Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus faecium
Abstract
:1. Introduction
2. Results
2.1. Bacterial Isolates
2.2. Checkerboard Analyses
2.3. Time-Kill Analyses
2.4. Bacteriophage and Antibiotic Resistance Testing
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Antimicrobial Agents and Media
4.3. Bacteriophage Source and Propagation
4.4. Biofilm Quantification Assay
4.5. Phage Sensitivity Assay
4.6. Antibiotic Susceptibility Testing
4.7. Modified Checkerboard for Antibiotic and Bacteriophage Synergy Screening
4.8. Time Kill Analyses
4.9. Resistance Testing
4.10. Phage Quantification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeli, Y.; Eliopoulos, G.; Mozaffari, E.; Samore, M. Health and Economic Outcomes of Vancomycin-Resistant Enterococci. Arch. Intern. Med. 2002, 162, 2223–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Driscoll, T.; Crank, C.W. Vancomycin-resistant enterococcal infections: Epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist. 2015, 8, 217–230. [Google Scholar]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States. 2019. Available online: https://stacks.cdc.gov/view/cdc/82532 (accessed on 3 May 2021).
- Chiang, H.-Y.; Perencevich, E.N.; Nair, R.; Nelson, R.E.; Samore, M.; Khader, K.; Chorazy, M.L.; Herwaldt, L.A.; Blevins, A.; Ward, M.A.; et al. Incidence and Outcomes Associated with Infections Caused by Vancomycin-Resistant Enterococci in the United States: Systematic Literature Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2017, 38, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Jabbari Shiadeh, S.M.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [Green Version]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control. Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paganelli, F.L.; Willems, R.J.L.; Jansen, P.; Hendrickx, A.; Zhang, X.; Bonten, M.J.M.; Leavis, H.L. Enterococcus faecium Biofilm Formation: Identification of Major Autolysin AtlA Efm, Associated Acm Surface Localization, and AtlA Efm-Independent Extracellular DNA Release. MBio 2013, 4, e00154-13. [Google Scholar] [CrossRef] [Green Version]
- Taglialegna, A.; Matilla-Cuenca, L.; Dorado-Morales, P.; Navarro, S.; Ventura, S.; Garnett, J.A.; Lasa, I.; Valle, J. The biofilm-associated surface protein Esp of Enterococcus faecalis forms amyloid-like fibers. NPJ Biofilms Microbiomes. 2020, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Ramadhan, A.A.; Hegedus, E. Biofilm formation and esp gene carriage in enterococci. J. Clin. Pathol. 2005, 58, 685–686. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Mosselhy, D.A.; Assad, M.; Sironen, T.; Elbahri, M. Nanotheranostics: A Possible Solution for Drug-Resistant Staphylococcus aureus and their Biofilms? Nanomaterials 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Kheir, M.M.; Tan, T.L.; Higuera, C.; George, J.; Valle Della, C.J.; Shen, M.; Parvizi, J. Periprosthetic Joint Infections Caused by Enterococci Have Poor Outcomes. J. Arthroplasty 2017, 32, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, A.; Rasmussen, M. Mature biofilms of Enterococcus faecalis and Enterococcus faecium are highly resistant to antibiotics. Diagn. Microbiol. Infect. Dis. 2016, 84, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Mascio, C.T.M.; Alder, J.D.; Silverman, J.A. Bactericidal Action of Daptomycin against Stationary-Phase and Nondividing Staphylococcus aureus Cells. Antimicrob. Agents Chemother. 2007, 51, 4255–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanbakhsh, S.; Singh, N.B.; Yim, J.; Kebriaei, R.; Smith, J.R.; Lev, K.; Tran, T.T.; Rose, W.E.; Arias, C.A.; Rybak, M.J. Impact of Daptomycin Dose Exposure Alone or in Combination with β-Lactams or Rifampin against Vancomycin-Resistant Enterococci in an In Vitro Biofilm Model. Antimicrob. Agents Chemother. 2020, 64, e02074-19. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.-G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, E7077–E7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S.; Davison, W.M.; Steenbergen, J.N. Daptomycin Rapidly Penetrates a Staphylococcus epidermidis Biofilm. Antimicrob. Agents Chemother. 2009, 53, 3505–3507. [Google Scholar] [CrossRef] [Green Version]
- Lellek, H.; Franke, G.C.; Ruckert, C.; Wolters, M.; Wolschke, C.; Christner, M.; Büttner, H.; Alawi, M.; Kröger, N.; Rohde, H. Emergence of daptomycin non-susceptibility in colonizing vancomycin-resistant Enterococcus faecium isolates during daptomycin therapy. Int. J. Med. Microbiol. 2015, 305, 902–909. [Google Scholar] [CrossRef]
- Munita, J.M.; Murray, B.E.; Arias, C.A. Daptomycin for the treatment of bacteraemia due to vancomycin-resistant enterococci. Int. J. Antimicrob. Agents 2014, 44, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, M.; Cohen, N.; Gilhuley, K.; Babady, N.E.; Seo, S.K.; Sepkowitz, K.A. Emergence of Daptomycin-Resistant VRE: Experience of a Single Institution. Infect. Control Hosp. Epidemiol. 2011, 32, 391–394. [Google Scholar] [CrossRef]
- Kebriaei, R.; Rice, S.A.; Singh, K.V.; Stamper, K.C.; Dinh, A.Q.; Rios, R.; Diaz, L.; Murray, B.E.; Munita, J.M.; Tran, T.T.; et al. Influence of Inoculum Effect on the Efficacy of Daptomycin Monotherapy and in Combination with β-Lactams against Daptomycin-Susceptible Enterococcus faecium Harboring LiaSR Substitutions. Antimicrob. Agents Chemother. 2018, 62, e00315-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, A.D.; Steed, M.E.; Arias, C.A.; Murray, B.E.; Rybak, M.J. Evaluation of Standard- and High-Dose Daptomycin versus Linezolid against Vancomycin-Resistant Enterococcus Isolates in an In Vitro Pharmacokinetic/Pharmacodynamic Model with Simulated Endocardial Vegetations. Antimicrob. Agents Chemother. 2012, 56, 3174–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prater, A.G.; Mehta, H.H.; Beabout, K.; Suoandy, A.; Miller, W.R.; Tran, T.T.; Arias, C.A.; Shamoo, Y. Daptomycin Resistance in Enterococcus faecium Can Be Delayed by Disruption of the LiaFSR Stress Response Pathway. Antimicrob. Agents Chemother. 2021, 65, e01317-20. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Tran, T.T.; Munita, J.M.; Miller, W.R.; Rincon, S.; Carvajal, L.P.; Wollam, A.; Reyes, J.; Panesso, D.; Rojas, N.L.; et al. Whole-Genome Analyses of Enterococcus faecium Isolates with Diverse Daptomycin MICs. Antimicrob. Agents Chemother. 2014, 58, 4527–4534. [Google Scholar] [CrossRef] [Green Version]
- Morrisette, T.; Kebriaei, R.; Lev, K.L.; Morales, S.; Rybak, M.J. Bacteriophage Therapeutics: A Primer for Clinicians on Phage-Antibiotic Combinations. Pharmacotherapy 2020, 40, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.; Abdelbary, H. Does Treatment Order Matter? Investigating the Ability of Bacteriophage to Augment Antibiotic Activity against Staphylococcus aureus Biofilms. Front. Microbiol. 2018, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Harper, D.R.; Parracho, H.M.R.T.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol. J. Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.-B.; Trampuz, A.; Luca, M.D. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 0842–0853. [Google Scholar] [CrossRef]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting biofilms using phages and their enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Dakheel, K.H.; Abdul Rahim, R.; Al-Obaidi, J.R.; Neela, V.K.; Hun, T.G.; Isa, M.N.M.; Razali, N.; Yusoff, K. Proteomic analysis revealed the biofilm-degradation abilities of the bacteriophage UPMK_1 and UPMK_2 against Methicillin-resistant Staphylococcus aureus. Biotechnol. Lett. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr. Opin. Microbiol 2019, 51, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, R.; Lev, K.; Morrisette, T.; Stamper, K.C.; Abdul-Mutakabbir, J.C.; Lehman, S.M.; Morales, S.; Rybak, M.J. Bacteriophage-Antibiotic Combination Strategy: An Alternative against Methicillin-Resistant Phenotypes of Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, e00461-20. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, R.A.; Leung, C.Y.; Chan, B.K.; Turner, P.E.; Weitz, J.S. Quantitative Models of Phage-Antibiotic Combination Therapy. mSystems 2020, 5, e00756-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef]
- Morrisette, T.; Lev, K.L.; Kebriaei, R.; Abdul-Mutakabbir, J.C.; Stamper, K.C.; Morales, S.; Lehman, S.M.; Canfield, G.S.; Duerkop, B.A.; Arias, C.A.; et al. Bacteriophage-Antibiotic Combinations for Enterococcus faecium with Varying Bacteriophage and Daptomycin Susceptibilities. Antimicrob. Agents Chemother. 2020, 64, e00993-20. [Google Scholar] [CrossRef]
- Shlezinger, M.; Coppenhagen-Glazer, S.; Gelman, D.; Beyth, N.; Hazan, R. Eradication of Vancomycin-Resistant Enterococci by Combining Phage and Vancomycin. Viruses 2019, 11, 954. [Google Scholar] [CrossRef] [Green Version]
- Canfield, G.S.; Chatterjee, A.; Espinosa, J.; Mangalea, M.R.; Sheriff, E.K.; Keidan, M.; McBride, B.D.; Hang, H.C.; Duerkop, B.A. Lytic Bacteriophages Facilitate Antibiotic Sensitization of Enterococcus faecium. Antimicrob. Agents Chemother. 2021, 65, e00143-21. [Google Scholar] [CrossRef]
- Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages Using the Small Drop Plaque Assay System. In Bacteriophages; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 81–85. Available online: http://link.springer.com/10.1007/978-1-60327-164-6_9 (accessed on 13 January 2022).
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, L.M.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceri, H.; Olson, M.; Morck, D.; Storey, D.; Read, R.; Olson, B. The MBEC assay system: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 2001, 337, 377–385. [Google Scholar] [PubMed]
- Kebriaei, R.; Stamper, K.C.; Singh, K.V.; Khan, A.; Rice, S.A.; Dinh, A.Q.; Tran, T.T.; Murray, B.E.; Arias, C.A.; Rybak, M.J. Mechanistic Insights Into the Differential Efficacy of Daptomycin Plus β-Lactam Combinations Against Daptomycin-Resistant Enterococcus faecium. J. Infect. Dis. 2020, 222, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Grein, F.; Müller, A.; Scherer, K.M.; Liu, X.; Ludwig, K.C.; Klöckner, A.; Strach, M.; Sahl, H.-G.; Kubitscheck, U.; Schneider, T. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 2020, 11, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehman, S.; Mearns, G.; Rankin, D.; Cole, R.A.; Smrekar, F.; Branston, S.D.; Morales, S. Design and Preclinical Development of a Phage Product for the Treatment of Antibiotic-Resistant Staphylococcus aureus Infections. Viruses 2019, 11, 88. [Google Scholar]
- Bonilla, N.; Rojas, M.I.; Netto Flores Cruz, G.; Hung, S.-H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. Peer J. 2016, 4, e2261. [Google Scholar] [CrossRef] [Green Version]
- Bose. J.L.; Lehman, M.K.; Fey, P.D.; Bayles, K.W. Contribution of the Staphylococcus aureus Atl AM and GL Murein Hydrolase Activities in Cell Division, Autolysis, and Biofilm Formation. PLoS ONE 2012, 7, e42244. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Ali, L.; Khambaty, F.; Diachenko, G. Investigating the suitability of the Calgary Biofilm Device for assessing the antimicrobial efficacy of new agents. Bioresour. Technol. 2006, 97, 1887–1893. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition. Available online: https://clsi.org/media/1632/m07a10_sample.pdf (accessed on 13 January 2022).
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef] [Green Version]
- Rice, L.B.; Carias, L.L.; Rudin, S.; Hutton, R.; Marshall, S.; Hassan, M.; Josseaume, N.; Dubost, L.; Marie, A.; Arthur, M. Role of Class A Penicillin-Binding Proteins in the Expression of β-Lactam Resistance in Enterococcus faecium. J. Bacteriol. 2009, 191, 3649–3656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhvania, P.; Hoyle, N.S.; Nadareishvili, L.; Nizharadze, D.; Kutateladze, M. Phage Therapy in a 16-Year-Old Boy with Netherton Syndrome. Front Med. 2017, 4, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, K.E.; Werth, B.J.; McRoberts, J.P.; Rybak, M.J. A Novel Approach Utilizing Biofilm Time-Kill Curves To Assess the Bactericidal Activity of Ceftaroline Combinations against Biofilm-Producing Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 2989–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, M.J.; Hershberger, E.; Moldovan, T.; Grucz, R.G. In Vitro Activities of Daptomycin, Vancomycin, Linezolid, and Quinupristin-Dalfopristin against Staphylococci and Enterococci, Including Vancomycin- Intermediate and -Resistant Strains. Antimicrob. Agents Chemother. 2000, 44, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- O’Flynn, G.; Ross, R.P.; Fitzgerald, G.F.; Coffey, A. Evaluation of a Cocktail of Three Bacteriophages for Biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2004, 70, 3417–3424. [Google Scholar] [CrossRef] [Green Version]
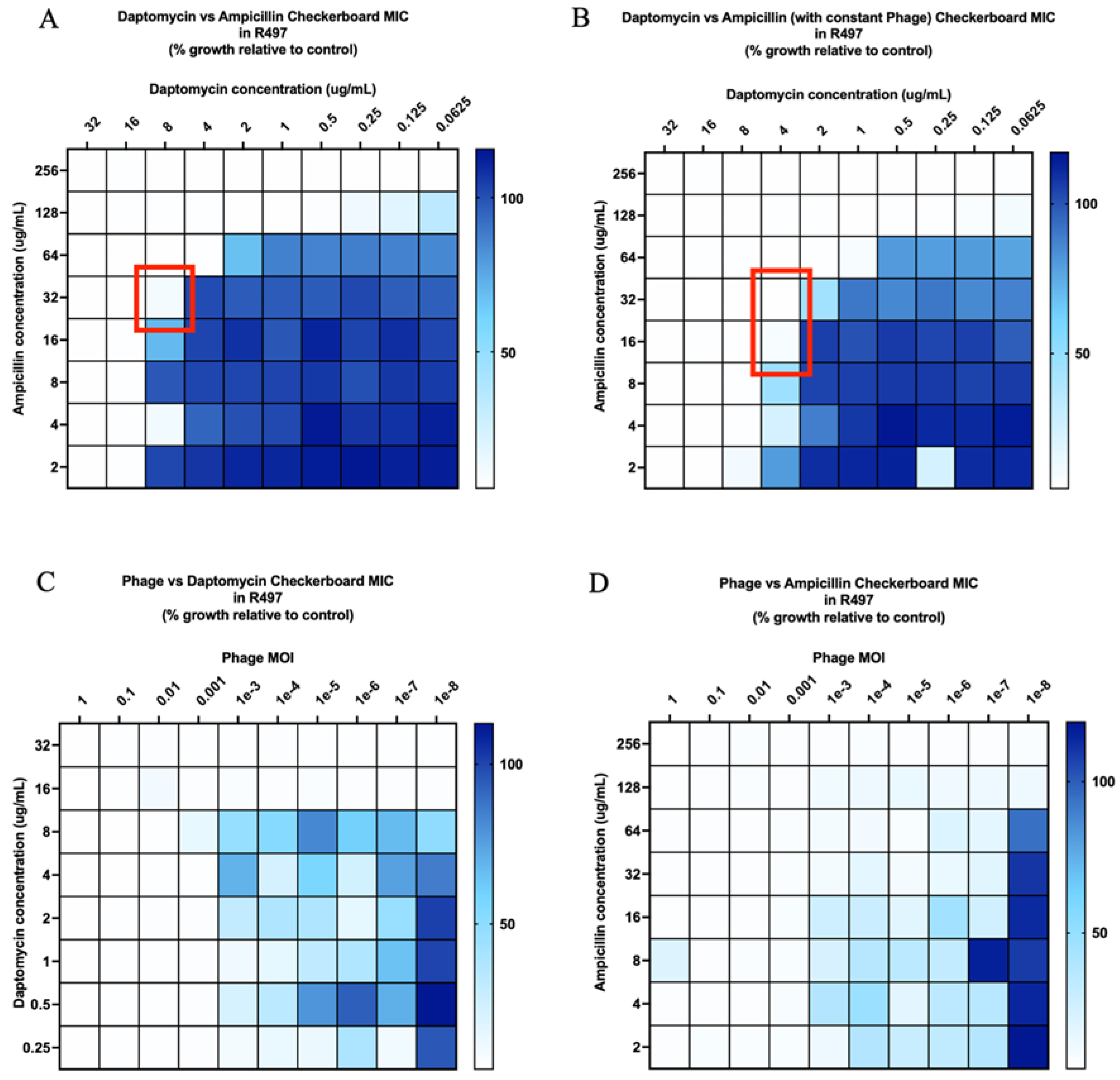
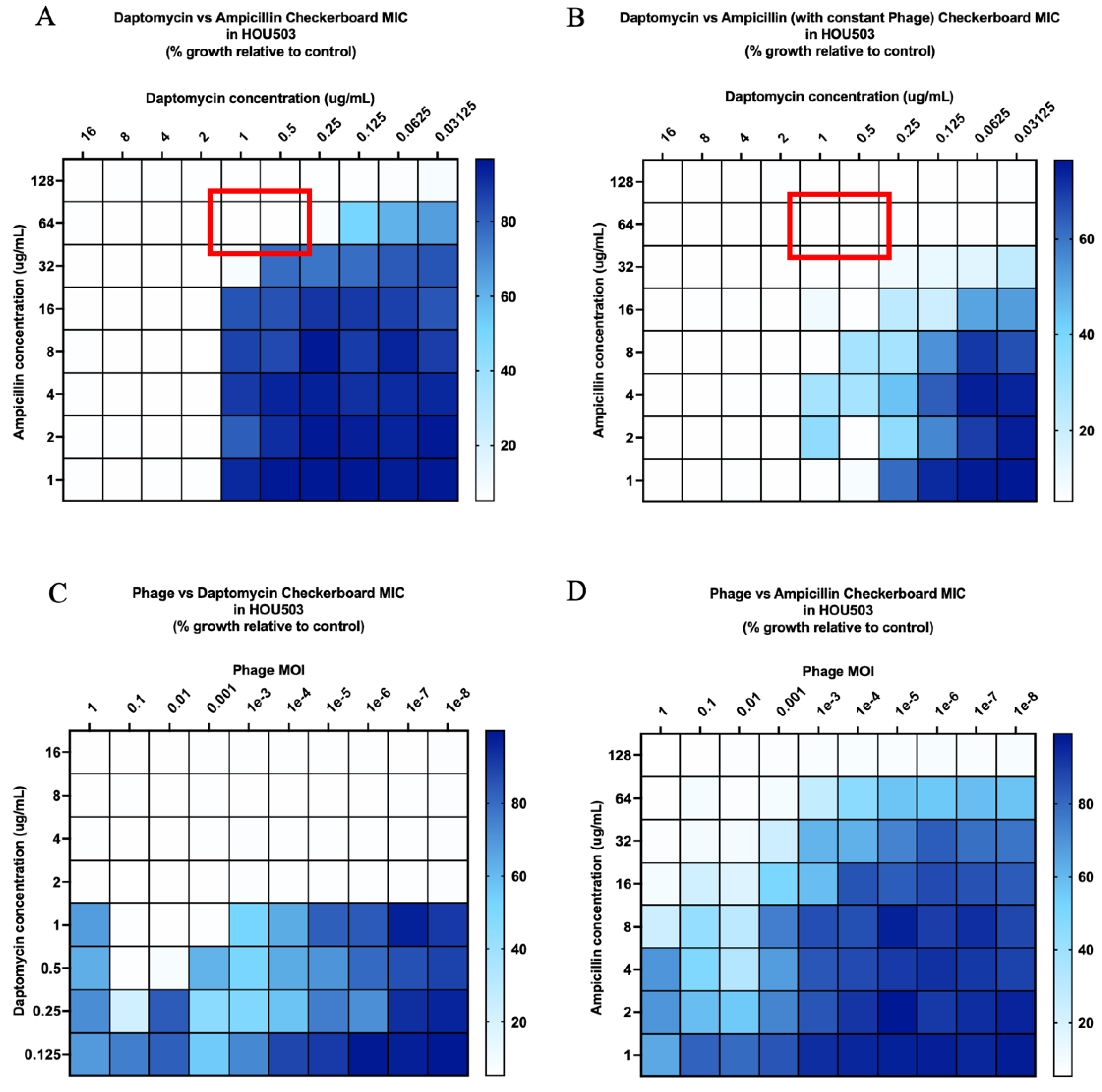

| Organism | Bacteriophage Susceptibility (PFU/mL Compared to Host) a | Biofilm Quantification (OD Compared to Control) b |
|---|---|---|
| R497 | High | Medium |
| HOU503 | Medium | High |
| S447 (55) | Low | None |
| SF12047 (56) | Low | None |
| 12311 (56) | Low | Low |
| Bacteriophage Resistance a | ||
|---|---|---|
| Regimen | R497 | HOU503 |
| Phage | R | R |
| Phage-DAP | S | R |
| Phage-AMP | S | R |
| Phage-CPT | S | R |
| Phage-ERT | R | R |
| Phage-DAP-AMP | S | S |
| Phage-DAP-CPT | S | S |
| Phage-DAP-ERT | S | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lev, K.; Kunz Coyne, A.J.; Kebriaei, R.; Morrisette, T.; Stamper, K.; Holger, D.J.; Canfield, G.S.; Duerkop, B.A.; Arias, C.A.; Rybak, M.J. Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus faecium. Antibiotics 2022, 11, 392. https://doi.org/10.3390/antibiotics11030392
Lev K, Kunz Coyne AJ, Kebriaei R, Morrisette T, Stamper K, Holger DJ, Canfield GS, Duerkop BA, Arias CA, Rybak MJ. Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus faecium. Antibiotics. 2022; 11(3):392. https://doi.org/10.3390/antibiotics11030392
Chicago/Turabian StyleLev, Katherine, Ashlan J. Kunz Coyne, Razieh Kebriaei, Taylor Morrisette, Kyle Stamper, Dana J. Holger, Gregory S. Canfield, Breck A. Duerkop, Cesar A. Arias, and Michael J. Rybak. 2022. "Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus faecium" Antibiotics 11, no. 3: 392. https://doi.org/10.3390/antibiotics11030392
APA StyleLev, K., Kunz Coyne, A. J., Kebriaei, R., Morrisette, T., Stamper, K., Holger, D. J., Canfield, G. S., Duerkop, B. A., Arias, C. A., & Rybak, M. J. (2022). Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus faecium. Antibiotics, 11(3), 392. https://doi.org/10.3390/antibiotics11030392






