Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial Susceptibility
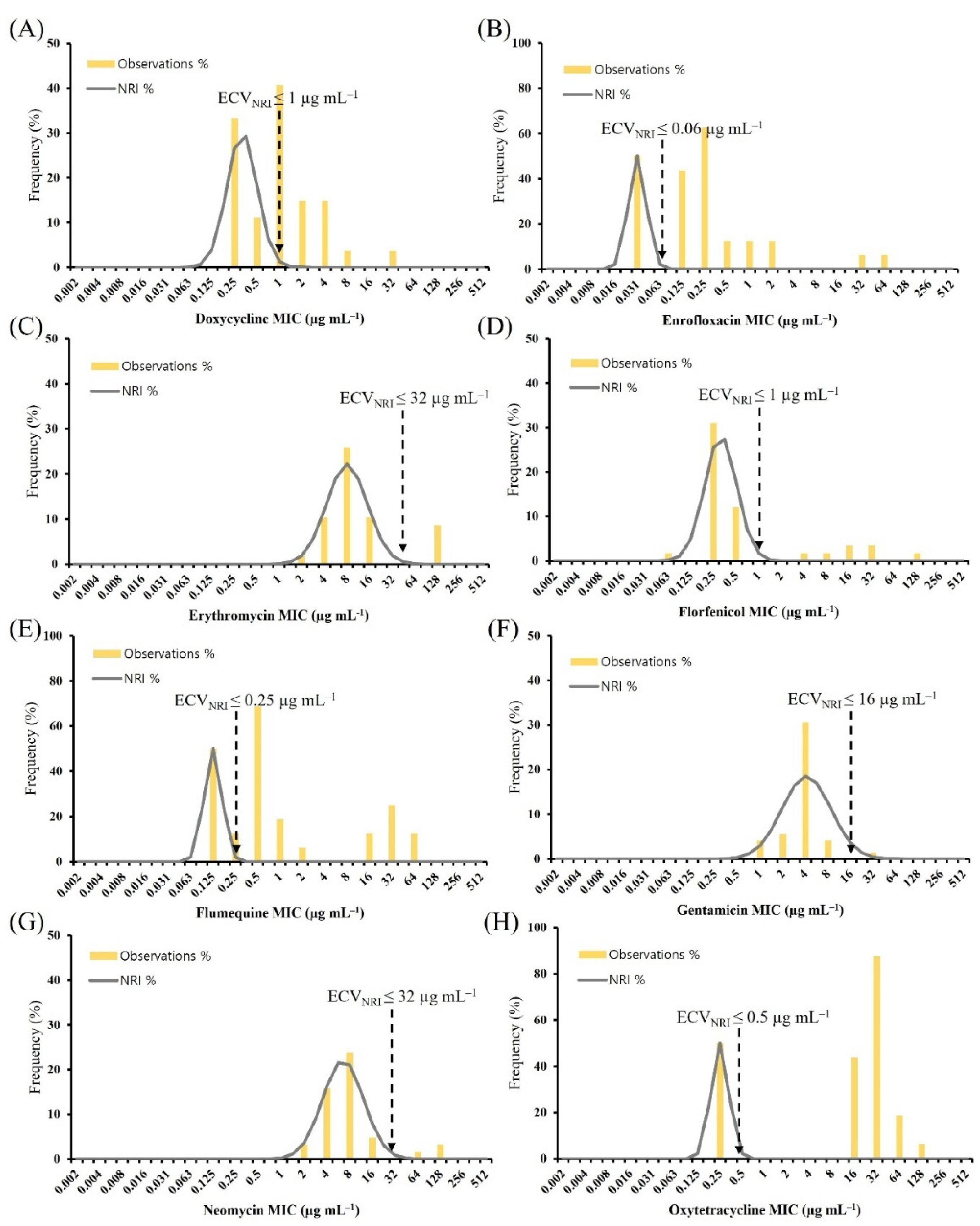
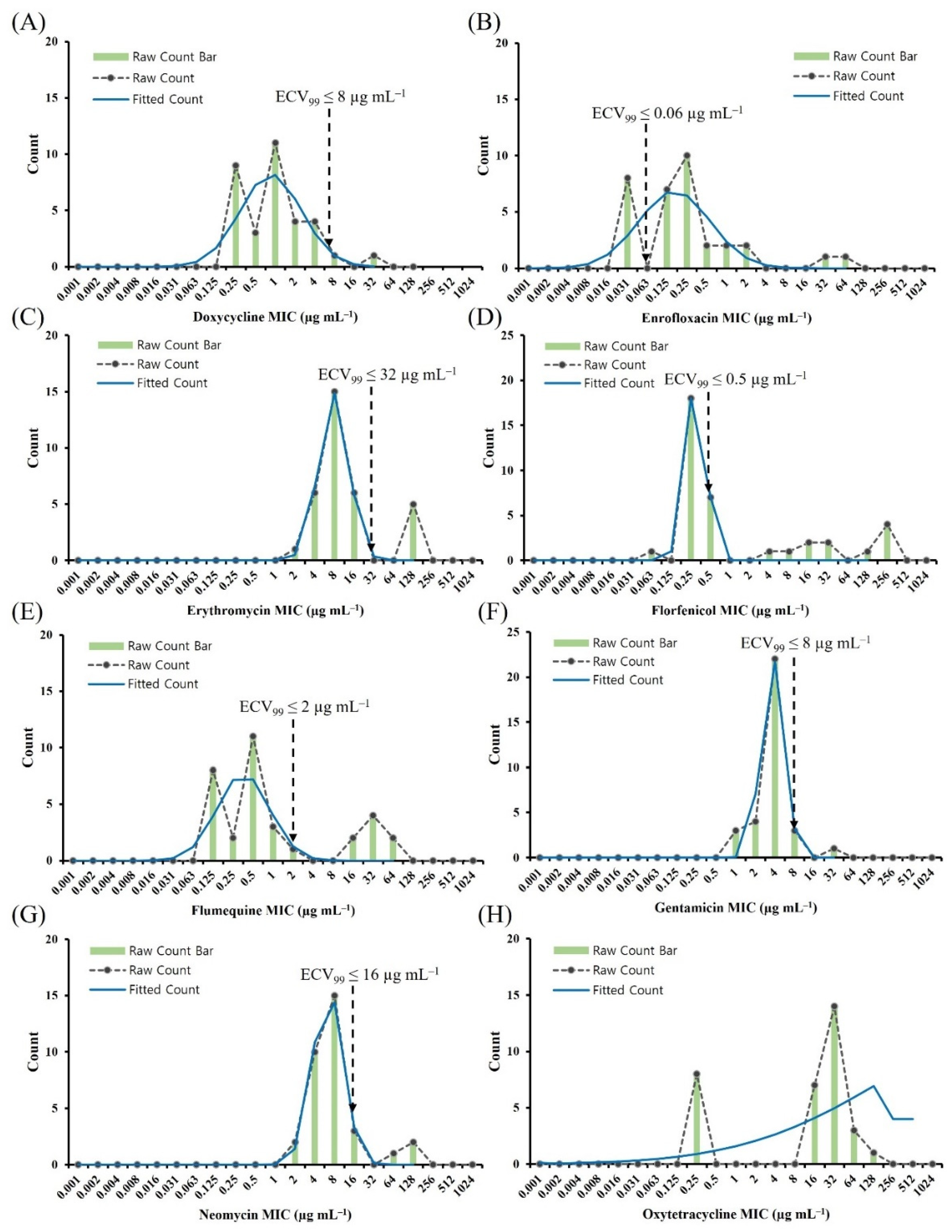
2.2. ECV Establishment Using Two Analytical Methods
2.3. Comparison of the ECVCLSI, ECVNRI, and ECV99
2.4. Presumptive Multidrug-Resistant (pMDR) Aeromonas spp. Isolates
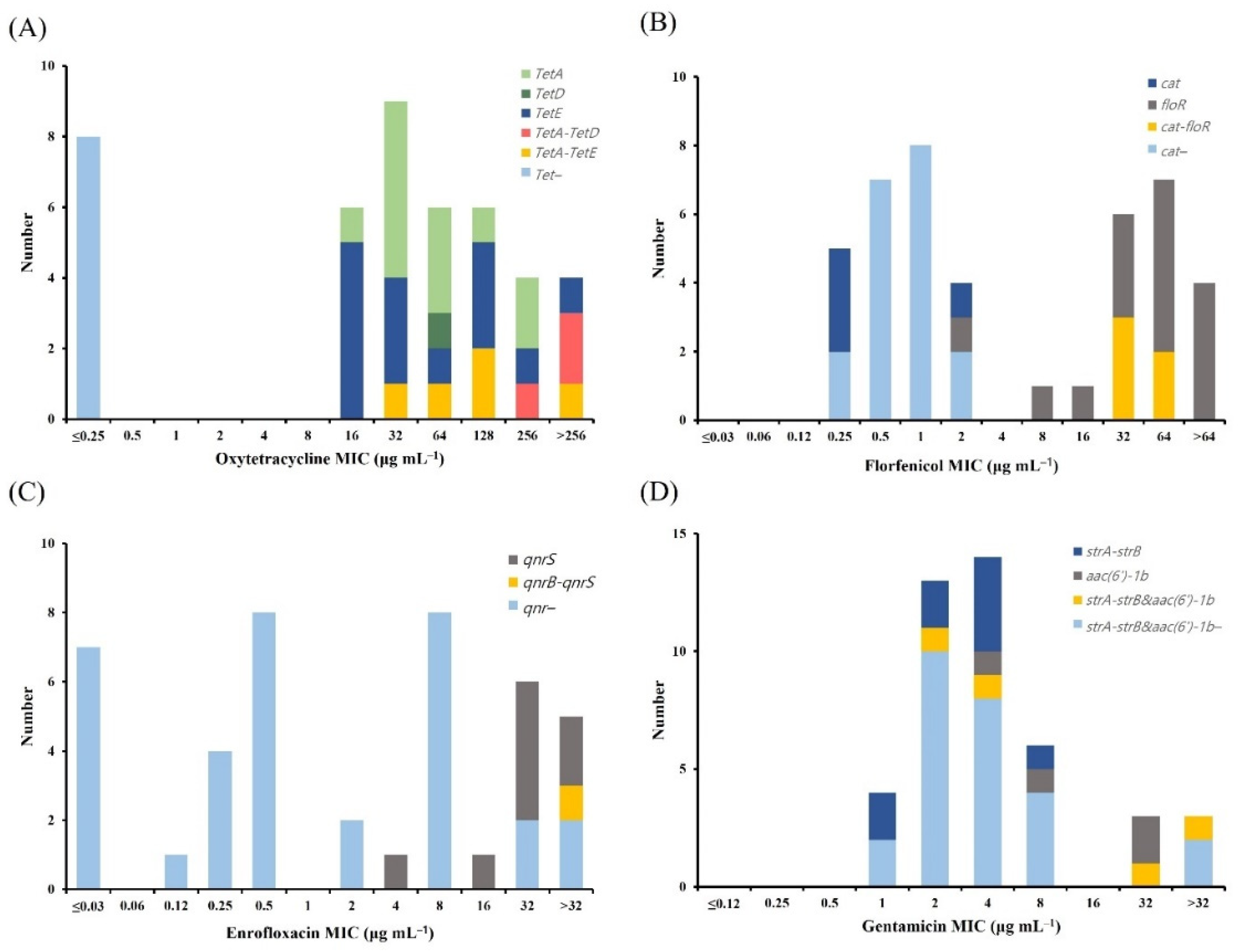
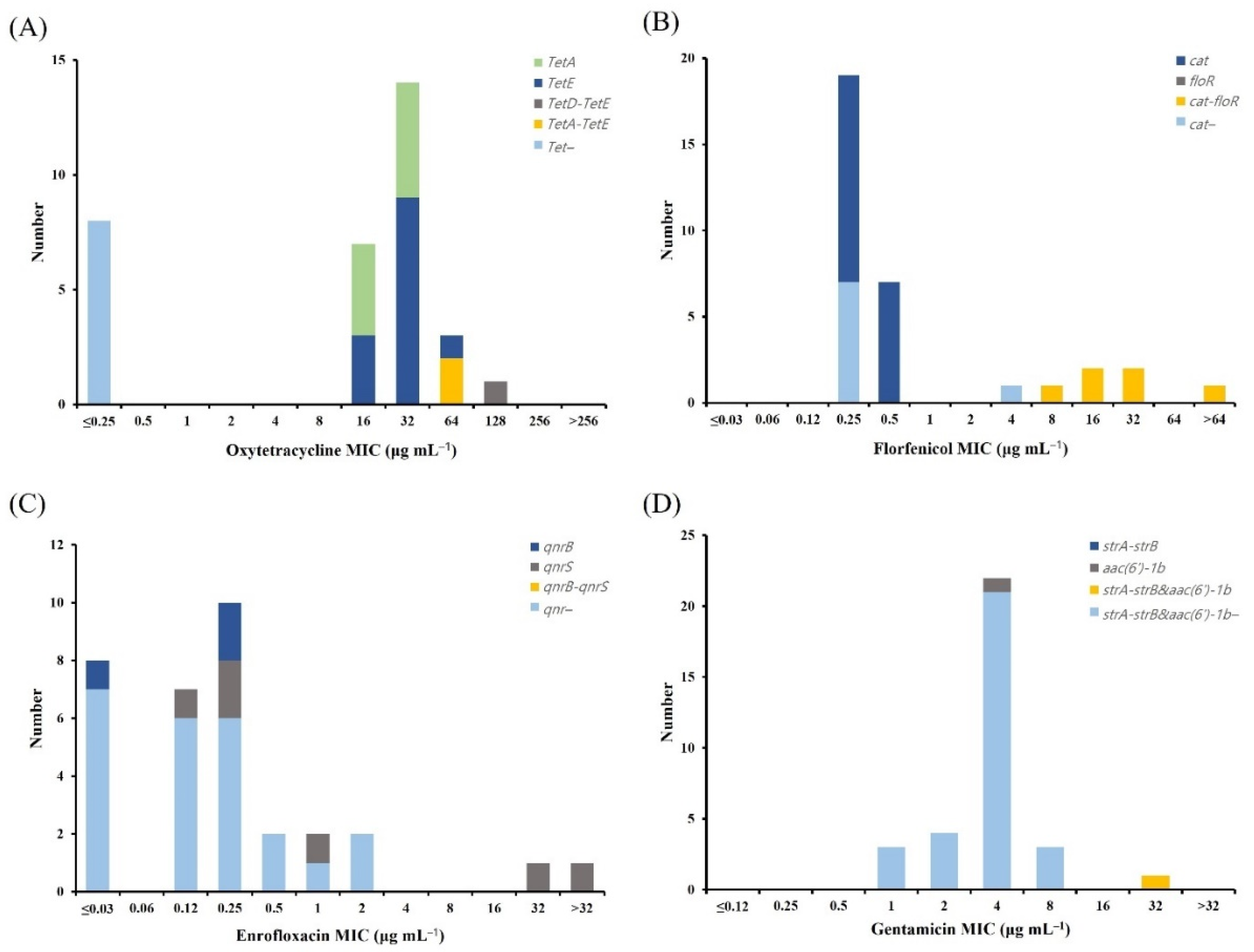
2.5. Distribution of Antimicrobial Resistance Genes (ARGs)
2.6. Quality Control (QC)
3. Discussion
4. Materials and Methods
4.1. Collection and Isolation of Aeromonas spp.
4.2. Molecular Identification
4.3. Antimicrobial Susceptibility Test
4.4. Determination of Provisional ECVs
4.5. Terminology
4.6. Analysis of ARGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khor, W.C.; Puah, S.M.; Koh, T.H.; Tan, J.A.M.A.; Puthucheary, S.D.; Chua, K.H. Comparison of clinical isolates of Aeromonas from Singapore and Malaysia with regard to molecular identification, virulence, and antimicrobial profiles. Microb. Drug Resist. 2018, 24, 469–478. [Google Scholar] [CrossRef]
- Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Popelka, P.; Toropilova, J. The use of probiotic bacteria against Aeromonas infections in salmonid aquaculture. Aquaculture 2017, 469, 42. [Google Scholar] [CrossRef]
- Mazumder, A.; Choudhury, H.; Dey, A.; Sarma, D. Isolation and characterization of two virulent Aeromonads associated with haemorrhagic septicaemia and tail-rot disease in farmed climbing perch Anabas testudineus. Sci. Rep. 2021, 11, 5826. [Google Scholar] [CrossRef]
- Sen, K.; Rodgers, M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: A PCR identification. J. Appl. Microbiol. 2004, 97, 1077–1086. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Abd-El-Rhman, A.M. Antagonism of Aeromonas hydrophila by propolis and its effect on the performance of Nile tilapia, Oreochromis niloticus. Fish. Shellfish Immunol. 2009, 27, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Frost, P.; Miest, J.; Pionnier, N.; Irnazarow, I.; Hoole, D. Reduced inflammatory response to Aeromonas salmonicida infection in common carp (Cyprinus carpio L.) fed with β-glucan supplements. Fish. Shellfish Immunol. 2012, 32, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bergh, P.; Frey, J. Aeromonas salmonicida subsp. salmonicida in the light of its type-three secretion system. Microb. Biotechnol. 2014, 7, 381–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Hu, X.; Lü, A.; Liu, R.; Sun, J.; Sung, Y.Y.; Song, Y. Transcriptome analysis in the skin of Carassius auratus challenged with Aeromonas hydrophila. Fish. Shellfish Immunol. 2019, 94, 510–516. [Google Scholar] [CrossRef]
- Mulyani, Y.; Aryantha, I.N.P.; Suhandono, S.; Pancoro, A. Intestinal bacteria of common carp (Cyprinus carpio L.) as a biological control agent for Aeromonas. J. Pure Appl. Microbiol. 2018, 12, 601–610. [Google Scholar] [CrossRef]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence factors of Aeromonas hydrophila: In the wake of reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Ramalivhana, J.N.; Obi, C.L.; Moyo, S.R. Prevalence of extended-spectrum β-lactamases producing Aeromonas hydrophila isolated from stool samples collected in the Limpopo province, South Africa. Afr. J. Microbiol. Res. 2010, 4, 1203–1208. [Google Scholar] [CrossRef]
- Chenia, H.Y. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from South African freshwater fish. Int. J. Food Microbiol. 2016, 231, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Dahanayake, P.S.; Heo, G.J. Distribution of antimicrobial resistance genes and class 1 integron gene cassette arrays in motile Aeromonas spp. isolated from goldfish (Carassius auratus). Microb. Drug Resist. 2018, 24, 1217–1225. [Google Scholar] [CrossRef]
- Aravena-Román, M.; Inglis, T.J.; Henderson, B.; Riley, T.V.; Chang, B.J. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob. Agents Chemother. 2012, 56, 1110–1112. [Google Scholar] [CrossRef] [Green Version]
- Boerlin, P.; Reid-Smith, R.J. Antimicrobial resistance: Its emergence and transmission. Anim. Health Res. Rev. 2008, 9, 115–126. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals; VET03/VET04-S2.; CLSI: Wayne, PA, USA, 2014; pp. 1–42. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing of Bacteria Isolated from Aquatic Animals, 3rd ed.; VET04.; CLSI: Wayne, PA, USA, 2020; pp. 1–88. [Google Scholar]
- Yao, Z.; Sun, L.; Wang, Y.; Lin, L.; Guo, Z.; Li, D.; Lin, W.; Lin, X. Quantitative proteomics reveals antibiotics resistance function of outer membrane proteins in Aeromonas hydrophila. Front. Cell. Infect. Microbiol. 2018, 8, 390. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, Y.; Li, X.; Lin, Z.; Lin, Y.; Srinivasan, R.; Lin, X. The characteristics of antibiotic resistance and phenotypes in 29 outer-membrane protein mutant strains in Aeromonas hydrophila. Environ. Microbiol. 2019, 21, 4614–4628. [Google Scholar] [CrossRef]
- Baron, S.; Granier, S.A.; Larvor, E.; Jouy, E.; Cineux, M.; Wilhelm, A.; Gassilloud, B.; Bouquin, S.L.; Kempf, I.; Chauvin, C. Aeromonas Diversity and Antimicrobial Susceptibility in Freshwater—An Attempt to Set Generic Epidemiological Cut-Off Values. Front. Microbiol. 2017, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Hayatgheib, N.; Calvez, S.; Fournel, C.; Pineau, L.; Pouliquen, H.; Moreau, E. Antimicrobial Susceptibility Profiles and Resistance Genes in Genus Aeromonas spp. Isolated from the Environment and Rainbow Trout of Two Fish Farms in France. Microorganisms 2021, 9, 1201. [Google Scholar] [CrossRef]
- Duman, M.; Saticioglu, I.B.; Altun, S. The determination of antimicrobial susceptibility by MIC and epidemiological cut-off values and the detection of resistance genes in Aeromonas species isolated from cultured fish. Lett. Appl. Microbiol. 2020, 71, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI guideline M45.; CLSI: Wayne, PA, USA, 2016; pp. 1–19. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI standard M07; CLSI: Wayne, PA, USA, 2018; pp. 1–13. [Google Scholar]
- Pessoa, R.B.G.; de Oliveira, W.F.; Marques, D.S.C.; dos Santos Correia, M.T.; de Carvalho, E.V.M.M.; Coelho, L.C.B.B. The genus Aeromonas: A general approach. Microb. Pathog. 2019, 130, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Goñi-Urriza, M.; Pineau, L.; Capdepuy, M.; Roques, C.; Caumette, P.; Quentin, C. Antimicrobial resistance of mesophilic Aeromonas spp. isolated from two European rivers. J. Antimicrob. Chemother. 2000, 46, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Saengsitthisak, B.; Chaisri, W.; Punyapornwithaya, V.; Mektrirat, R.; Klayraung, S.; Bernard, J.K.; Pikulkaew, S. Occurrence and antimicrobial susceptibility profiles of multidrug-resistant aeromonads isolated from freshwater ornamental fish in Chiang Mai province. Pathogens 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Čížek, A.; Dolejská, M.; Sochorová, R.; Strachotová, K.; Piačková, V.; Veselý, T. Antimicrobial resistance and its genetic determinants in aeromonads isolated in ornamental (koi) carp (Cyprinus carpio koi) and common carp (Cyprinus carpio). Vet. Microbiol. 2010, 142, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Nhinh, D.T.; Le, D.V.; Van, K.V.; Huong Giang, N.T.; Dang, L.T.; Hoai, T.D. Prevalence, Virulence Gene Distribution and Alarming the Multidrug Resistance of Aeromonas hydrophila Associated with Disease Outbreaks in Freshwater Aquaculture. Antibiotics 2021, 10, 532. [Google Scholar] [CrossRef]
- Piotrowska, M.; Przygodzińska, D.; Matyjewicz, K.; Popowska, M. Occurrence and variety of β-lactamase genes among Aeromonas spp. isolated from urban wastewater treatment plant. Front. Microbiol. 2017, 8, 863. [Google Scholar] [CrossRef] [Green Version]
- Goñi-Urriza, M.; Capdepuy, M.; Arpin, C.; Raymond, N.; Caumette, P.; Quentin, C. Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas spp. Appl. Environ. Microbiol. 66, 125–132. [CrossRef] [Green Version]
- Skwor, T.; Shinko, J.; Augustyniak, A.; Gee, C.; Andraso, G. Aeromonas hydrophila and Aeromonas veronii predominate among potentially pathogenic ciprofloxacin-and tetracycline-resistant Aeromonas isolates from Lake Erie. Appl. Environ. Microbiol. 2014, 80, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Zdanowicz, M.; Mudryk, Z.J.; Perliński, P. Abundance and antibiotic resistance of Aeromonas isolated from the water of three carp ponds. Vet. Res. Commun. 2020, 44, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekedar, H.C.; Arick, M.A.; Hsu, C.Y.; Thrash, A.; Blom, J.; Lawrence, M.L.; Abdelhamed, H. Identification of antimicrobial resistance determinants in Aeromonas veronii strain MS-17-88 recovered from channel catfish (Ictalurus punctatus). Front. Cell. Infect. Microbiol. 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Food and Drug Safety Evaluation (NIFDS). National Antimicrobial Resistance Surveillance on the Domestic and Imported Meat and Fishery Products; NIFDS: Cheongju, Korea, 2019; pp. 1–161. [Google Scholar]
- National Institute of Fisheries Science (NIFS). Aquatic Medicine Catalog; NIFS: Busan, Korea, 2020; pp. 12–102. [Google Scholar]
- McIntosh, D.; Cunningham, M.; Ji, B.; Fekete, F.A.; Parry, E.M.; Clark, S.E.; Zalinger, Z.B.; Gilg, I.C.; Danner, R.D.; Johnson, K.A.; et al. Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J. Antimicrob. Chemother. 2008, 61, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, L.; Cloeckaert, A.; Doublet, B.; Schwarz, S.; Bouju-Albert, A.; Ganière, J.P.; Le Bris, H.; Le Flèche-Matéos, A.; Giraud, E. Complete sequence of the floR-carrying multiresistance plasmid pAB5S9 from freshwater Aeromonas bestiarum. J. Antimicrob. Chemother. 2008, 62, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Sezer, A.D.; Akbuğa, J.; Baş, A.L. In vitro evaluation of enrofloxacin-loaded MLV liposomes. Drug Deliv. 2007, 14, 47–53. [Google Scholar] [CrossRef]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Heo, G.J. Prevalence of virulence and antimicrobial resistance genes in Aeromonas species isolated from marketed cockles (Tegillarca granosa) in Korea. Lett. Appl. Microbiol. 2020, 71, 94–101. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Larsen, J.L. Incidence, distribution, and spread of tetracycline resistance determinants and integron-associated antibiotic resistance genes among motile aeromonads from a fish farming environment. Appl. Environ. Microbiol. 2001, 67, 5675–5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koksal, F.; Oguzkurt, N.; Samastı, M.; Altas, K. Prevalence and antimicrobial resistance patterns of Aeromonas strains isolated from drinking water samples in Istanbul, Turkey. Chemotherapy 2007, 53, 30–35. [Google Scholar] [CrossRef]
- Borella, L.; Salogni, C.; Vitale, N.; Scali, F.; Moretti, V.M.; Pasquali, P.; Alborali, G.L. Motile aeromonads from farmed and wild freshwater fish in northern Italy: An evaluation of antimicrobial activity and multidrug resistance during 2013 and 2016. Acta Vet. Scand. 2020, 62, 1–8. [Google Scholar] [CrossRef]
- Igbinosa, I.H. Antibiogram profiling and pathogenic status of Aeromonas species recovered from Chicken. Saudi J. Biol. Sci. 2014, 21, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawal, I.; Joshi, H.; Chaudhary, L. Isolation, identification, and antibiotics resistance of Aeromonas spp. from lakes of Udaipur (Rajasthan), India. Asian J. Pharm. 2016, 10, 132–136. [Google Scholar] [CrossRef]
- Lin, H.T.; Bavro, V.N.; Barrera, N.P.; Frankish, H.M.; Velamakanni, S.; van Veen, H.W.; Robinson, C.; Borges-Walmsley, M.I.; Walmsley, A.R. MacB ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA. J. Biol. Chem. 2009, 284, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Azzam-Sayuti, M.; Ina-Salwany, M.Y.; Zamri-Saad, M.; Yusof, M.T.; Annas, S.; Najihah, M.Y.; Liles, M.R.; Monir, M.S.; Zaidi, Z.; Amal, M.N.A. The prevalence, putative virulence genes and antibiotic resistance profiles of Aeromonas spp. isolated from cultured freshwater fishes in peninsular Malaysia. Aquaculture 2021, 540, 736719. [Google Scholar] [CrossRef]
- Hernould, M.; Gagné, S.; Fournier, M.; Quentin, C.; Arpin, C. Role of the AheABC efflux pump in Aeromonas hydrophila intrinsic multidrug resistance. Antimicrob. Agents Chemother. 2008, 52, 1559–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Ramanathan, S.; Chen, L.; Zeng, F.; Li, X.; Zhao, Y.; Lin, L.; Monaghan, S.J.; Lin, X.; Pang, H. Comparative transcriptomic analysis reveals the molecular mechanisms related to oxytetracycline-resistance in strains of Aeromonas hydrophila. Aquac. Rep. 2021, 21, 100812. [Google Scholar] [CrossRef]
- Smith, P.; Kronvall, G. How many strains are required to set an epidemiological cut-off value for MIC values determined for bacteria isolated from aquatic animals? Aquac. Int. 2015, 23, 465–470. [Google Scholar] [CrossRef]
- Yanez, M.A.; Catalán, V.; Apráiz, D.; Figueras, M.J.; Martínez-Murcia, A.J. Phylogenetic analysis of members of the genus Aeromonas based on gyrB gene sequences. Int. J. Syst. Evol. Microbiol. 2003, 53, 875–883. [Google Scholar] [CrossRef] [Green Version]
- Kronvall, G. Normalized resistance interpretation as a tool for establishing epidemiological MIC susceptibility breakpoints. J. Clin. Microbiol. 2010, 48, 4445–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnidge, J.; Kahlmeter, G.; Kronvall, G. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006, 12, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silley, P. Susceptibility testing methods, resistance and breakpoints: What do these terms really mean? OIE Rev. Sci. Tech. 2012, 31, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.J.; Jeong, J.B.; Huh, M.D.; Chung, J.K.; Choi, D.L.; Lee, C.H.; Jeong, H.D. Detection of tetracycline-resistance determinants by multiplex polymerase chain reaction in Edwardsiella tarda isolated from fish farms in Korea. Aquaculture 2004, 240, 89–100. [Google Scholar] [CrossRef]
- Akinbowale, O.L.; Peng, H.; Barton, M.D. Diversity of tetracycline resistance genes in bacteria from aquaculture sources in Australia. J. Appl. Microbiol. 2007, 103, 2016–2025. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Sunde, M.; Norström, M. The genetic background for streptomycin resistance in Escherichia coli influences the distribution of MICs. J. Antimicrob. Chemother. 2005, 56, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Robicsek, A.; Jacoby, G.A.; Sahm, D.; Hooper, D.C. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 2006, 50, 3953–3955. [Google Scholar] [CrossRef] [Green Version]


| Antimicrobials | No. of Isolates with MIC a (µg mL−1) | MIC50 | MIC90 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | |||
| Doxycycline | 8 | 3 | 5 | 4 | 6 | 9 | 3 | 2 | 2 | 1 | 4 | 32 | |||||
| Enrofloxacin | 7 | 0 | 1 | 4 | 8 | 8 | 2 | 1 | 0 | 1 | 6 | 5 | 1 | 32< | |||
| Erythromycin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 8 | 4 | 3 | 23 | 64< | 64< | ||
| Florfenicol | 0 | 0 | 0 | 5 | 7 | 7 | 5 | 0 | 1 | 1 | 5 | 5 | 7 | 2 | 64< | ||
| Flumequine | 7 | 0 | 1 | 2 | 2 | 0 | 2 | 5 | 7 | 6 | 7 | 4 | 32 | 128 | |||
| Gentamicin | 0 | 0 | 0 | 4 | 13 | 14 | 6 | 0 | 3 | 3 | 4 | 32 | |||||
| Neomycin | 0 | 0 | 3 | 10 | 4 | 3 | 3 | 3 | 17 | 32 | 64< | ||||||
| Oxytetracycline | 8 | 0 | 0 | 0 | 0 | 0 | 6 | 9 | 6 | 6 | 4 | 4 | 64 | 256 | |||
| Antimicrobials | No. of Isolates with MIC a (µg mL−1) | MIC50 | MIC90 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | |||
| Doxycycline | 9 | 3 | 11 | 4 | 4 | 1 | 0 | 1 | 0 | 0 | 1 | 4 | |||||
| Enrofloxacin | 8 | 0 | 7 | 10 | 2 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 0.25 | 2 | |||
| Erythromycin | 0 | 0 | 0 | 00 | 0 | 0 | 1 | 6 | 15 | 6 | 0 | 0 | 5 | 8 | 64< | ||
| Florfenicol | 0 | 1 | 0 | 18 | 7 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 0.25 | 16 | ||
| Flumequine | 8 | 2 | 11 | 3 | 1 | 0 | 0 | 2 | 4 | 2 | 0 | 0 | 0.5 | 16 | |||
| Gentamicin | 0 | 0 | 0 | 3 | 4 | 22 | 3 | 0 | 1 | 0 | 4 | 8 | |||||
| Neomycin | 0 | 0 | 2 | 10 | 15 | 3 | 0 | 1 | 2 | 8 | 16 | ||||||
| Oxytetracycline | 8 | 0 | 0 | 0 | 0 | 0 | 7 | 14 | 3 | 1 | 0 | 0 | 32 | 64 | |||
| Species | Antimicrobial | ECVCLSI (µg mL−1) | WT(%) | NWT(%) | ECVNRI (µg mL−1) | WT(%) | NWT(%) | ECV99 (µg mL−1) | WT(%) | NWT(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A. hydrophila | Doxycycline | ND | - | - | 2 | 46.5 | 53.5 | 128 | 100.0 | 0.0 |
| Enrofloxacin | 0.03 | 16.3 | 83.7 | 32 # | 88.4 | 11.6 | 16 | 74.4 | 25.6 | |
| Erythromycin | 64 | 46.5 | 53.5 | 64 | 46.5 | 53.5 | ND | - | - | |
| Florfenicol | 2 | 55.8 | 44.2 | 1 | 44.2 | 55.8 | 4 | 55.8 | 44.2 | |
| Flumequine | ND | - | - | 64 # | 74.4 | 25.6 | ND | - | - | |
| Gentamicin | 4 | 72.1 | 27.9 | 16 | 86.0 | 14.0 | 16 | 86.0 | 14.0 | |
| Neomycin | ND | - | - | 16 | 46.5 | 53.5 | ND | - | - | |
| Oxytetracycline | 0.25 | 18.6 | 62.8 | ND | - | - | ND | - | - |
| Species | Antimicrobial | ECVCLSI (µg mL−1) | WT(%) | NWT(%) | ECVNRI (µg mL−1) | WT(%) | NWT(%) | ECV99 (µg mL−1) | WT(%) | NWT(%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A. veronii | Doxycycline | ND | - | - | 1 | 69.7 | 30.3 | 8 | 97.0 | 3.0 |
| Enrofloxacin | ND | - | - | 0.06 | 24.2 | 75.8 | 0.06 | 24.2 | 75.8 | |
| Erythromycin | ND | - | - | 32 | 84.8 | 15.2 | 32 | 84.8 | 15.2 | |
| Florfenicol | ND | - | - | 1 | 78.8 | 21.2 | 0.5 | 78.8 | 21.2 | |
| Flumequine | ND | - | - | 0.25 | 30.3 | 69.7 | 2 | 75.8 | 24.2 | |
| Gentamicin | ND | - | - | 16 | 97.0 | 3.0 | 8 | 97.0 | 3.0 | |
| Neomycin | ND | - | - | 32 | 90.9 | 9.1 | 16 | 90.9 | 9.1 | |
| Oxytetracycline | ND | - | - | 0.5 | 24.2 | 75.8 | ND | - | - |
| Strain | Isolate No. | Host | Year | Phenotype |
|---|---|---|---|---|
| A. hydrophila | 20FBAer0358 | Anguilla japonica | 2020 | E, Er, F, Fl, G, N, O |
| 20FBAer0371 | Anguilla japonica | 2020 | Er, F, Fl, G, N, O | |
| 20FBAer0351 | Anguilla japonica | 2020 | E, F, G, O | |
| 19FBAHy0001 | Silurus asotus | 2019 | E, Er, F, Fl, N, O | |
| 18FBAHy0001 | Silurus asotus | 2018 | E, Er, F, Fl, N, O | |
| 18FBAhy0003 | Anguilla japonica | 2018 | E, Er, F, Fl, N, O | |
| 17FBAHy0013 | Salmo salar | 2017 | E, Er, F, Fl, N, O | |
| 17FBAHy0006 | Misgurnus mizolepis | 2017 | F, G, N, O | |
| A. veronii | 20FBAer0306 | Anguilla japonica | 2020 | E, F, G, N, O |
| 20FBAer0374 | Oncorhynchus mykiss | 2020 | E, Er, N, O | |
| 21FBAer0172 | Cyprinus carpio nudus | 2018 | E, Er, F, Fl, N, O | |
| 21FBAer0163 | Cyprinus carpio nudus | 2018 | E, F, Fl, O | |
| 21FBAer0164 | Carassius carassius | 2018 | E, F, Fl, O | |
| 21FBAer0171 | Cyprinus carpio nudus | 2018 | E, F, O | |
| FP3978 | Cyprinus carpio nudus | 2010 | D, E, Er, Fl, O | |
| FP3973 | Cyprinus carpio nudus | 2010 | E, F, O |
| Tetracycline | Florfenicol | Quinolone | Aminoglycoside | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tetA | tetB | tetD | tetE | Others * | cat | floR | Others * | qnrA | qnrB | qnrS | Others * | strA-strB | aac(6′)-1b | Others * | |
| A. hydrophila | 12 | - | 1 | 14 | 8 | 4 | 15 | 5 | - | - | 8 | 1 | 9 | 4 | 4 |
| A. veronii | 9 | - | 0 | 13 | 3 | 19 | - | 6 | - | 3 | 6 | - | - | 1 | 1 |
| Class | Primer | Sequence (5′–3′) | AT * (°C) | Size(bp) | Reference |
|---|---|---|---|---|---|
| Tetracycline | tetA-F | GCG CTN TAT GCG TTG ATG CA | 53 | 387 | [59] |
| tetA-R | ACA GCC CGT CAG GAA ATT | ||||
| tetB-F | CTC AGT ATT CCA AGC CTT TG | 58 | 400 | [60] | |
| tetB-R | CTA AGC ACT TGT CTC CTG TT | ||||
| tetD-F | GCG CTN TAT GCG TTG ATG CA | 50 | 484 | [59] | |
| tetD-R | CCA GAG GTT TAA GCA GTG T | ||||
| tetE-F | GCG CTN TAT GCG TTG ATG CA | 50 | 246 | [59] | |
| tetE-R | ATG TGT CCT GGA TTC CT | ||||
| Phenicol | cat-F | AGC GCA ACG TCC TCT ATC AC | 55 | 378 | This study (PMU05929.1) |
| cat-R | TGT CGT CGT CAA AGC GGT AG | ||||
| floR-F | GCC CGC TAT GAT CCA ACT CA | 55 | 289 | This study (QEV84023.1) | |
| floR-R | AAG GCC GTA GAT GAC GAC AC | ||||
| Quinolone | qnrA-F | AGA GGA TTT CTC ACG CCA GG | 56 | 580 | [61] |
| qnrA-R | TGC CAG GCA CAG ATC TTG AC | ||||
| qnrB-F | GAT CGT GAA AGC CAG AAA GG | 53 | 496 | [61] | |
| qnrB-R | ACG ATG CCT GGT AGT TGT CC | ||||
| qnrS-F | GCA AGT TCA TTG AAC AGG GT | 56 | 428 | [61] | |
| qnrS-R | TCT AAA CCG TCG AGT TCG GCG | ||||
| Aminoglycoside | strA-strB-F | TAT CTG CGA TTG GAC CCT CTG | 55 | 538 | [62] |
| strA-strB-R | CAT TGC TCA TCA TTT GAT CGG CT | ||||
| aac(6′)-1b-F | TTG CGA TGC TCT ATG AGT GGC TA | 55 | 482 | [63] | |
| aac(6′)-1b-R | CTC GAA TGC CTG GCG TGT TT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.-J.; Kim, M.-S.; Jeong, M.-G.; Do, M.-Y.; Hwang, S.-D.; Kim, W.-J. Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals. Antibiotics 2022, 11, 343. https://doi.org/10.3390/antibiotics11030343
Woo S-J, Kim M-S, Jeong M-G, Do M-Y, Hwang S-D, Kim W-J. Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals. Antibiotics. 2022; 11(3):343. https://doi.org/10.3390/antibiotics11030343
Chicago/Turabian StyleWoo, Soo-Ji, Myoung-Sug Kim, Min-Gyeong Jeong, Mi-Young Do, Sung-Don Hwang, and Woo-Jin Kim. 2022. "Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals" Antibiotics 11, no. 3: 343. https://doi.org/10.3390/antibiotics11030343






