Routine Postoperative Antibiotic Prophylaxis Offers No Benefit after Hepatectomy—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
- Population: patients undergoing liver resections
- Intervention: postoperative antibiotics beyond the first postoperative day (POD1) (POA)
- Comparison: no postoperative antibiotics beyond POD1 (control)
- Outcome: SSI (overall, superficial, and deep/organ), remote infections, sepsis, PHLF, bile leakage/bilioma, and microbiota changes
- Study design: comparative studies
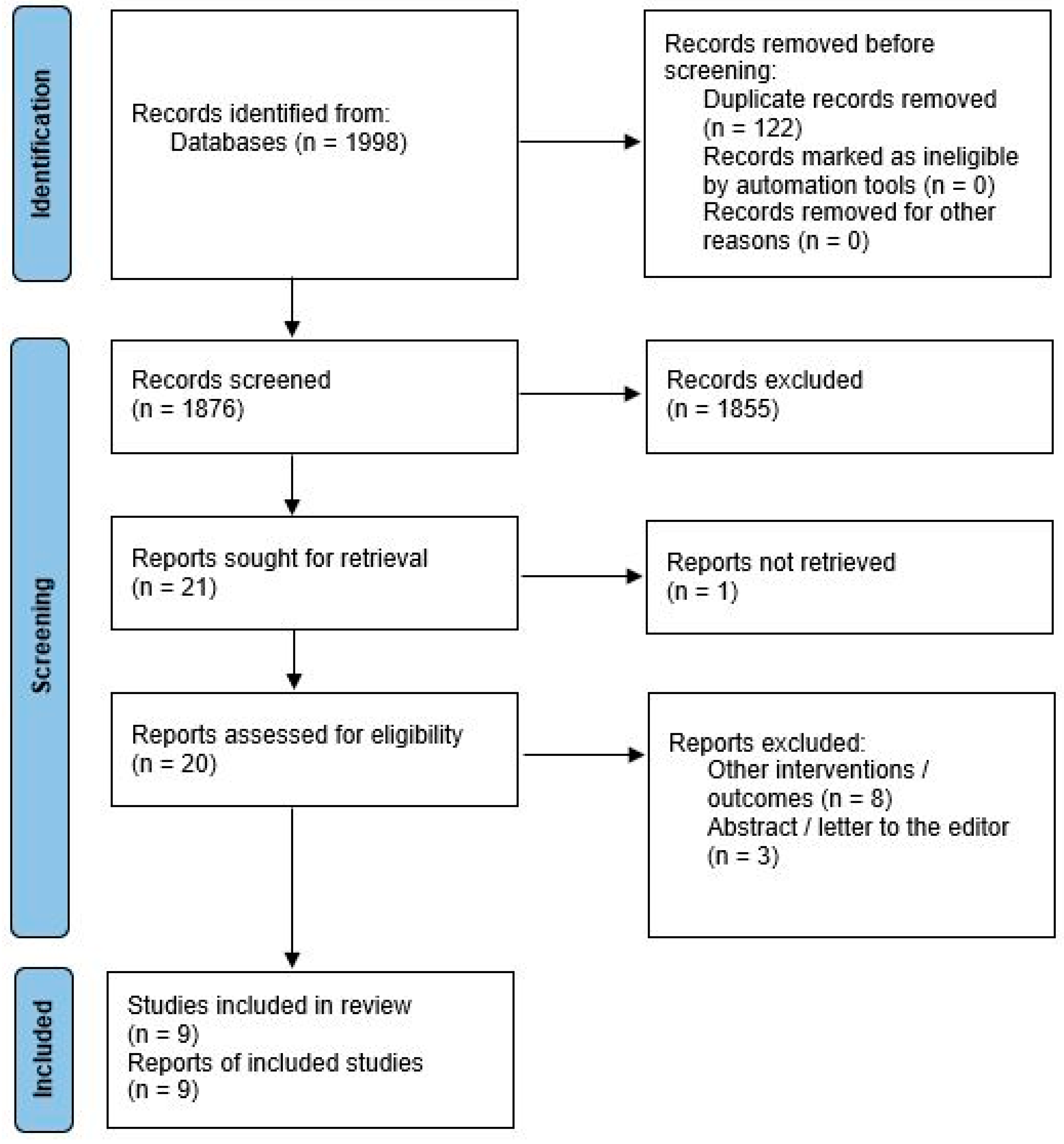
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Critical Appraisal of Included Studies
3. Results
3.1. Critical Appraisal of Included Studies
3.2. Patient Demographics
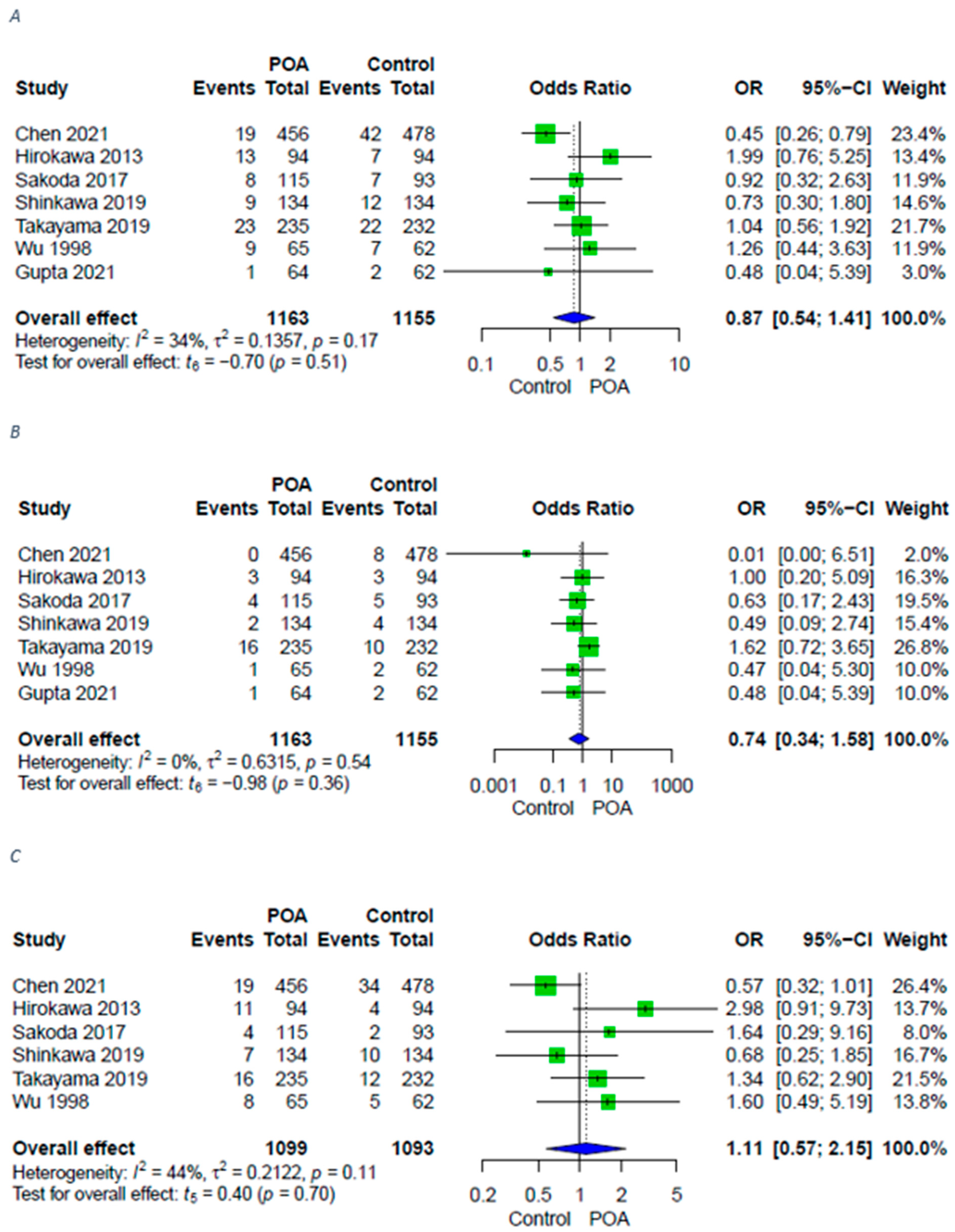
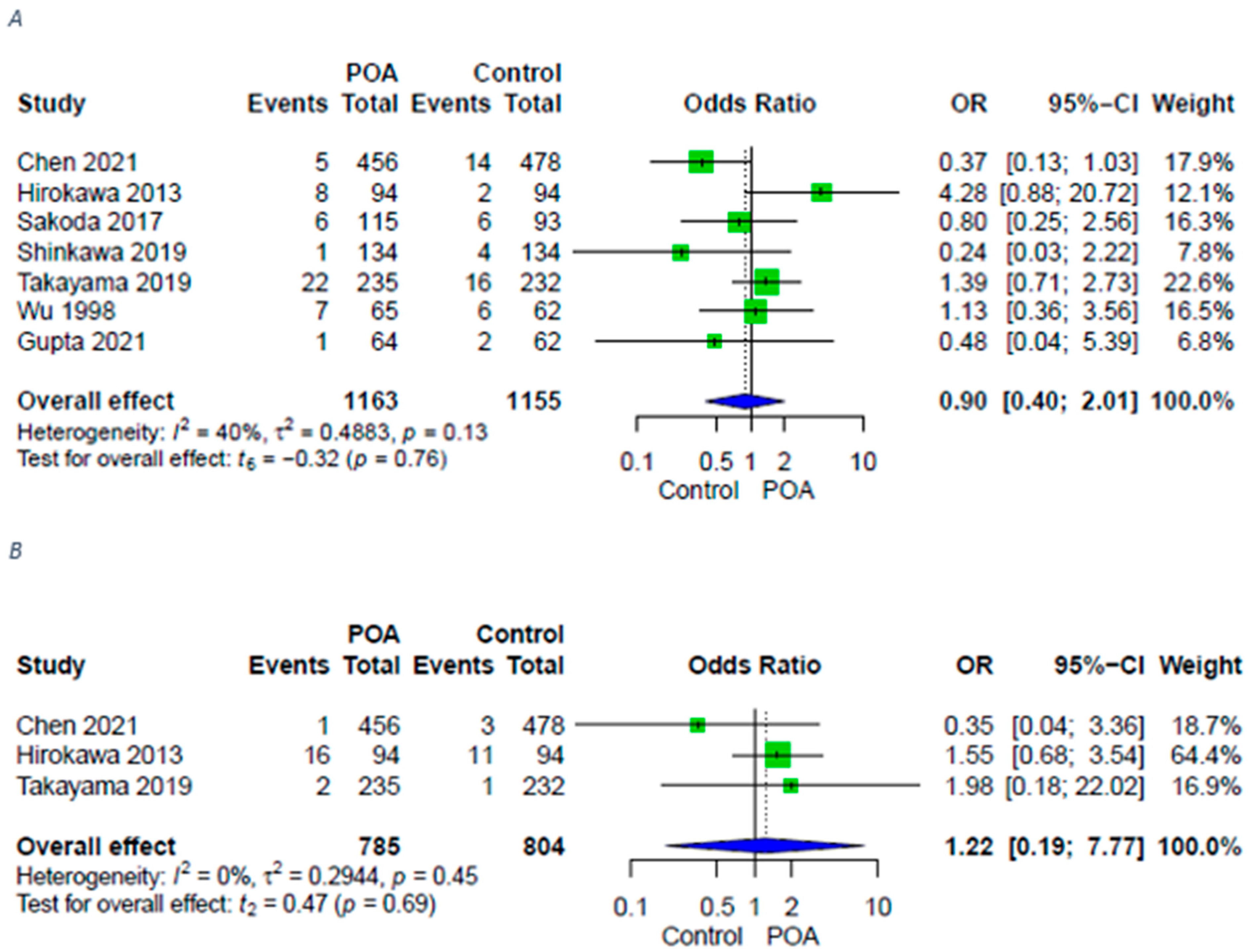
3.3. Infective Complications
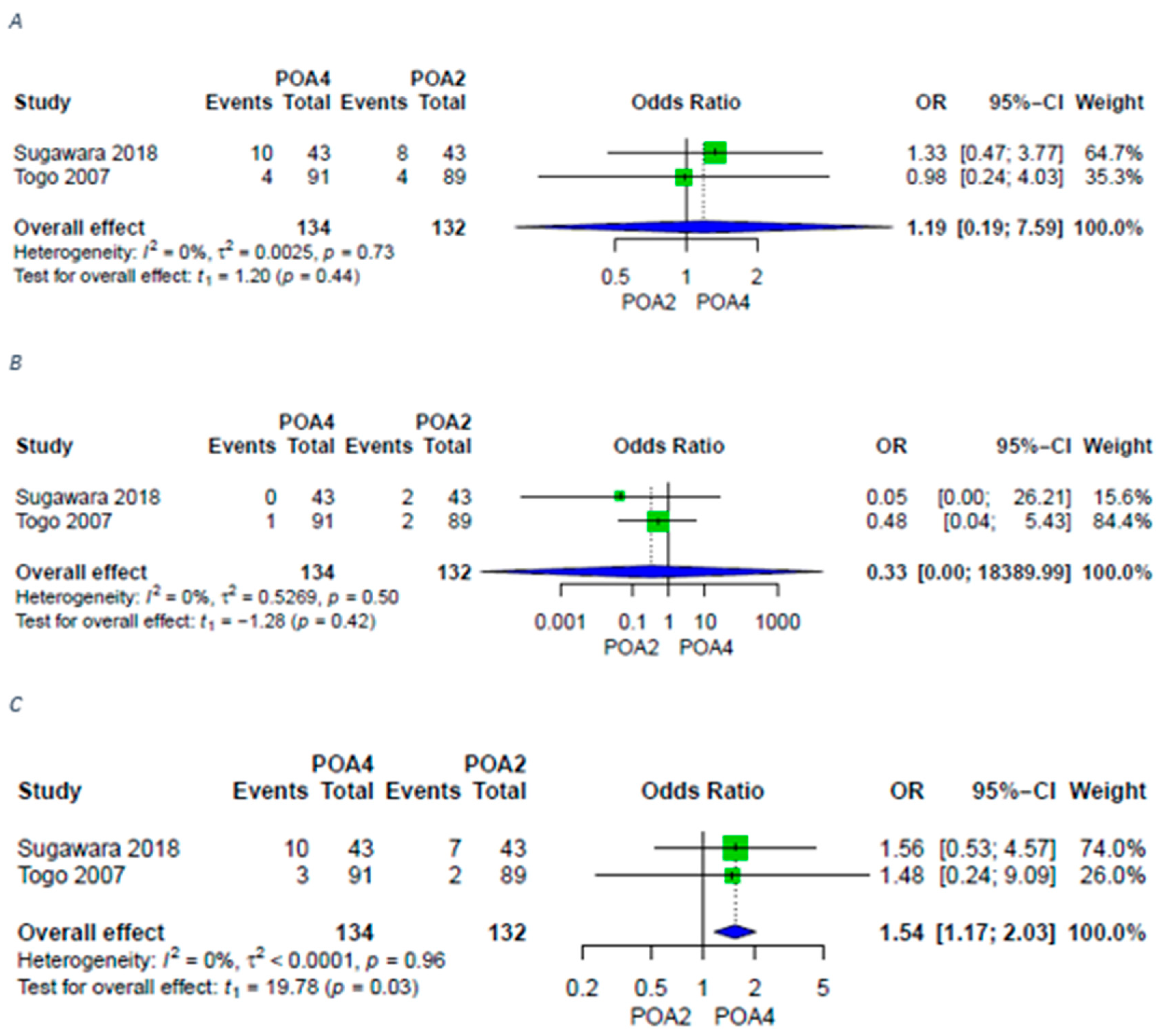
3.4. Liver-Specific Complications
3.5. Pathogenic Isolates
3.6. Certainty of Evidence
4. Discussion
Certainty of Evidence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HCC | Hepatocellular Carcinoma |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| OR | Odds Ratio |
| PHLF | Post-Hepatectomy Liver Failure |
| PICOS | Patients, Intervention, Comparison, Outcome, Study design |
| POA | Postoperative Antibiotics |
| POD | Postoperative Day |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| RCT | Randomized Controlled Trial |
| RoB2 | Risk of Bias |
| ROBINS-I | Risk of Bias in Non-randomized Studies of Interventions |
| SSI | Surgical Site Infections |
References
- Young, P.Y.; Khadaroo, R.G. Surgical Site Infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef]
- Cheadle, W.G. Risk factors for surgical site infection. Surg. Infect. 2006, 7 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Hao, J.; Li, Y.; Liu, A.; Wu, Y.; Cai, M. Impact of the Antibiotic Stewardship Program on Prevention and Control of Surgical Site Infection during Peri-Operative Clean Surgery. Surg. Infect. 2018, 19, 326–333. [Google Scholar] [CrossRef]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70 (Suppl. 2), 3–10. [Google Scholar] [CrossRef]
- Ahn, B.K.; Lee, K.H. Single-dose antibiotic prophylaxis is effective enough in colorectal surgery. ANZ J. Surg. 2013, 83, 641–645. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Siu, J.; McCall, J.; Connor, S. Systematic review of pathophysiological changes following hepatic resection. HPB 2014, 16, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Murtha-Lemekhova, A.; Teoerde, M.; Fuchs, J.; Hoffmann, K. Postoperative Antibiotics in Hepatectomy—A Systematic Review and Meta-Analysis. PROSPERO 2021. CRD42021288510. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021288510 (accessed on 9 May 2022).
- Kalkum, E.; Klotz, R.; Seide, S.; Hüttner, F.J.; Kowalewski, K.-F.; Nickel, F.; Khajeh, E.; Knebel, P.; Diener, M.K.; Probst, P. Systematic reviews in surgery-recommendations from the Study Center of the German Society of Surgery. Langenbecks Arch. Surg. 2021, 406, 1723–1731. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; Wang, Y.; Liang, R.; Xu, L.; Lai, J.; Shen, J.; Li, J.; Li, D.; Li, S.; et al. Three-day postoperative antibiotics reduces post-hepatectomy infection rate in hepatitis B virus-related hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021, 36, 2531–2539. [Google Scholar] [CrossRef]
- Gupta, S.; Sinha, P.K.; Patil, N.S.; Mohapatra, N.; Sindwani, G.; Garg, N.; Khillan, V.; Pamecha, V. Randomized control trial on perioperative antibiotic prophylaxis in live liver donors: Are three doses enough? J. Hepatobiliary Pancreat. Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K.; Nishimura, Y. Evaluation of postoperative antibiotic prophylaxis after liver resection: A randomized controlled trial. Am. J. Surg. 2013, 206, 8–15. [Google Scholar] [CrossRef]
- Sakoda, M.; Iino, S.; Mataki, Y.; Kawasaki, Y.; Kurahara, H.; Maemura, K.; Ueno, S.; Natsugoe, S. Influence of a Shorter Duration of Post-Operative Antibiotic Prophylaxis on Infectious Complications in Patients Undergoing Elective Liver Resection. Surg. Infect. 2017, 18, 149–156. [Google Scholar] [CrossRef]
- Shinkawa, H.; Tanaka, S.; Takemura, S.; Amano, R.; Kimura, K.; Nishioka, T.; Ito, T.; Miyazaki, T.; Ishihara, A.; Kubo, S. Giving short-term prophylactic antibiotics in patients undergoing open and laparoscopic hepatic resection. Ann. Gastroenterol. Surg. 2019, 3, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, G.; Yokoyama, Y.; Ebata, T.; Mizuno, T.; Yagi, T.; Ando, M.; Nagino, M. Duration of Antimicrobial Prophylaxis in Patients Undergoing Major Hepatectomy with Extrahepatic Bile Duct Resection: A Randomized Controlled Trial. Ann. Surg. 2018, 267, 142–148. [Google Scholar] [CrossRef]
- Takayama, T.; Aramaki, O.; Shibata, T.; Oka, M.; Itamoto, T.; Shimada, M.; Isaji, S.; Kanematsu, T.; Kubo, S.; Kusunoki, M.; et al. Antimicrobial prophylaxis for 1 day versus 3 days in liver cancer surgery: A randomized controlled non-inferiority trial. Surg. Today 2019, 49, 859–869. [Google Scholar] [CrossRef]
- Togo, S.; Tanaka, K.; Matsuo, K.; Nagano, Y.; Ueda, M.; Morioka, D.; Endo, I.; Shimada, H. Duration of antimicrobial prophylaxis in patients undergoing hepatectomy: A prospective randomized controlled trial using flomoxef. J. Antimicrob. Chemother. 2007, 59, 964–970. [Google Scholar] [CrossRef]
- Wu, C.C.; Yeh, D.C.; Lin, M.C.; Liu, T.J.; P’Eng, F.K. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br. J. Surg. 1998, 85, 489–493. [Google Scholar] [CrossRef]
- Hoffmann, K.; Bulut, S.; Tekbas, A.; Hinz, U.; Büchler, M.W.; Schemmer, P. Is Hepatic Resection for Non-colorectal, Non-neuroendocrine Liver Metastases Justified? Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S1083–S1092. [Google Scholar] [CrossRef]
- Murtha-Lemekhova, A.; Fuchs, J.; Schulz, E.; Sterkenburg, A.; Probst, P.; Hoffmann, K. Pushing the limit of liver regeneration—Safety and survival after monosegment-ALPPS: Systematic review and individual patient data meta-analysis. HPB 2021, 24, 353–358. [Google Scholar] [CrossRef]
- Cipriani, F.; Ratti, F.; Cardella, A.; Catena, M.; Paganelli, M.; Aldrighetti, L. Laparoscopic Versus Open Major Hepatectomy: Analysis of Clinical Outcomes and Cost Effectiveness in a High-Volume Center. J. Gastrointest. Surg. 2019, 23, 2163–2173. [Google Scholar] [CrossRef]
- Kimbrell, A.R.; Novosel, T.J.; Collins, J.N.; Weireter, L.J.; Terzian, H.W.; Adams, R.T.; Beydoun, H.A. Do postoperative antibiotics prevent abscess formation in complicated appendicitis? Am. Surg. 2014, 80, 878–883. [Google Scholar] [CrossRef]
- Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; Cousson, J.; et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial. Intensive Care Med. 2018, 44, 300–310. [Google Scholar] [CrossRef]
- Van Rossem, C.C.; Schreinemacher, M.H.; van Geloven, A.A.; Bemelman, W.A. Antibiotic Duration After Laparoscopic Appendectomy for Acute Complicated Appendicitis. JAMA Surg. 2016, 151, 323–329. [Google Scholar] [CrossRef]
- Guo, T.; Ding, R.; Yang, J.; Wu, P.; Liu, P.; Liu, Z.; Li, Z. Evaluation of different antibiotic prophylaxis strategies for hepatectomy: A network meta-analysis. Medicine 2019, 98, e16241. [Google Scholar] [CrossRef]
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef]

| Report | Study Design | Indications for Hepatectomy | Type of Hepatectomy | Method of Access | Number of Patients in Intervention Group | Number of Patients in Control Group | Duration of Intervention Regimen | Duration of Control Regimen | Antibiotic Investigated |
|---|---|---|---|---|---|---|---|---|---|
| Chen 2021 | Retrospective | HCC | Unspecified | Unspecified | 456 | 478 | Various | None | Cephalosporins |
| Hirokawa 2013 | RCT | Various | Major/Minor | Unspecified | 94 | 94 | 3 days | None | Flomoxef sodium |
| Sakoda 2017 | Retrospective | Various | Major/Minor | Open/Laparoscopic | 115 | 93 | 3 days | None | Cefotiam |
| Shinkawa 2019 | RetrospectiveSubgroups propensity score matched | Various | Major/Minor | Open/Laparoscopic | 75 | 173 | 3 days | None | Flomoxef sodium |
| Sugawara 2018 | RCT | Various | Major | Unspecified | 43 | 43 | 4 days | 2 days | Various |
| Takayama 2019 | RCT | HCC | Major/Minor | Open | 235 | 232 | 3 days | None | Flomoxef sodium |
| Togo 2007 | RCT | Various | Major/Minor | Unspecified | 91 | 89 | 5 days | 2 days | Flomoxef sodium |
| Wu 1998 | RCT | Various | Major/Minor | Unspecified | 65 | 62 | 7 days | None | Cephazolin/gentamicin |
| Gupta 2021 | RCT | Live liver donors | Major/Minor | Open/Laparoscopic | 64 | 62 | 9 doses | 3 doses | Piperacillin/Tazobactam |
| Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results | Overall | |
|---|---|---|---|---|---|---|
| Hirokawa 2013 [9] |  |  |  |  |  |  |
| Sugawara 2018 [14] |  |  |  |  |  |  |
| Takayama 2019 [12] |  |  |  |  |  |  |
| Togo 2007 [15] |  |  |  |  |  |  |
| Wu 1998 [13] |  |  |  |  |  |  |
| Gupta 2021 [8] |  |  |  |  |  |  |
 Low risk
Low risk  Some concern
Some concern  High risk.
High risk.| Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Results | Overall | |
|---|---|---|---|---|---|---|---|---|
| Chen 2021 [7] | ||||||||
| Sakoda 2017 [10] | ||||||||
| Shinkawa 2019 [11] |
| No AB | POAs | Level of Significance ** | |
|---|---|---|---|
| Age * | 63.4 ± 11.9 | 62.5 ± 13.4 | 0.519 |
| Gender | 0.130 | ||
| 580 | 485 | |
| 228 | 226 | |
| Indication | 0.726 | ||
| 782 | 809 | |
| 3 | 5 | |
| 48 | 40 | |
| 63 | 68 | |
| 10 | 14 | |
| Type of surgery | 0.215 | ||
| 231 | 226 | |
| 565 | 481 | |
| Mode of surgery | 0.274 | ||
| 512 | 454 | |
| 130 | 98 |
| Outcome | № of Included Studies | Certainty of the Evidence (GRADE) | Relative Effect(95% CI) |
|---|---|---|---|
| Surgical site infections | 7 (4 RCTs, 3 retrospective) | ⨁⨁⨁◯ Moderate | OR 0.87 [0.54; 1.41] |
| Superficial surgical site infections | 7 (4 RCTs, 3 retrospective) | ⨁⨁⨁◯ Moderate | OR 0.74 [0.34; 1.58] |
| Deep surgical site infections | 6 (3 RCTs, 3 retrospective) | ⨁⨁⨁◯ Moderate | OR 1.11 [0.57; 2.15] |
| Remote infections | 7 (4 RCTs, 3 retrospective) | ⨁⨁⨁◯ Moderate | OR 0.90 [0.40; 2.01] |
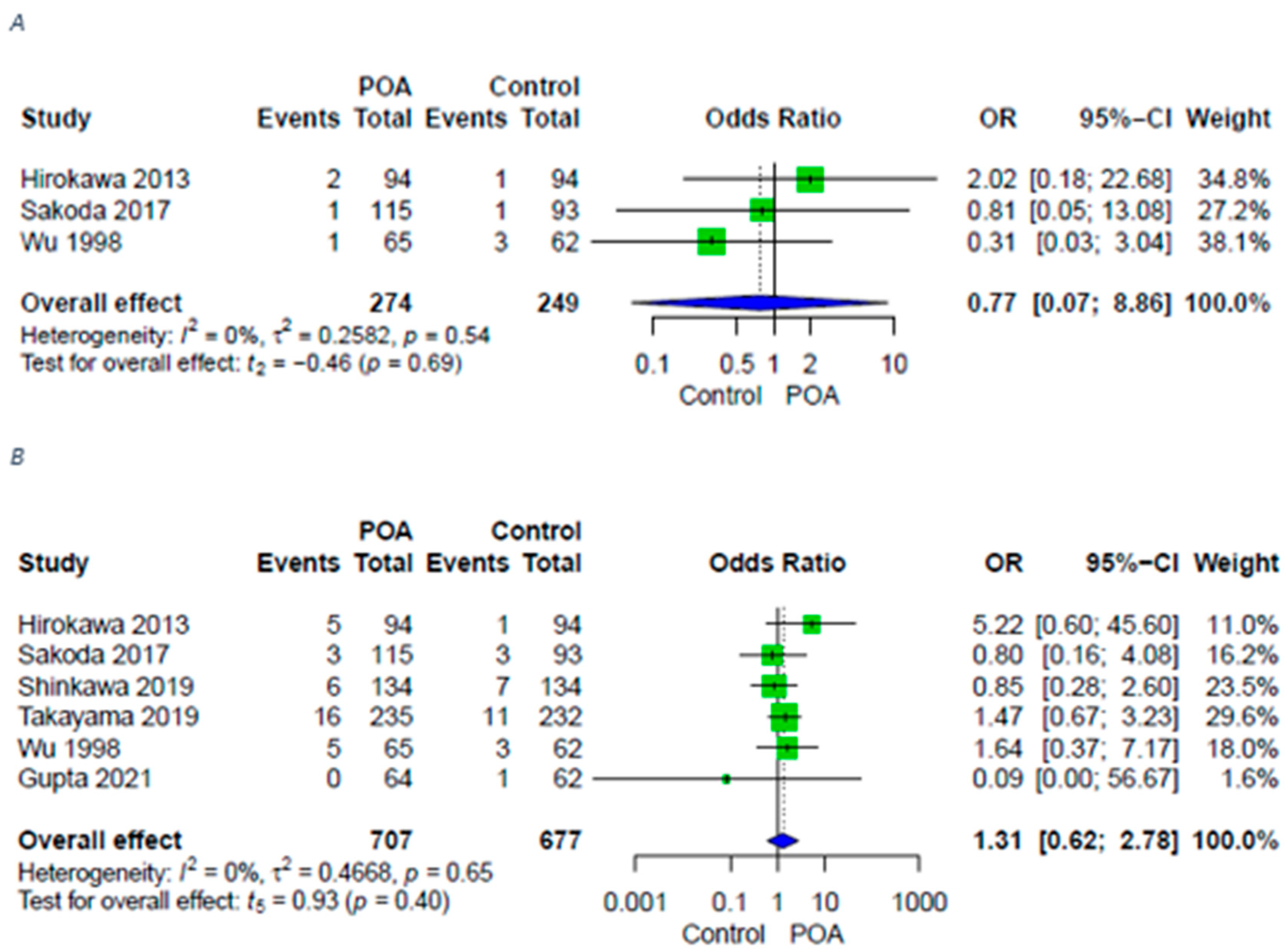
| Sepsis | 3 (2 RCTs, 1 retrospective) | ⨁⨁◯◯ LOW | OR 1.22 [0.19; 7.77] |
| PHLF | 3 (2 RCTs, 1 retrospective) | ⨁⨁◯◯ LOW | OR 0.77 [0.07; 8.86] |
| Bile leakage | 6 (4 RCTs 2 retrospective) | ⨁⨁⨁◯ Moderate | OR 1.31 [0.62; 2.78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtha-Lemekhova, A.; Fuchs, J.; Teroerde, M.; Chiriac, U.; Klotz, R.; Hornuss, D.; Larmann, J.; Weigand, M.A.; Hoffmann, K. Routine Postoperative Antibiotic Prophylaxis Offers No Benefit after Hepatectomy—A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 649. https://doi.org/10.3390/antibiotics11050649
Murtha-Lemekhova A, Fuchs J, Teroerde M, Chiriac U, Klotz R, Hornuss D, Larmann J, Weigand MA, Hoffmann K. Routine Postoperative Antibiotic Prophylaxis Offers No Benefit after Hepatectomy—A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(5):649. https://doi.org/10.3390/antibiotics11050649
Chicago/Turabian StyleMurtha-Lemekhova, Anastasia, Juri Fuchs, Miriam Teroerde, Ute Chiriac, Rosa Klotz, Daniel Hornuss, Jan Larmann, Markus A. Weigand, and Katrin Hoffmann. 2022. "Routine Postoperative Antibiotic Prophylaxis Offers No Benefit after Hepatectomy—A Systematic Review and Meta-Analysis" Antibiotics 11, no. 5: 649. https://doi.org/10.3390/antibiotics11050649
APA StyleMurtha-Lemekhova, A., Fuchs, J., Teroerde, M., Chiriac, U., Klotz, R., Hornuss, D., Larmann, J., Weigand, M. A., & Hoffmann, K. (2022). Routine Postoperative Antibiotic Prophylaxis Offers No Benefit after Hepatectomy—A Systematic Review and Meta-Analysis. Antibiotics, 11(5), 649. https://doi.org/10.3390/antibiotics11050649








