “enterococcus”[All Fields]) OR “vancomycin resistant enterococcus”[All Fields] OR “VRE”[All Fields] OR (“vancomycin resistant enterococci”[MeSH Terms] OR (“vancomycin resistant”[All Fields] AND “enterococci”[All Fields]) OR “vancomycin resistant enterococci”[All Fields] OR (“vancomycin”[All Fields] AND “resistant”[All Fields] AND “enterococcus”[All Fields]) OR “vancomycin resistant enterococcus”[All Fields])) AND (“poultry”[MeSH Terms] OR “poultry”[All Fields] OR “poultries”[All Fields] OR “poultry s”[All Fields]) AND (“malaysia”[MeSH Terms] OR “malaysia”[All Fields] OR “malaysia”[All Fields]). In addition, references and titles from included articles were utilized as a supplementary search tool. Two authors carried out the search to minimize bias.
4.3. Inclusion and Exclusion Criteria for Studies
All cross-sectional or cohort studies that reported the prevalence of VRE isolates or numbers of VRE and total enterococci isolates in poultry, poultry environment and poultry products in Malaysia were included. In addition, studies published or reported in English in which the standard method (method approved for use according to the Clinical and Laboratory Standards Institute (CLSI) and other guidelines) was used to detect VRE were included. Studies with insufficient information, studies on antimicrobial susceptibility tests other than vancomycin, studies not reporting enterococcal isolates separately (no population denominator), reviews, comments and duplications, case report studies and studies that did not report the prevalence of VRE in poultry in Malaysia were excluded.
4.4. Data Extraction
Identification of studies was conducted based on our exclusion criteria and studies to be included were analyzed according to their title, abstract and full text. The first author’s name, publication year, study year, isolate sources, study region, number of cases involved in the studies, detection method, sample size, Enterococcus species isolated and the prevalence of VRE in poultry were extracted from the manuscripts. Two independent reviewers extracted all data from the included articles, and the results were reviewed by a third reviewer. Discrepancies between the reviewers were resolved by a consensus.
4.5. Study Quality Assessment
The Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence data [27] (Supplementary File S2) was used to evaluate the quality of the included studies. This appraisal checklist contains 9 items that assess: (1) appropriate sampling frame; (2) proper sampling technique; (3) adequate sample size; (4) study subject and setting description; (5) sufficient data analysis; (6) use of valid methods for the identified conditions; (7) valid measurement for all participants; (8) using appropriate statistical analysis; and (9) adequate response rate. Each item is graded as yes, no, unclear or not applicable. A score of 1 was allotted for the ‘yes’ response, while 0 scores were provided for ‘no’ and ‘unclear’ responses. Finally, the mean score was calculated for each article. Then, studies with scores below and above the mean were characterized as poor and good quality, respectively [24]. Studies were included in the analysis if consensus was reached among two reviewers. The quality of the 13 included studies is given in (Supplementary File S3).
4.6. Data Analysis
Prevalence of VRE in poultry in Malaysia was calculated, and subgroup analyses were performed according to the study year, study region, the isolate sources and detection method. Where the prevalence was not reported by a study, they were back-calculated. DerSimonian and Laird method of meta-analysis [28,29] was used to determine the pooled prevalence. The random-effect model was used because heterogeneity was anticipated given that the studies were carried out in diverse locations and settings.
4.7. Bias and Heterogeneity Analysis
Within-study biases were evaluated by the study region, study year, isolate sources, and detection method. Small study effects or bias were examined by funnel plots and trim-and-fill plots. The heterogeneities of study-level estimates were assessed by Cochran’s Q test. Non-significant heterogeneity was accepted if the ratio of Q and the degrees of freedom (Q/df) was less than one. The percentage of the variation in prevalence estimates attributable to heterogeneity was measured by the inverse variance index (I2), and I2 values of 25%, 50% and 75% were considered to be of low, moderate and high heterogeneity, respectively [29]. The sources of heterogeneity were analyzed using the sensitivity analysis (leave-one-out meta-analysis), subgroup analysis and meta-regression. Meta-analysis was performed using OpenMeta Analyst software [30] and Comprehensive meta-analysis version 2 [31].
5. Conclusions
There is abundant proof that drug-resistant bacteria exist in poultry and can be transmitted to humans. A systematic review and meta-analysis of studies reporting the prevalence of VRE in poultry in Malaysia was conducted and a pooled prevalence of 24.0% was obtained. However, given the observed relatively high heterogeneity, it is hard to conclude that this estimate reflects the real point estimate. Nonetheless, we believe the estimate gives a good idea of the prevalence of VRE in poultry in Malaysia. With the emergence of drug-resistant bacterial strains, including vancomycin, there is a need to investigate newer antimicrobials in veterinary medicine. Based on our study, regular monitoring of VRE in poultry would aid policymakers in developing effective control measures and design AMR surveillance capacity building in Malaysia. Further, livestock farmers should be educated on antibiotics resistance and trained on responsible utilization of antibiotics. Awareness on antimicrobial resistance should be raise by all stakeholders and lastly, reduction of contamination by encouraging proper hygiene in animal husbandry, food production and processing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11020171/s1, File S1: PRISMA-P 2015 Checklist; File S2: The Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence data. File S3: The quality of the 13 included studies.
Author Contributions
Y.W. conceived and designed the study; Y.W., A.A.I. and C.Y.Y. selected and assessed the quality of studies, extracted and analyzed data; Y.W., H.A.A., A.R.Z. and A.H. interpreted results, drafted the manuscript; Y.W., M.W., A.R.Z. selected and assessed the quality of studies, extracted data and interpreted results; A.R.Z., C.Y.Y., R.H.S. and A.H. interpreted the results, reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This meta-analysis and systematic review were supported by the RUI Universiti Sains Malaysia (USM) grant with number 1001.PPSP.8012259. YW and AAI are supported by the USM Fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are included in the manuscript.
Acknowledgments
We acknowledge the USM Fellowship Scheme.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wada, Y.; Binti Harun, A.; Chan Yean, Y.; Mohamad Nasir, N.S.; Abdul Rahman, Z. Prevalence of vancomycin-resistant Enterococcus in Malaysia: A meta-analysis and systematic review. Int. J. Infect. Dis. 2020, 101, 20. [Google Scholar] [CrossRef]
- Wada, Y.; Harun, A.B.; Yean, C.Y.; Zaidah, A.R. Vancomycin-Resistant Enterococci (VRE) in Nigeria: The First Systematic Review and Meta-Analysis. Antibiotics 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Harun, A.B.; Yean, C.Y.; Zaidah, A.R. Vancomycin-resistant enterococcus: Issues in human health, animal health, resistant mechanisms and the malaysian paradox. Adv. Anim. Vet. Sci. 2019, 7, 1021–1034. [Google Scholar] [CrossRef]
- Getachew, Y.; Hassan, L.; Zakaria, Z.; Abdul Aziz, S. Genetic Variability of Vancomycin-Resistant Enterococcus faecium and Enterococcus faecalis Isolates from Humans, Chickens, and Pigs in Malaysia. Appl. Environ. Microbiol. 2013, 79, 4528–4533. [Google Scholar] [CrossRef]
- Wada, Y.; Irekeola, A.A.; Engku Nur Syafirah, E.A.R.; Yusof, W.; Huey, L.L.; Muhammad, S.L.; Harun, A.; Yean, C.Y.; Zaidah, A.R. Prevalence of vancomycin-resistant Enterococcus (VRE) in companion animals: The first meta-analysis and systematic review. Antibiotics 2021, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Hassan, L.; Wan Mohd Zain, W.N.A.; Saleha, A.A.; Ramanoon, S.Z. Epidemiological features of vancomycin-resistant enterococci in broiler farms in Malaysia. In Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, Cairns, Australia, 6–11 August 2006. [Google Scholar]
- Ooi, W.L.; Haryiani, Y.; Lesley, M.B.; Tunung, R.; Jurin, W.G.; Wong, C.M.V.L.; Cheah, Y.K.; Son, R. Vancomycin Resistant Enterococci (VRE) in Poultry, Vegetable, Environmental and Clinical Sources. Malays. Appl. Biol. 2006, 35, 49–56. [Google Scholar]
- Chan, Y.Y.; Abd Nasir, M.H.B.; Yahaya, M.A.B.; Salleh, N.M.A.B.; Md Dan, A.D.B.; Musa, A.M.B.; Ravichandran, M. Low prevalence of vancomycin- and bifunctional aminoglycoside-resistant enterococci isolated from poultry farms in Malaysia. Int. J. Food Microbiol. 2008, 122, 221–226. [Google Scholar] [CrossRef]
- Radu, S.; Toosa, H.; Rahim, R.A.; Reezal, A.; Ahmad, M.; Rusul, G.; Nishibuchi, M. Occurrence of the vanA and vanC2/C3 genes in Enterococcus species isolated from poultry sources in Malaysia. Diagn. Microbiol. Infect. Dis. 2001, 39, 145–153. [Google Scholar] [CrossRef]
- Toosa, H.; Radu, S.; Rusul, G.; Reezal Abdul Latif, A.; Abdul Rahim, R.; Ahmad, N.; Wai Ling, O. Detection of Vancomycin-Resistant Enterococcus Spp. (Vre) from Poultry. Malays. J. Med. Sci. 2001, 8, 53–58. [Google Scholar]
- Ong, C.H.S.; Asaad, M.; Lim, K.C.; Ngeow, Y.F. Infrequent occurrence of vancomycin-resistant enterococci in poultry from Malaysian wet markets. Malays. J. Pathol. 2002, 24, 91–94. [Google Scholar]
- Shah-Majid, M.; Azlina, A.M.M.; Ana Maria, A.R.; Zaharah, B.; Norhaliza, A.H. Occurrence of vancomycin-resistant enterococci in ducks in Malaysia. Vet. Rec. 2004, 155, 680–681. [Google Scholar] [CrossRef] [PubMed]
- Yew, T.D.; Kew, W.S.; Jaganathan, S.; Singh, J.P. Screening and characterization of vancomycin-resistant enterococci (vre) isolated from poultry in Malaysia. In 18th Veterinary Association Malaysia Scientific Congress; Seri Pacific Hotel: Kuala Lumpur, Malaysia, 2006. [Google Scholar]
- Shah-Majid, M.; Maria, A.R.A.; Shahidayani, S.; Salwani, A.M.D.; Khairani, S. Occurrence of vancomycin-resistant enterococci in chickens in Malaysia. Vet. Rec. 2007, 160, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Getachew, Y.M.; Hassan, L.; Zakaria, Z. Vancomycin-Resistant Enterococci and Vancomycin-Resistance Genes in Pigs and Poultry Isolates of Malaysia. Int. J. Infect. Dis. 2008, 12, e138. [Google Scholar] [CrossRef][Green Version]
- Getachew, Y.M.; Hassan, L.; Zakaria, Z.; Saleha, A.A.; Kamaruddin, M.I.; Che Zalina, M.Z. Characterization of vancomycin-resistant Enterococcus isolates from broilers in Selangor, Malaysia. Trop. Biomed. 2009, 26, 280–288. [Google Scholar]
- Getachew, Y.; Hassan, L.; Zakaria, Z.; Zaid, C.Z.M.; Yardi, A.; Shukor, R.A.; Marawin, L.T.; Embong, F.; Aziz, S.A. Characterization and risk factors of vancomycin-resistant Enterococci (VRE) among animal-affiliated workers in Malaysia. J. Appl. Microbiol. 2012, 113, 1184–1195. [Google Scholar] [CrossRef]
- Veloo, Y.; Thahir, S.S.A.; Shaharudin, R.; Rajendiran, S.; Hock, L.K.; Muthu, V.; Ahmad, N. Prevalence of Antibiotic-Resistant Bacteria in the Environment of Poultry Farms. 2020. Available online: https://assets.researchsquare.com/files/rs-42662/v1/3fd3aa33-2023-474a-abb8-3b9afad54729.pdf?c=1631846457 (accessed on 8 December 2021).
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Farman, M.; Yasir, M.; Al-Hindi, R.R.; Farraj, S.A.; Jiman-Fatani, A.A.; Alawi, M.; Azhar, E.I. Genomic analysis of multidrug-resistant clinical Enterococcus faecalis isolates for antimicrobial resistance genes and virulence factors from the western region of Saudi Arabia. Antimicrob. Resist. Infect. Control 2019, 8, 55. [Google Scholar] [CrossRef]
- Hashem, Y.A.; Abdelrahman, K.A.; Aziz, R.K. Phenotype–Genotype Correlations and Distribution of Key Virulence Factors in Enterococcus faecalis Isolated from Patients with Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 1713–1723. [Google Scholar] [CrossRef]
- Daniel, D.S.; Lee, S.M.; Gan, H.M.; Dykes, G.A.; Rahman, S. Genetic diversity of Enterococcus faecalis isolated from environmental, animal and clinical sources in Malaysia. J. Infect. Public Health 2017, 10, 617–623. [Google Scholar] [CrossRef]
- Gumphol, W.; Vanaporn, W.; Soawapak, H.; Nicholas, P.J.D.; Direk, L. Antibiotic use in poultry: A survey of eight farms in Thailand. Bull. World Health Organ. 2018, 96, 94–100. [Google Scholar]
- Yam, E.; Hsu, L.; Yap, E.; Yeo, T.; Lee, V.; Schlundt, J.; Lwin, M.O.; Limmathurotsakul, D.; Jit, M.; Dedon, P.; et al. Antimicrobial Resistance in the Asia Pacific region: A meeting report. Antimicrob. Resist. Infect. Control 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Marni, S.; Marzura, M.R.; Eddy, A.A.; Suliana, A.K. Veterinary drug residues in chicken, pork and beef in Peninsular Malaysia in the period 2010–2016. Malays. J. Vet. Res. 2017, 8, 71–77. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Joanna Briggs Institute. Checklist for Prevalence Studies. 2017. Available online: https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Prevalence_Studies2017_0.pdf (accessed on 8 December 2021).
- George, B.J.; Aban, I.B. An application of meta-analysis based on DerSimonian and Laird method. J. Nucl. Cardiol. 2016, 23, 690–692. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Comprehensive Meta-Analysis Software (CMA). Available online: https://www.meta-analysis.com/ (accessed on 21 December 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).