Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection and Study Variables
2.5. Data Management and Statistical Analysis
2.6. Ethical Consideration
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Antibiotics Usage According to Demographic and Clinical Characteristics
3.3. Antimicrobials Used
3.4. Cephalosporin Groups
3.5. Other Groups of Antibiotics in Different Departments
3.6. Number of Antibiotics Used for Treating Patients after Admission
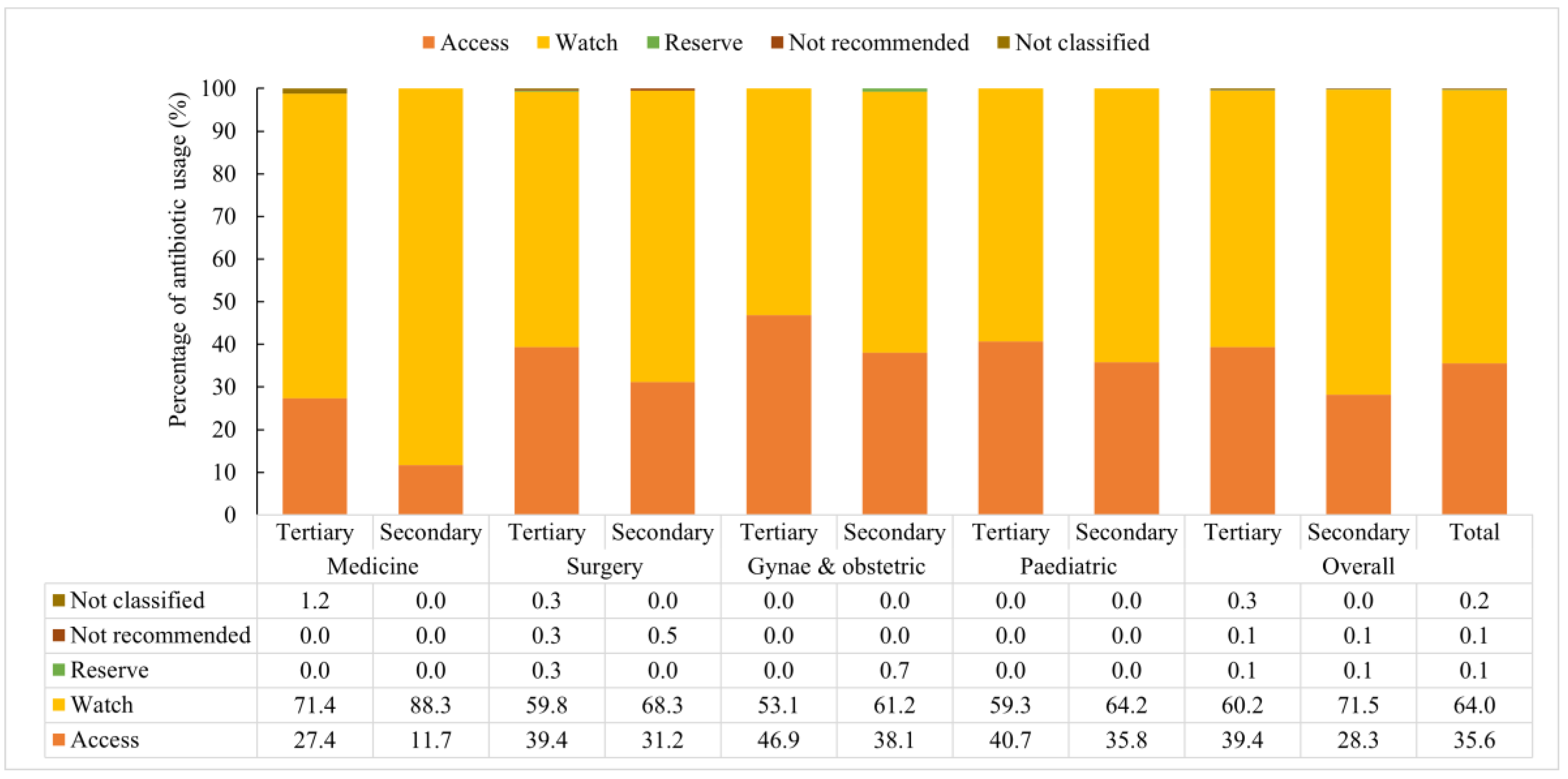
3.7. Antibiotics Used According to AWaRE Category
3.8. Assiciation between Antibiotics Use (According Aware Classification) and Characteristics of Patients and Hospitals
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Jansen, W.T.M.; Van der Bruggen, J.T.; Verhoef, J.; Fluit, A.C. Bacterial resistance: A sensitive issue Complexity of the challenge and containment strategy in Europe. Drug Resist. Updates 2006, 9, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Mark, S.; Smolinski, M.A.H.; Lederberg, J. (Eds.) Microbial Threats to Health: Emergence, Detection, and Response; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Singh, S.K.; Sengupta, S.; Antony, R.; Bhattacharya, S.; Mukhopadhyay, C.; Ramasubramanian, V.; Sharma, A.; Sahu, S.; Nirkhiwale, S.; Gupta, S.; et al. Variations in antibiotic use across India: Multi-centre study through Global Point Prevalence survey. J. Hosp. Infect. 2019, 103, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Ahmed, S.M.; Naher, N.; Islam, M.A.; Rousham, E.K.; Islam, B.Z.; Hassan, S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE 2020, 15, e0227947. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Korpe, P.; Ahmed, T.; Chisti, M.J.; Faruque, A.S.G. Burden and Risk Factors of Antimicrobial Use in Children Less Than 5 Years of Age with Diarrheal Illness in Rural Bangladesh. Am. J. Trop. Med. Hyg. 2018, 98, 1571–1576. [Google Scholar] [CrossRef]
- Biswas, M.; Roy, M.N.; Manik, M.I.N.; Hossain, M.S.; Tapu, S.T.A.; Moniruzzaman, M.; Sultana, S. Self medicated antibiotics in Bangladesh: A cross-sectional health survey conducted in the Rajshahi City. BMC Public Health 2014, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.E., Jr. Antimicrobial Resistance in Hospital Organisms and Its Relation to Antibiotic Use. Rev. Infect. Dis. 1983, 5, 1033–1048. [Google Scholar] [CrossRef]
- WHO. The Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/254352/sea-cd-308.pdf?sequence=1&isAllowed=y (accessed on 22 May 2022).
- WHO. WHO Access, Watch, Reserve (AWaRe) Classification of Antibiotics for Evaluation and Monitoring of Use. 2021. Available online: https://apps.who.int/iris/rest/bitstreams/1374989/retrieve (accessed on 22 May 2022).
- Hillock, N.T.; Connor, E.; Wilson, C.; Kennedy, B. Comparative analysis of Australian hospital antimicrobial utilization, using the WHO AWaRe classification system and the adapted Australian Priority Antimicrobial List (PAL). JAC Antimicrob. Resist. 2021, 3, dlab017. [Google Scholar] [CrossRef]
- Mugada, V.; Mahato, V.; Andhavaram, D.; Vajhala, S.M. Evaluation of Prescribing Patterns of Antibiotics Using Selected Indicators for Antimicrobial Use in Hospitals and the Access, Watch, Reserve (AWaRe) Classification by the World Health Organization. Turk. J. Pharm. Sci 2021, 18, 282–288. [Google Scholar] [CrossRef]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629. [Google Scholar] [CrossRef] [Green Version]
- International Experts Join Forces against Superbugs at the 4th World Forum on Healthcare-Associated Infections and Antimicrobial Resistance. 2013. Available online: https://www.biomerieux.com/content/dam/biomerieux-com/investor/regulated-informations/2013/releases/pr_biomerieux_world_hai_forum_20130627_en.pdf.coredownload.pdf (accessed on 22 May 2022).
- Goossens, H.; Nathwani, D. Global Point Prevalence Survey of Antimicrobial Consumption and Resistance. 2019. Available online: https://www.global-pps.com/wp-content/uploads/2019/02/Global-PPS-2019-protocol.pdf (accessed on 22 May 2022).
- WHO. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals, Version 1.1. 2018. Available online: https://apps.who.int/iris/rest/bitstreams/1175969/retrieve (accessed on 22 May 2022).
- Le, N.K.; Hf, W.; Vu, P.D.; Khu, D.T.K.; Le, H.T.; Hoang, B.T.N.; Vo, V.T.; Lam, Y.M.; Vu, D.T.V.; Nguyen, T.H.; et al. High prevalence of hospital-acquired infections caused by gram-negative carbapenem resistant strains in Vietnamese pediatric ICUs: A multi-centre point prevalence survey. Medicine 2016, 95, e4099. [Google Scholar] [CrossRef] [PubMed]
- Munckhof, W. Antibiotics for surgical prophylaxis. Aust. Prescr. 2005, 28, 38–40. [Google Scholar] [CrossRef] [Green Version]
- Management Information System. HEALTH BULLETIN 2019. 2020. Available online: https://old.dghs.gov.bd/images/docs/Publicaations/Health%20Bulletin%202019%20Print%20Version%20(2)-Final.pdf (accessed on 22 May 2022).
- Panditrao, A.M.; Shafiq, N.; Chatterjee, S.; Pathak, A.; Trivedi, N.; Sadasivam, B.; Kshirsagar, N.; Kaul, R.; Biswal, M.; Kakkar, A.; et al. A multicentre point prevalence survey (PPS) of antimicrobial use amongst admitted patients in tertiary care centres in India. J. Antimicrob. Chemother. 2021, 76, 1094–1101. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H.; Global, P.P.S.n. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef]
- Labi, A.K.; Obeng-Nkrumah, N.; Nartey, E.T.; Bjerrum, S.; Adu-Aryee, N.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Antibiotic use in a tertiary healthcare facility in Ghana: A point prevalence survey. Antimicrob. Resist. Infect. Control 2018, 7, 15. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Oyawole, M.R.; Odunuga, P.T.; Kalejaye, F.; Yinka-Ogunleye, A.F.; Olalekan, A.; Ogundele, S.O.; Ebruke, B.E.; Kalada Richard, A.; Anand Paramadhas, B.D.; et al. A multicentre point prevalence study of antibiotics utilization in hospitalized patients in an urban secondary and a tertiary healthcare facilities in Nigeria: Findings and implications. Expert Rev. Anti-Infect. Ther. 2022, 20, 297–306. [Google Scholar] [CrossRef]
- Anand Paramadhas, B.D.; Tiroyakgosi, C.; Mpinda-Joseph, P.; Morokotso, M.; Matome, M.; Sinkala, F.; Gaolebe, M.; Malone, B.; Molosiwa, E.; Shanmugam, M.G.; et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev. Anti-Infect. Ther. 2019, 17, 535–546. [Google Scholar] [CrossRef]
- Saleem, Z.; Hassali, M.A.; Versporten, A.; Godman, B.; Hashmi, F.K.; Goossens, H.; Saleem, F. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: Findings and implications. Expert Rev. Anti-Infect. Ther. 2019, 17, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Boone, K.; Morris, S.K.; Doshi, S.; Black, J.; Mohsin, M.; Ahmed, T.; Al Mahmud, A.; Roth, D.E. Antimicrobial Prescribing during Infant Hospital Admissions in a Birth Cohort in Dhaka, Bangladesh. J. Trop. Pediatr. 2021, 67, fmaa093. [Google Scholar] [CrossRef]
- Nguyen, N.V.; Do, N.T.T.; Nguyen, C.T.K.; Tran, T.K.; Ho, P.D.; Nguyen, H.H.; Vu, H.T.L.; Wertheim, H.F.L.; van Doorn, H.R.; Lewycka, S. Community-level consumption of antibiotics according to the AWaRe (Access, Watch, Reserve) classification in rural Vietnam. JAC Antimicrob. Resist. 2020, 2, dlaa048. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2019 to 2020. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1069632/espaur-report-2020-to-2021-16-Nov-FINAL-v2.pdf (accessed on 22 May 2022).
- Akhtar, Z.; Mah-E-Muneer, S.; Islam, M.A.; Chowdhury, S.; Rashid, M.M.; Ahmed, M.S.; Khan, Z.; Hassan, M.Z.; Parveen, S.; Rahman, N.D.M.; et al. Antibiotics Use and Its Knowledge in the Community: A Mobile Phone Survey during the COVID-19 Pandemic in Bangladesh. Antibiotics 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Mah-E-Muneer, S.; Hassan, M.Z.; Biswas, M.A.A.J.; Rahman, F.; Akhtar, Z.; Das, P.; Islam, M.A.; Chowdhury, F. Use of Antimicrobials among Suspected COVID-19 Patients at Selected Hospitals, Bangladesh: Findings from the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 738. [Google Scholar] [CrossRef] [PubMed]
- Desk, Tribune. ‘No Mask, No Service’. Dhaka Tribune. 2020. Available online: https://archive.dhakatribune.com/bangladesh/government-affairs/2020/10/31/no-mask-no-entry (accessed on 22 May 2022).
- Ioannou, P.; Karakonstantis, S.; Schouten, J.; Kostyanev, T.; Charani, E.; Vlahovic-Palcevski, V.; Kofteridis, D.P. Indications for medical antibiotic prophylaxis and potential targets for antimicrobial stewardship intervention: A narrative review. Clin. Microbiol. Infect. 2022, 28, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Crader, M.F.; Varacallo, M. Preoperative Antibiotic Prophylaxis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ierano, C.; Nankervis, J.M.; James, R.; Rajkhowa, A.; Peel, T.; Thursky, K. Surgical antimicrobial prophylaxis. Aust. Prescr. 2017, 40, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaser, M.; Aljabri, A.K.; Alsaadi, F.N.; Rizk, L.M.; Alahmadi, R.Y.; Aljuhani, S.R.; Aljohani, S.H.; Al Thagfan, S.S.; Alamuddin, W.A.; Alonazie, W.S.; et al. A prospective antibiotic point prevalence survey in two primary referral hospitals during and after pilgrims stay in Madinah, Saudi Arabia. Trop. J. Pharm. Res. 2020, 19, 391–399. [Google Scholar] [CrossRef]
- Segala, F.V.; Murri, R.; Taddei, E.; Giovannenze, F.; Del Vecchio, P.; Birocchi, E.; Taccari, F.; Cauda, R.; Fantoni, M. Antibiotic appropriateness and adherence to local guidelines in perioperative prophylaxis: Results from an antimicrobial stewardship intervention. Antimicrob. Resist. Infect. Control 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Larramendy, S.; Gaultier, A.; Fournier, J.P.; Caillon, J.; Moret, L.; Beaudeau, F. Local characteristics associated with higher prevalence of ESBL-producing Escherichia coli in community-acquired urinary tract infections: An observational, cross-sectional study. J. Antimicrob. Chemother. 2021, 76, 789–795. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [Green Version]
- Crump, J.A.; Sjolund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [Green Version]
- Robert, W.; Frenck, J.A.M.; Nakhla, I.; Sultan, Y.; Putnam, S.; Wierzba, T.; Knirsch, M.M.C. Short-Course Azithromycin for the Treatment of Uncomplicated Typhoid Fever in Children and Adolescents. Clin. Infect. Dis. 2004, 38, 951–957. [Google Scholar]
- Lubell, Y.; Turner, P.; Ashley, E.A.; White, N.J. Susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asia to macrolide antibiotics. Trop. Med. Int. Health 2011, 16, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- WHO. In the Face of Slow Progress, WHO Offers a New Tool and Sets a Target to Accelerate Action against Antimicrobial Resistance. 2019. Available online: https://www.who.int/news/item/18-06-2019-in-the-face-of-slow-progress-who-offers-a-new-tool-and-sets-a-target-to-accelerate-action-against-antimicrobial-resistance (accessed on 22 May 2022).
- WHO. The Selection and Use of Essential Medicines. 2017. Available online: https://www.who.int/publications/i/item/9789241210157 (accessed on 22 May 2022).
- Budd, E.; Cramp, E.; Sharland, M.; Hand, K.; Howard, P.; Wilson, P.; Wilcox, M.; Muller-Pebody, B.; Hopkins, S. Adaptation of the WHO Essential Medicines List for national antibiotic stewardship policy in England: Being AWaRe. J. Antimicrob. Chemother. 2019, 74, 3384–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | Tertiary Level Hospitals (N = 892) | Secondary Level Hospitals (N = 525) | Overall (N = 1417) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sex | |||
| Female | 455 (51.0) | 276 (52.6) | 731 (51.6) |
| Male | 437 (48.9) | 249 (47.43) | 686 (48.4) |
| Age group | |||
| 0–14 years age group | 251 (28.1) | 148 (28.2) | 399 (28.2) |
| 15–64 years age group | 561 (62.9) | 326 (62.1) | 887 (62.6) |
| 65 years and above | 80 (9.0) | 51 (9.7) | 131 (9.2) |
| Mean age ± SD | 31.2 (23.1) | 30.9 (23.5) | 31.1 (23.3) |
| Education level | |||
| No education | 453 (50.8) | 270 (51.4) | 723 (51.0) |
| Primary | 181 (20.3) | 80 (15.2) | 261 (18.4) |
| Secondary | 176 (19.7) | 116 (22.1) | 292 (20.6) |
| Higher | 82 (9.2) | 59 (11.2) | 141 (10.0) |
| Patient transfer from another hospital | 218 (24.4) | 39 (7.4) | 257 (18.1) |
| Patient hospitalized in last 3 months | 138 (15.5) | 63 (12.0) | 201 (14.2) |
| Invasive device used | |||
| Central vascular catheter | 11 (1.2) | 4 (1.0) | 15 (1.1) |
| Peripheral vascular catheter | 649 (73.0) | 398 (75.8) | 1047 (73.9) |
| Urinary catheter | 103 (11.6) | 27 (5.1) | 130 (9.2) |
| Intubation tube | 4 (0.5) | 1 (0.2) | 5 (0.4) |
| Had surgery after admission | 165 (18.5) | 57 (10.9) | 222 (15.7) |
| Patients on antimicrobials | 716 (80.3) | 441 (84.0) | 1157 (81.7) |
| Patients on antibiotics | 669 (75.0) | 430 (81.9) | 1099 (77.6) |
| Average number of antimicrobials used among patients since admission | |||
| Mean (±SD) | 1.9 (±1.06) | 1.6 (±0.80) | 1.8 (±0.98) |
| Average number of antibiotics used among patients since admission | |||
| Mean (±SD) | 1.3 (±1.56) | 1.2 (±0.46) | 1.2 (±0.53) |
| Indications for antimicrobial used (n = 1099) | |||
| Medical prophylaxis | 317 (44.3) | 235 (53.3) | 552 (47.7) |
| Community-acquired infection | 234 (32.7) | 134 (30.4) | 368 (31.8) |
| Hospital-acquired infection | 5 (0.7) | 4 (0.9) | 9 (0.8) |
| Surgical prophylaxis | 191 (26.7) | 73 (16.6) | 264 (22.8) |
| Dose and duration of antibiotics used as surgical prophylaxis (SP) | |||
| One dose on 1 day/multiple days (SP1) | 5 (2.6) | 2 (2.7) | 7 (2.7) |
| Multiple doses on 1 day (SP2) | 3 (1.6) | 9 (12.3) | 12 (4.6) |
| Multiple doses > 1 day (SP3) | 183 (95.8) | 62 (84.9) | 245 (92.8) |
| Characteristics | Antibiotic Use in Tertiary Level Hospitals (N = 892) n (%) | Antibiotic Use in Secondary Level Hospitals (N = 525) n (%) | p-Value | Overall Antibiotic Use (N = 1417) n (%) |
|---|---|---|---|---|
| Sex of patients | ||||
| Female | 338 (74.3) | 227 (82.3) | 0.013 | 565 (77.3) |
| Male | 331 (75.7) | 203 (81.5) | 0.080 | 534 (77.8) |
| Age group | ||||
| 0–14 years | 214 (85.3) | 145 (98.0) | 0.000 | 359 (90.0) |
| 15–64 years | 407 (72.6) | 248 (76.1) | 0.249 | 655 (73.8) |
| 65 years and above | 48 (60.0) | 37 (72.6) | 0.142 | 85 (64.9) |
| Departments | ||||
| Medicine | 168 (62.5) | 145 (71.1) | 0.050 | 313 (66.2) |
| Surgery | 189 (75.0) | 123 (83.1) | 0.059 | 312 (78.0) |
| Gynae and Obstetrics | 118 (78.7) | 66 (86.8) | 0.136 | 184 (81.4) |
| Pediatrics | 194 (87.8) | 96 (99.0) | 0.001 | 290 (91.2) |
| Had surgery after admission * | 161 (97.6) | 57 (100.0) | 0.236 | 218 (98.2) |
| Use of Devices | ||||
| Peripheral vascular catheter | 545 (84.0) | 340 (85.4) | 0.528 | 885 (84.5) |
| Urinary catheter | 90 (87.4) | 23 (85.2) | 0.763 | 113 (86.9) |
| Central vascular catheter | 7 (63.6) | 4 (100.0) | 0.159 | 11 (73.3) |
| Intubation device | 4 (100.0) | 0 (0.0) | 0.025 | 4 (80.0) |
| History of transfer or hospitalization | ||||
| Patients transferred from another hospital | 180 (82.6) | 35 (89.7) | 0.264 | 215 (83.7) |
| Hospitalization within the last 90 days | 97 (70.3) | 47 (74.6) | 0.529 | 144 (71.6) |
| Indication for antimicrobial use | ||||
| Medical Prophylaxis | 293 (92.4) | 223 (94.9) | 0.246 | 516 (93.5) |
| Community-Acquired Infections | 213 (91.0) | 130 (97.0) | 0.028 | 343 (93.2) |
| Surgical Prophylaxis | 189 (99.0) | 73 (100.0) | 0.380 | 262 (99.2) |
| Hospital-Acquired Infections | 5 (100.0) | 4 (100.0) | 9 (100.0) |
| Antimicrobials Used | WHO AWaRe Classification | Number of Antimicrobial Agents Used According to Different Departments | Number of Antimicrobial Agents Used According to the Types of Hospitals | Overall Antimicrobial Agents Used (N = 2138) | ||||
|---|---|---|---|---|---|---|---|---|
| Medicine (N = 463) | Surgery (N = 611) | Gynae and Obstetrics (N = 479) | Pediatrics (N = 585) | Tertiary Level Hospitals (N = 1416) | Secondary Level Hospitals (N = 722) | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Antibiotics (In total) | 455 (98.3) | 607 (99.3) | 478 (99.8) | 572 (97.8) | 1397 (98.7) | 715 (99.0) | 2112 (98.8) | |
| Cephalosporins Group (In total) | 241 (52.1) | 347 (56.8) | 213 (44.5) | 285 (48.7) | 693 (48.9) | 393 (54.4) | 1086 (51.4) | |
| 1st-generation cephalosporins | Access | 0 (0.0) | 0 (0.0) | 23 (4.8) | 0 (0.0) | 23 (1.6) | 0 (0.0) | 23 (1.1) |
| 2nd-geneneration cephalosporins | Watch | 22 (4.8) | 37 (6.1) | 31 (6.5) | 3 (0.5) | 45 (3.2) | 48 (6.6) | 93 (4.3) |
| 3rd-geneneration cephalosporins | Watch | 217 (46.9) | 304 (49.8) | 158 (33.0) | 275 (47.0) | 622 (43.9) | 332 (46.0) | 954 (44.6) |
| 4th-geneneration cephalosporins | Watch | 2 (0.4) | 4 (0.7) | 1 (0.2) | 7 (1.2) | 2 (0.1) | 12 (1.7) | 14 (0.7) |
| 2nd-geneneration cephalosporins + beta lactamase inhibitors | Not recommended | 0 (0.0) | 2 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Imidazoles | Access | 34 (7.3) | 86 (14.1) | 121 (25.3) | 12 (2.1) | 172 (12.1) | 81 (11.2) | 253 (11.8) |
| Penicillins | Access | 11 (2.4) | 116 (19.0) | 56 (11.7) | 80 (13.7) | 167 (11.8) | 96 (13.3) | 263 (12.3) |
| Aminoglycosides | Access | 2 (0.4) | 13 (2.1) | 7 (1.5) | 132 (22.6) | 137 (9.7) | 17 (2.4) | 154 (7.2) |
| Macrolides | Watch | 82 (17.7) | 3 (0.5) | 31 (6.5) | 8 (1.4) | 60 (4.2) | 64 (8.9) | 124 (5.8) |
| Fluoroquinolones | Watch | 25 (5.4) | 23 (3.8) | 40 (8.4) | 8 (1.4) | 53 (3.7) | 43 (6.0) | 96 (4.5) |
| Carbapenems | Watch | 9 (1.9) | 10 (1.6) | 4 (0.8) | 41 (7.0) | 52 (3.7) | 12 (1.7) | 64 (3.0) |
| Beta lactam-beta lactamase inhibitors | Access | 41 (8.9) | 2 (0.3) | 1 (0.2) | 1 (0.2) | 42 (3.0) | 3 (0.4) | 45 (2.1) |
| Glycopeptides | Watch | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.9) | 5 (0.4) | 0 (0.0) | 5 (0.2) |
| Lincosamide | Access | 2 (0.4) | 3 (0.5) | 4 (0.8) | 0 (0.0) | 5 (0.4) | 4 (0.6) | 9 (0.4) |
| Furadantin | Access | 3 (0.6) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 1 (0.1) | 4 (0.2) |
| Anti-tubercular agents | Not- classified | 3 (0.6) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 4 (0.3) | 0 (0.0) | 4 (0.2) |
| Rifamycins | Watch | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Oxazolidinones | Reserve | 0 (0.0) | 1 (0.2) | 1 (0.2) | 0 (0.0) | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Trimethoprim-sulfonamide combinations | Access | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (0.0) |
| Antivirals | Non-Antibiotics | 6 (1.3) | 2 (0.3) | 1 (0.2) | 10 (1.7) | 15 (1.1) | 4 (0.6) | 19 (0.9) |
| Antifungals | Non-Antibiotics | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Antiparasitics | Non-Antibiotics | 2 (0.4) | 0 (0.2) | 0 (0.0) | 2 (0.3) | 1 (0.1) | 3 (0.4) | 5 (0.2) |
| Total | 463 (100.0) | 611 (100.0) | 479 (100.0) | 585 (100.0) | 1416 (100.0) | 722 (100.0) | 2138 (100.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.M.; Akhtar, Z.; Chowdhury, S.; Islam, M.A.; Parveen, S.; Ghosh, P.K.; Rahman, A.; Khan, Z.H.; Islam, K.; Debnath, N.; et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics 2022, 11, 810. https://doi.org/10.3390/antibiotics11060810
Rashid MM, Akhtar Z, Chowdhury S, Islam MA, Parveen S, Ghosh PK, Rahman A, Khan ZH, Islam K, Debnath N, et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics. 2022; 11(6):810. https://doi.org/10.3390/antibiotics11060810
Chicago/Turabian StyleRashid, Md. Mahbubur, Zubair Akhtar, Sukanta Chowdhury, Md. Ariful Islam, Shahana Parveen, Probir Kumar Ghosh, Aninda Rahman, Zobaidul Haque Khan, Khaleda Islam, Nitish Debnath, and et al. 2022. "Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh" Antibiotics 11, no. 6: 810. https://doi.org/10.3390/antibiotics11060810
APA StyleRashid, M. M., Akhtar, Z., Chowdhury, S., Islam, M. A., Parveen, S., Ghosh, P. K., Rahman, A., Khan, Z. H., Islam, K., Debnath, N., Rahman, M., & Chowdhury, F. (2022). Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics, 11(6), 810. https://doi.org/10.3390/antibiotics11060810







