Altered Gut Structure and Anti-Bacterial Defense in Adult Mice Treated with Antibiotics during Early Life
Abstract
:1. Introduction
2. Results
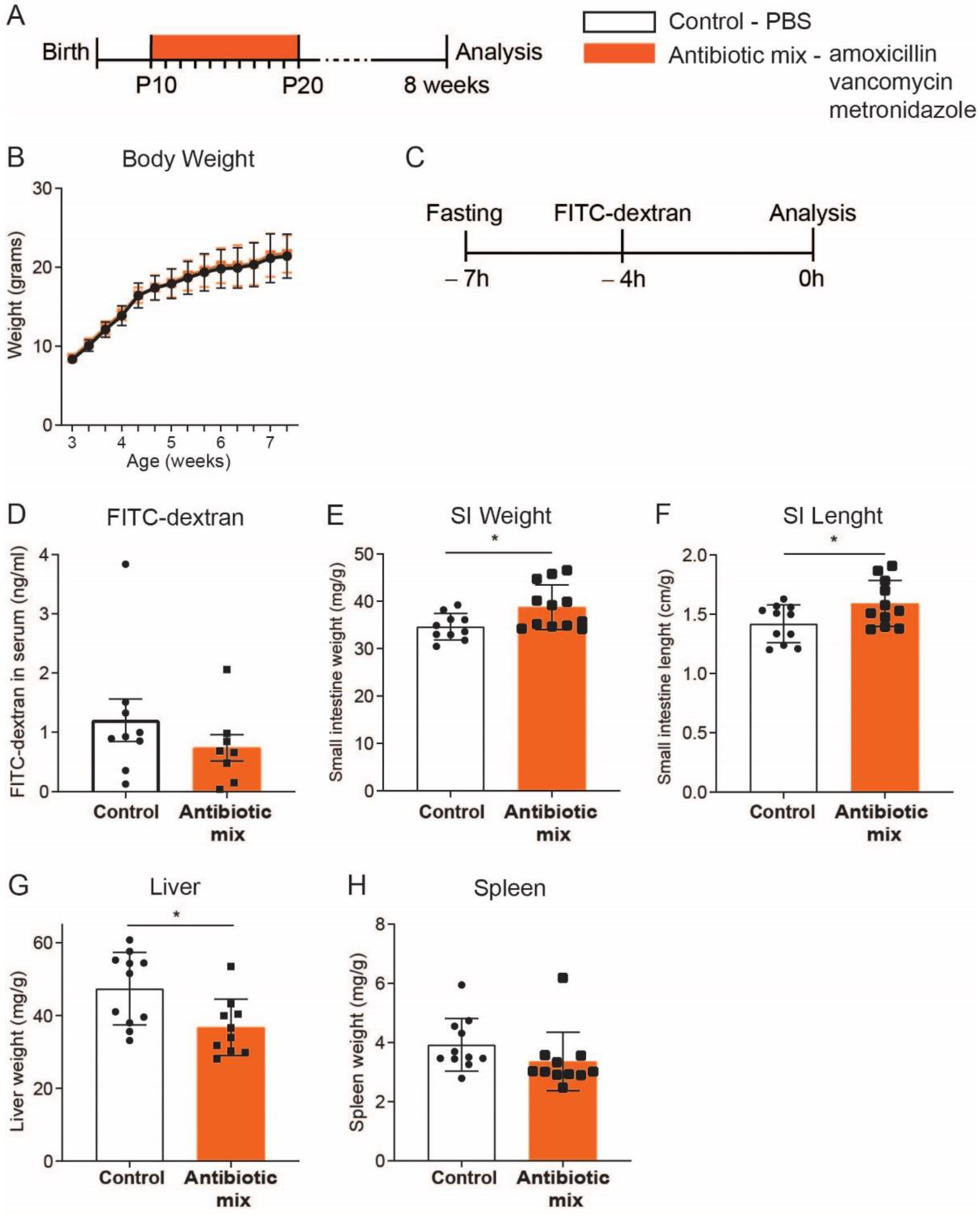
2.1. Adult Mice Treated with Early Life Antibiotics Show Altered Small Intestine Morphology
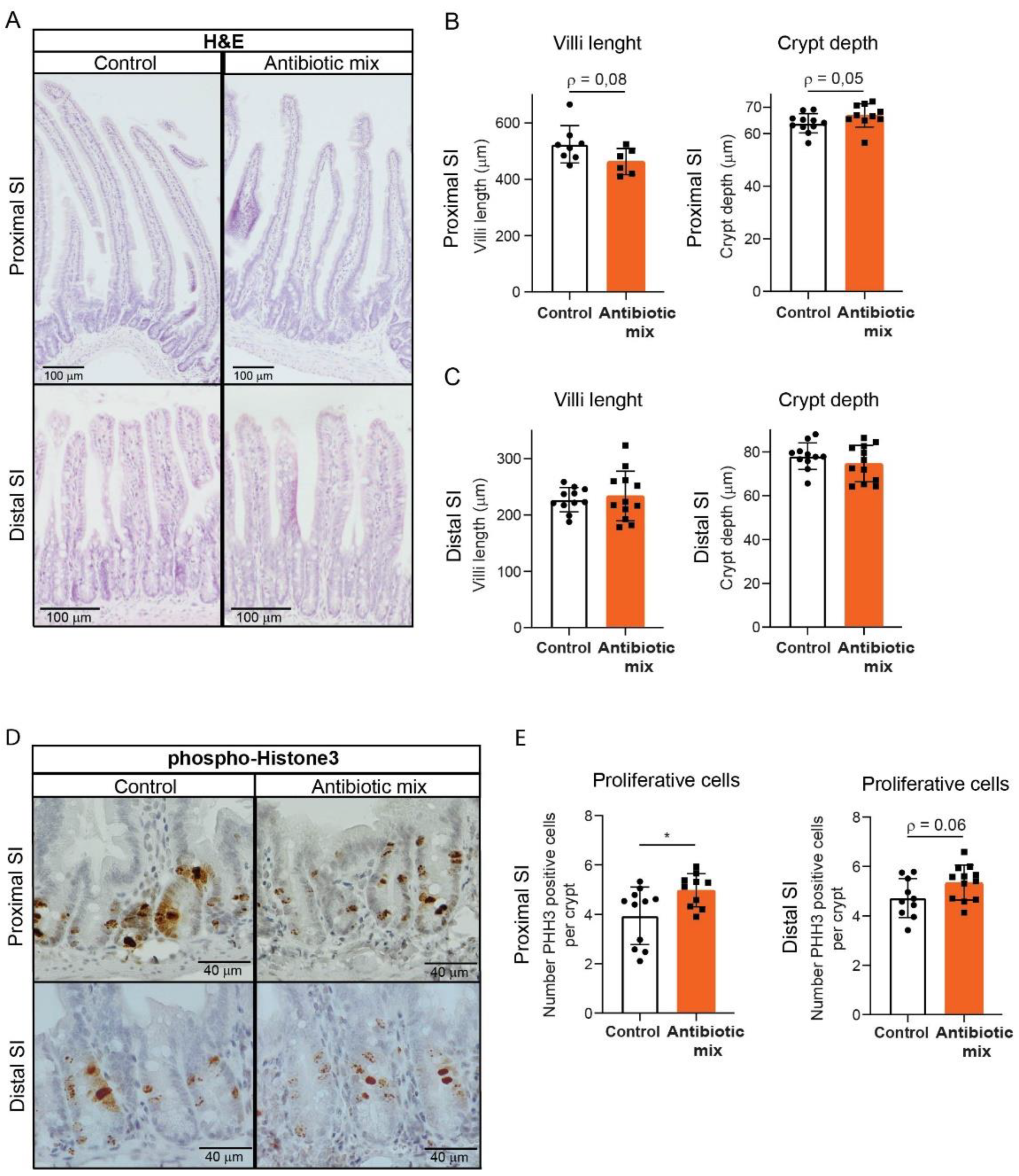
2.2. Early Life Antibiotics Induce Hyperproliferative Crypts in Adult Proximal Small Intestine
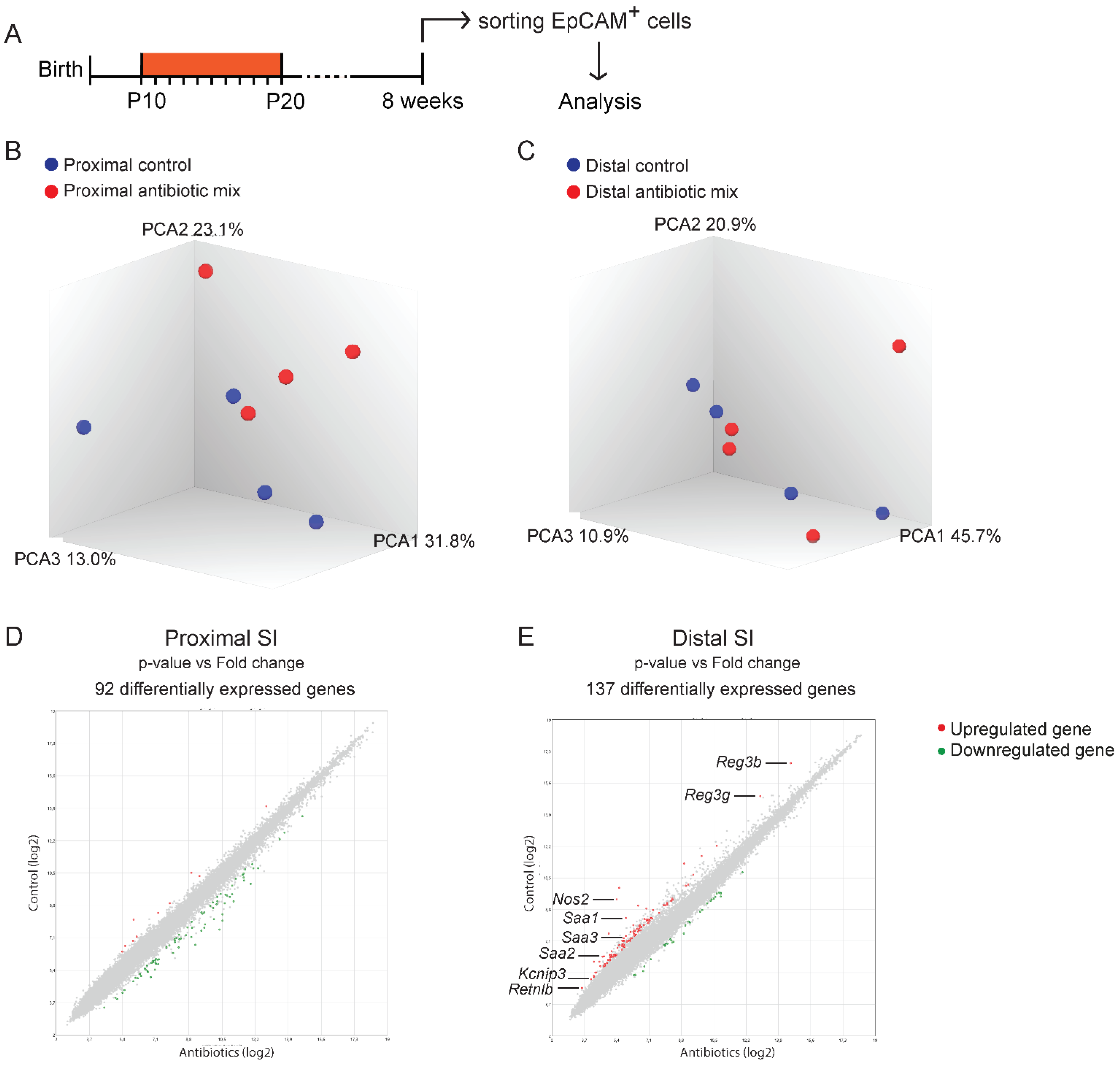
2.3. The Majority of the Direct Effects Induced by Antibiotics on the Neonatal Small Intestine Do Not Persist into Adulthood
2.4. Genome-Wide Gene Expression Analysis Reveals Modest Differences in Epithelial Cells of Adult Mice Treated with Antibiotics in Early Life
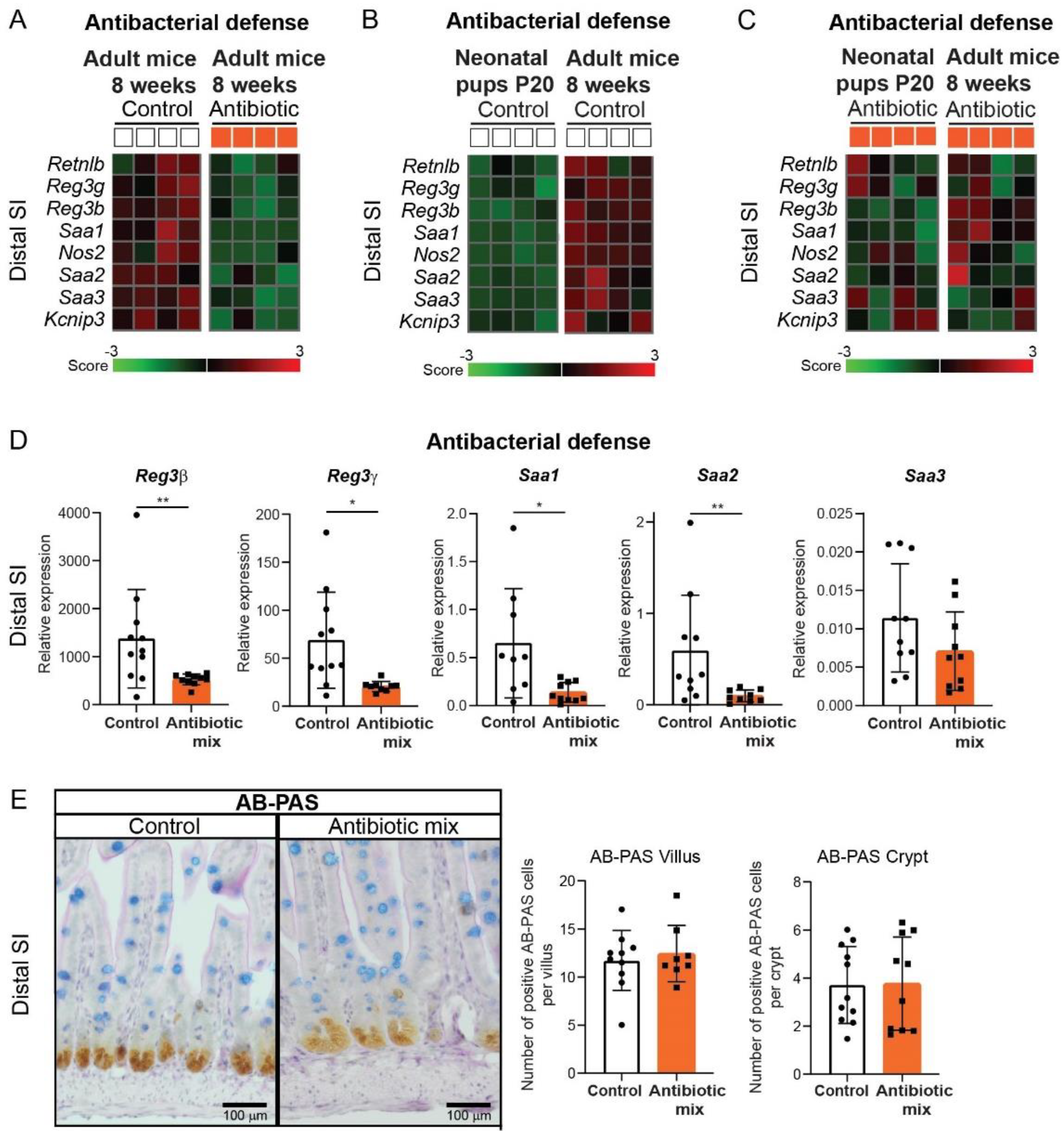
2.5. Intestinal Antibacterial Defense Is Reduced in Distal Small Intestine of Adult Mice That Received Antibiotics in Early Life
3. Discussions
4. Materials and Methods
4.1. In Vivo Studies
4.2. FITC-Dextran In Vivo Permeability Assay
4.3. Immunostaining
4.4. Epithelial Cells FACS-Sorting
4.5. RNA Isolation and qRT-PCR
- Rpl4: FW-CCTTCTCCTCTCCCCGTCA ; RV-GCATAGGGCTGTCTGTTGTTT
- Ppib: FW-GCCAACGATAAGAAGAAGGGA; RV-TCCAAAGAGTCCAAAGACGAC
- Sis: FW-TGCCTGCTGTGGAAGAAGTAA; RV-CAGCCACGCTCTTCACATTT
- Arg2: FW-TAGGGTAATCCCCTCCCTGC; RV-AGCAAGCCAGCTTCTCGAAT
- Lyz: FW-GGATGGCTACCGTGGTGTCAAGC; RV-TCCCATAGTCGGTGCTTCGGTC
- Reg3β: FW-TGGGAATGGAGTAACAAT; RV-GGCAACTTCACCTCACAT
- Reg3γ: FW-CCATCTTCACGTAGCAGC; RV-CAAGATGTCCTGAGGGC
- Gip: FW-AACTGTTGGCTAGGGGACAC; RV-TGATGAAAGTCCCCTCTGCG
- Gcg: FW-CTTCCCAGAAGAAGTCGCCA; RV-GTGACTGGCACGAGATGTTG
- Pyy: FW-ACGGTCGCAATGCTGCTAAT; RV-GCTGCGGGGACATCTCTTTTT
- Sst: FW-GACCTGCGACTAGACTGACC; RV-CCAGTTCCTGTTTCCCGGTG
- Sct: FW-GACCCCAAGACACTCAGACG; RV-TTTTCTGTGTCCTGCTCGCT
- Cck: FW-GAAGAGCGGCGTATGTCTGT; RV-CCAGAAGGAGCTTTGCGGA
- ChgA: FW-GTCTCCAGACACTCAGGGCT; RV-ATGACAAAAGGGGACACCAA
- Saa1: FW-GGTCTTCTGCTCCCTGCTC; RV-AGCAGCATCATAGTTCCCCC
- Saa2: FW-CAGCCTGGTCTTCTGCTCC; RV-CACATGTCTCCAGCCCCTTG
- Saa3: FW-AGTAGGCTCGCCACATGTCT; RV-TCCATTGCCATCATTCTTTG
4.6. Transcriptome Profiling
4.7. Software
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renz, H.; Skevaki, C. Early life microbial exposures and allergy risks: Opportunities for prevention. Nat. Rev. Immunol. 2021, 21, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hamalainen, A.M.; Harkonen, T.; Ryhanen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Egan, M.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. A good start in life is important-perinatal factors dictate early microbiota development and longer term maturation. FEMS Microbiol. Rev. 2020, 44, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F. Establishment of the early-life microbiome: A DOHaD perspective. J. Dev. Orig. Health Dis. 2020, 11, 201–210. [Google Scholar] [CrossRef]
- Sturkenboom, M.C.; Verhamme, K.M.; Nicolosi, A.; Murray, M.L.; Neubert, A.; Caudri, D.; Picelli, G.; Sen, E.F.; Giaquinto, C.; Cantarutti, L.; et al. Drug use in children: Cohort study in three European countries. BMJ 2008, 337, a2245. [Google Scholar] [CrossRef] [Green Version]
- Fink, G.; D’Acremont, V.; Leslie, H.H.; Cohen, J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: A cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect. Dis. 2020, 20, 179–187. [Google Scholar] [CrossRef]
- Flannery, D.D.; Ross, R.K.; Mukhopadhyay, S.; Tribble, A.C.; Puopolo, K.M.; Gerber, J.S. Temporal Trends and Center Variation in Early Antibiotic Use Among Premature Infants. JAMA Netw. Open 2018, 1, e180164. [Google Scholar] [CrossRef] [Green Version]
- Hsia, Y.; Sharland, M.; Jackson, C.; Wong, I.C.K.; Magrini, N.; Bielicki, J.A. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2019, 19, 67–75. [Google Scholar] [CrossRef]
- Ramasethu, J.; Kawakita, T. Antibiotic stewardship in perinatal and neonatal care. Semin. Fetal Neonatal. Med. 2017, 22, 278–283. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-Negative Early-Onset Neonatal Sepsis—At the Crossroad Between Efficient Sepsis Care and Antimicrobial Stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef] [Green Version]
- Schulfer, A.F.; Schluter, J.; Zhang, Y.; Brown, Q.; Pathmasiri, W.; McRitchie, S.; Sumner, S.; Li, H.; Xavier, J.B.; Blaser, M.J. The impact of early-life sub-therapeutic antibiotic treatment (STAT) on excessive weight is robust despite transfer of intestinal microbes. ISME J. 2019, 13, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobel, Y.R.; Cox, L.M.; Kirigin, F.F.; Bokulich, N.A.; Yamanishi, S.; Teitler, I.; Chung, J.; Sohn, J.; Barber, C.M.; Goldfarb, D.S.; et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015, 6, 7486. [Google Scholar] [CrossRef]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 16140. [Google Scholar] [CrossRef]
- Candon, S.; Perez-Arroyo, A.; Marquet, C.; Valette, F.; Foray, A.P.; Pelletier, B.; Milani, C.; Ventura, M.; Bach, J.F.; Chatenoud, L. Antibiotics in early life alter the gut microbiome and increase disease incidence in a spontaneous mouse model of autoimmune insulin-dependent diabetes. PLoS ONE 2015, 10, e0125448. [Google Scholar] [CrossRef]
- Ungaro, R.; Bernstein, C.N.; Gearry, R.; Hviid, A.; Kolho, K.L.; Kronman, M.P.; Shaw, S.; van Kruiningen, H.; Colombel, J.F.; Atreja, A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 1728–1738. [Google Scholar] [CrossRef]
- Schulfer, A.F.; Battaglia, T.; Alvarez, Y.; Bijnens, L.; Ruiz, V.E.; Ho, M.; Robinson, S.; Ward, T.; Cox, L.M.; Rogers, A.B.; et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat. Microbiol. 2018, 3, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.S.; Li, J.; Krautkramer, K.A.; Badri, M.; Battaglia, T.; Borbet, T.C.; Koh, H.; Ng, S.; Sibley, R.A.; Li, Y.; et al. Antibiotic-induced acceleration of type 1 diabetes alters maturation of innate intestinal immunity. eLife 2018, 7, e37816. [Google Scholar] [CrossRef]
- Ruiz, V.E.; Battaglia, T.; Kurtz, Z.D.; Bijnens, L.; Ou, A.; Engstrand, I.; Zheng, X.; Iizumi, T.; Mullins, B.J.; Muller, C.L.; et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat. Commun. 2017, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.M.; van Roest, M.; Vermeulen, J.L.M.; Meisner, S.; Smit, W.L.; Silva, J.; Koelink, P.J.; Koster, J.; Faller, W.J.; Wildenberg, M.E.; et al. Early Life Antibiotics Influence In Vivo and In Vitro Mouse Intestinal Epithelium Maturation and Functioning. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 943–981. [Google Scholar] [CrossRef] [PubMed]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Dejardin, F.; Sparwasser, T.; Berard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Bobe, A.M.; Miyoshi, S.; Huang, Y.; Hubert, N.; Delmont, T.O.; Eren, A.M.; Leone, V.; Chang, E.B. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep. 2017, 20, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheer, S.; Medina, T.S.; Murison, A.; Taves, M.D.; Antignano, F.; Chenery, A.; Soma, K.K.; Perona-Wright, G.; Lupien, M.; Arrowsmith, C.H.; et al. Early-life antibiotic treatment enhances the pathogenicity of CD4(+) T cells during intestinal inflammation. J. Leukoc. Biol. 2017, 101, 893–900. [Google Scholar] [CrossRef]
- Shaw-Smith, C.J.; Walters, J.R. Regional expression of intestinal genes for nutrient absorption. Gut 1997, 40, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderle, P.; Sengstag, T.; Mutch, D.M.; Rumbo, M.; Praz, V.; Mansourian, R.; Delorenzi, M.; Williamson, G.; Roberts, M.A. Changes in the transcriptional profile of transporters in the intestine along the anterior-posterior and crypt-villus axes. BMC Genom. 2005, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Udager, A.M.; Hu, C.; Qiao, X.T.; Richards, N.; Gumucio, D.L. Dynamic patterning at the pylorus: Formation of an epithelial intestine-stomach boundary in late fetal life. Dev. Dyn. 2009, 238, 3205–3217. [Google Scholar] [CrossRef] [Green Version]
- Kayisoglu, O.; Schlegel, N.; Bartfeld, S. Gastrointestinal epithelial innate immunity-regionalization and organoids as new model. J. Mol. Med. 2021, 99, 517–530. [Google Scholar] [CrossRef]
- Garcia, T.M.; Navis, M.; Wildenberg, M.E.; van Elburg, R.M.; Muncan, V. Recapitulating Suckling-to-Weaning Transition In Vitro using Fetal Intestinal Organoids. J. Vis. Exp. 2019, 153, e60470. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.; Nutten, S.; Donnicola, D.; Comelli, E.M.; Mansourian, R.; Cherbut, C.; Corthesy-Theulaz, I.; Garcia-Rodenas, C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol. Genom. 2005, 23, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, E.R.; Witta, J.; Zhong, J.; Arsenescu, R.; Arsenescu, V.; Wang, Y.; Ghoshal, S.; de Beer, M.C.; de Beer, F.C.; de Villiers, W.J. Intestinal epithelial serum amyloid A modulates bacterial growth in vitro and pro-inflammatory responses in mouse experimental colitis. BMC Gastroenterol. 2010, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger-van Paassen, N.; Loonen, L.M.; Witte-Bouma, J.; Korteland-van Male, A.M.; de Bruijn, A.C.; van der Sluis, M.; Lu, P.; van Goudoever, J.B.; Wells, J.M.; Dekker, J.; et al. Mucin Muc2 deficiency and weaning influences the expression of the innate defense genes Reg3beta, Reg3gamma and angiogenin-4. PLoS ONE 2012, 7, e38798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.E6. [Google Scholar] [CrossRef] [Green Version]
- Arevalo Sureda, E.; Westrom, B.; Pierzynowski, S.G.; Prykhodko, O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS ONE 2016, 11, e0164775. [Google Scholar] [CrossRef]
- Gookin, J.L.; Stauffer, S.H.; Stone, M.R. Induction of arginase II by intestinal epithelium promotes the uptake of L-arginine from the lumen of Cryptosporidium parvum-infected porcine ileum. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Talavera, M.M.; Nuthakki, S.; Cui, H.; Jin, Y.; Liu, Y.; Nelin, L.D. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Front Cell Dev. Biol. 2017, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Ozkul, C.; Ruiz, V.E.; Battaglia, T.; Xu, J.; Roubaud-Baudron, C.; Cadwell, K.; Perez-Perez, G.I.; Blaser, M.J. A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis. Genome Med. 2020, 12, 65. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Reimann, F.; Gribble, F.M. Models and Tools for Studying Enteroendocrine Cells. Endocrinology 2018, 159, 3874–3884. [Google Scholar] [CrossRef]
- Crooks, B.; Stamataki, N.S.; McLaughlin, J.T. Appetite, the enteroendocrine system, gastrointestinal disease and obesity. Proc. Nutr. Soc. 2021, 80, 50–58. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A. Enteroendocrine Regulation of Nutrient Absorption. J. Nutr. 2020, 150, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Posovszky, C.; Wabitsch, M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 2: Therapeutic potential of enteroendocrine cells in the treatment of obesity. Horm. Res. Paediatr. 2015, 83, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.H.; Allin, K.H.; Knop, F.K. Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: A review of the literature. Diabetes Obes. Metab. 2016, 18, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.H.; Knop, F.K.; Frost, M.; Hallas, J.; Pottegard, A. Use of Antibiotics and Risk of Type 2 Diabetes: A Population-Based Case-Control Study. J. Clin. Endocrinol. Metab. 2015, 100, 3633–3640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Soltesz, V.; Axelson, J.; Andersson, R. Cholecystokinin increases small intestinal motility and reduces enteric bacterial overgrowth and translocation in rats with surgically induced acute liver failure. Digestion 1996, 57, 67–72. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Lehotzky, R.E.; Partch, C.L.; Mukherjee, S.; Cash, H.L.; Goldman, W.E.; Gardner, K.H.; Hooper, L.V. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proc. Natl. Acad. Sci. USA 2010, 107, 7722–7727. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.X.; et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamphorst, K.; van Daele, E.; Vlieger, A.M.; Daams, J.G.; Knol, J.; van Elburg, R.M. Early life antibiotics and childhood gastrointestinal disorders: A systematic review. BMJ Paediatr. Open 2021, 5, e001028. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins Garcia, T.; van Roest, M.; Vermeulen, J.L.M.; Meisner, S.; Koster, J.; Wildenberg, M.E.; van Elburg, R.M.; Muncan, V.; Renes, I.B. Altered Gut Structure and Anti-Bacterial Defense in Adult Mice Treated with Antibiotics during Early Life. Antibiotics 2022, 11, 267. https://doi.org/10.3390/antibiotics11020267
Martins Garcia T, van Roest M, Vermeulen JLM, Meisner S, Koster J, Wildenberg ME, van Elburg RM, Muncan V, Renes IB. Altered Gut Structure and Anti-Bacterial Defense in Adult Mice Treated with Antibiotics during Early Life. Antibiotics. 2022; 11(2):267. https://doi.org/10.3390/antibiotics11020267
Chicago/Turabian StyleMartins Garcia, Tnia, Manon van Roest, Jacqueline L. M. Vermeulen, Sander Meisner, Jan Koster, Manon E. Wildenberg, Ruurd M. van Elburg, Vanesa Muncan, and Ingrid B. Renes. 2022. "Altered Gut Structure and Anti-Bacterial Defense in Adult Mice Treated with Antibiotics during Early Life" Antibiotics 11, no. 2: 267. https://doi.org/10.3390/antibiotics11020267






