Bacterial Co-Infection in Patients with COVID-19 Hospitalized (ICU and Not ICU): Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Result of the Study Identification Process
2.2. Overall Co-Infections Result
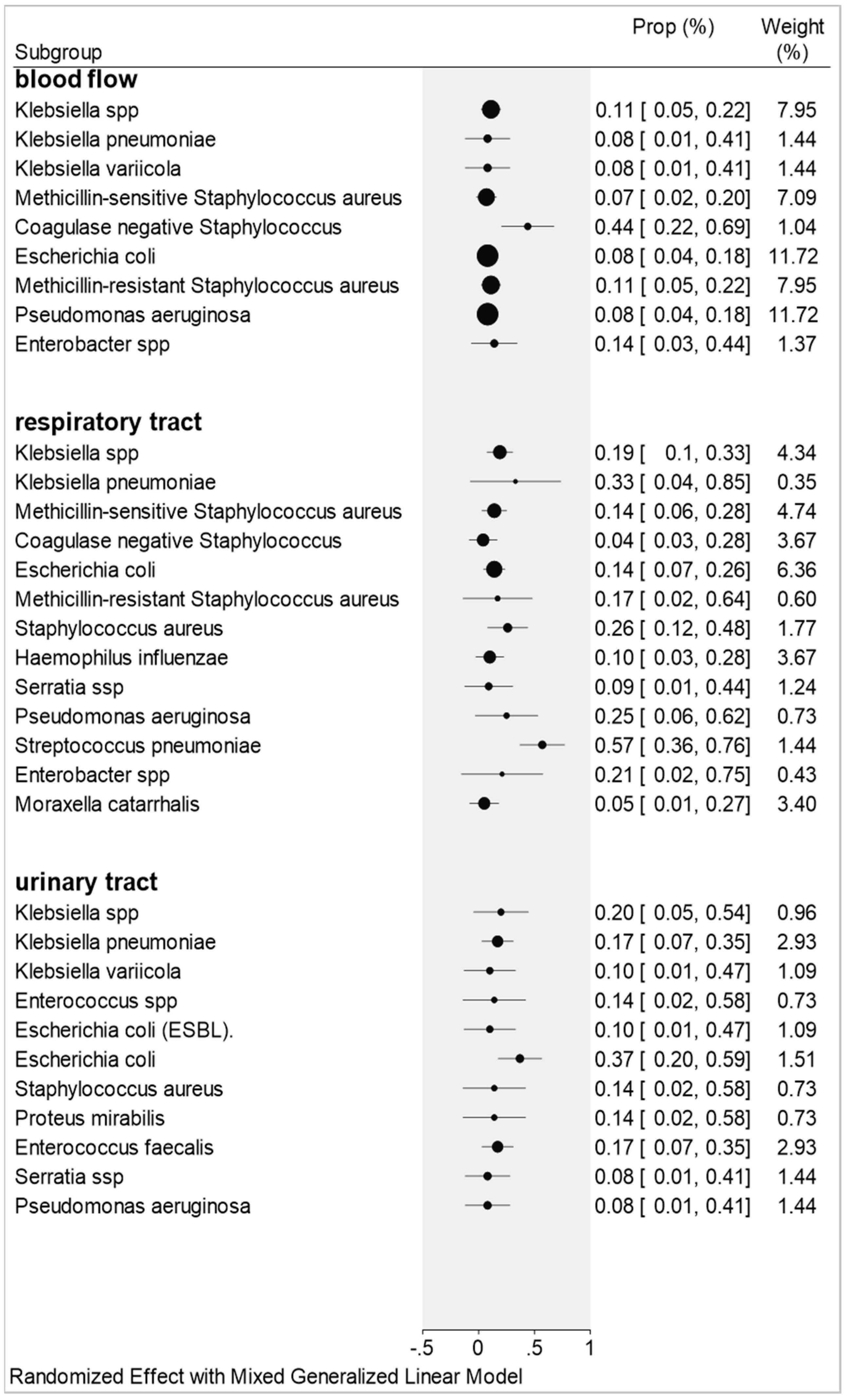
2.3. Co-Infections by Blood Flow, Urinary Tract, and Respiratory Tract Samples
2.3.1. Blood Flow
2.3.2. Respiratory Tract
2.3.3. Urinary Tract
3. Discussion
3.1. Blood Flow
3.2. Urinary Tract
3.3. Respiratory Tract
4. Materials and Methods
4.1. Study Identification
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mina, M.; Klugman, K.P. The role of influenza in the severity and transmission of respiratory bacterial disease. Lancet Respir. Med. 2014, 2, 750–763. [Google Scholar] [CrossRef] [Green Version]
- Bashir, A.; Abdullah, M.S.; Momin, N.R.; Chong, P.L.; Asli, R.; Ivan, B.M.; Chong, V.H. Prevalence of primary bacterial co-infections among patients with COVID-19 in Brunei Darussalam. West. Pac. Surveill. Response J. 2021, 12, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, D.; Giovanetti, M.; Ciccozzi, A.; Spoto, S.; Angeletti, S.; Ciccozzi, M. The 2019-new coronavirus epidemic: Evidence for virus evolution. J. Med. Virol. 2020, 92, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W. The Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Joseph, C.; Togawa, Y.; Shindo, N. Bacterial and viral infections associated with influenza. Influenza Other Respir. Viruses 2013, 7, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, G.A. COINFECÇÃO BACTERIANA EM PACIENTES COM COVID-19. Rev. Multidiscip. Em Saúde 2021, 2, 14. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Mirzaei, R.; Goodarzi, P.; Asadi, M.; Soltani, A.; Aljanabi, H.A.A.; Jeda, A.S.; Dashtbin, S.; Jalalifar, S.; Mohammadzadeh, R.; Teimoori, A.; et al. Bacterial co-infections with SARS-CoV-2. IUBMB Life 2020, 72, 2097–2111. [Google Scholar] [CrossRef]
- Paget, C.; Trottein, F. Mechanisms of bacterial superinfection post-influenza: A role for unconventional T cells. Front. Immunol. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xie, K.; Zhao, J.; Cao, D.; Liang, Y.; Hou, X.; Wang, L.; Li, Z. Mechanisms of severe mortality-associated bacterial co-infections following influenza virus infection. Front. Cell. Infect. Microbiol. 2017, 7, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quah, J.; Jiang, B.; Tan, P.C.; Siau, C.; Tan, T.Y. Impact of microbial Aetiology on mortality in severe community-acquired pneumonia. BMC Infect. Dis. 2018, 18, 451. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, X. Co-infection of tuberculosis and parasitic diseases in humans: A systematic review. Parasites Vectors 2013, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Wei, D.; Zhang, X.; Wu, Y.; Li, Q.; Zhou, M.; Qu, J. Clinical features predicting mortality risk in patients with viral pneumonia: The MuLBSTA Score. Front. Microbiol. 2019, 10, 2752. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; on behalf of the Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [Green Version]
- Prasso, J.E.; Deng, J.C. Post viral Complications: Bacterial Pneumonia. Clin. Chest Med. 2017, 38, 127–138. [Google Scholar] [CrossRef]
- Wang, R.; Kong, L.; Xu, Q.; Yang, P.; Wang, X.; Chen, N.; Li, L.; Jiang, S.; Lu, X. On-ward participation of clinical pharmacists in a Chinese intensive care unit for patients with COVID-19: A retrospective, observational study. Res. Soc. Adm. Pharm. 2021, 17, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lima-Setta, F.; Magalhães-Barbosa, M.C.; Rodrigues-Santos, G.; Figueiredo, E.A.N.; Jacques, M.L.; Zeitel, R.S.; Sapolnik, R.; Borges, C.T.S.; Lanziotti, V.S.; Castro, R.E.V.; et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: A multicenter, prospective cohort study. J. Pediatr. 2021, 9, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Chopra, T.; Kaye, K.S. Pathogens resistant to antibacterial agents. Infect. Dis. Clin. 2009, 23, 817–845. [Google Scholar] [CrossRef] [PubMed]
- Vatopoulos, A.C.; Kalapothaki, V.; Legakis, N.J. An electronic network for the surveillance of antimicrobial resistance in bacterial nosocomial isolates in Greece. The greek network for the surveillance of antimicrobial resistance. Bull. World Health Organ 1999, 77, 595–601. [Google Scholar]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Available online: https://www.who.int/publications/i/item/9789240005587 (accessed on 20 April 2021).
- Smith, A.M.; McCullers, J.A. Secondary bacterial infections in influenza virus infection pathogenesis. In Influenza Pathogenesis and Control—Volume I; Springer: Berlin/Heidelberg, Germany, 2014; Volume 385, pp. 327–356. [Google Scholar] [CrossRef]
- Abbasi, S.; Pendergrass, L.B.; Leggiadro, R.J. Influenza complicated by Moraxella catarrhalis bacteremia. Pediatr. Infect. Dis. J. 1994, 13, 937–938. [Google Scholar] [CrossRef]
- Jacobs, J.H.; Viboud, C.; Tchetgen, E.T.; Schwartz, J.; Steiner, C.; Simonsen, L.; Lipsitch, M. The association of meningococcal disease with influenza in the United States, 1989–2009. PLoS ONE 2014, 9, e107486. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; McLoughlin, R.M. Staphylococcus aureus and influenza A virus: Partners in coinfection. mBio 2016, 7, e02068-16. [Google Scholar] [CrossRef]
- Rizzo, C.; Caporali, M.G.; Rota, M.C. Pandemic influenza and pneumonia due to Legionella pneumophila: A frequently underestimated coinfection. Clin. Infect. Dis. 2010, 51, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertow, D.S.; Memoli, M.J. Bacterial coinfection in influenza: A grand rounds review. JAMA 2013, 309, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, C.S.; Wunderink, R.G. Respiratory Viral and Atypical Pneumonias. Clin. Chest Med. 2017, 38, xiii–xiv. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Amin, A.K.; Khanna, P.; Aali, A.; McGregor, A.; Bassett, P.; Rao, G.G. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021, 76, 796–803. [Google Scholar] [CrossRef]
- Hoque, M.N.; Rahman, M.S.; Ahmed, R.; Hossain, M.S.; Islam, S.; Islam, T.; Hossain, M.A.; Siddiki, A.Z. Diversity and Genomic Determinants of the Microbiomes Associated with COVID-19 and Non-COVID respiratory diseases. Resp. Dis. 2020, 23, 101200. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. Lung microbiome and coronavirus disease 2019 (COVID-19): Possible link and implications. Hum. Microbiome J. 2020, 17, 100073. [Google Scholar] [CrossRef]
- Peddu, V.; Shean, R.C.; Xie, H.; Shrestha, L.; Perchetti, G.A.; Minot, S.S.; Roychoudhury, P.; Huang, M.-L.; Nalla, A.; Reddy, S.B.; et al. Metagenomic analysis reveals clinical SARS-CoV-2 infection and bacterial or viral superinfection and colonization. Clin. Chem. 2020, 66, 966–972. [Google Scholar] [CrossRef]
- Martinez-Guerra, B.A.; Gonzalez-Lara, M.F.; Leon-Cividanes, N.A.; Tamez-Torres, K.M.; Roman-Montes, C.M.; Rajme-Lopez, S.; Villalobos-Zapata, G.I.; Lopez-Garcia, N.I.; Martínez-Gamboa, A.; Sifuentes-Osornio, J.; et al. Antimicrobial Resistance Patternsand Antibiotic Use during Hospital Conversion in the COVID-19 Pandemic. Antibiotics 2021, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet, F.P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e110. [Google Scholar] [CrossRef]
- Mahmoudi, H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS Hyg. Infect. Control. 2020, 15, Doc35. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020, 26, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Bufalino, A.; Sturm, L.; Huang, R.H.; Ottenbacher, A.; Saake, K.; Winegar, A.; Fogel, R.; Cacchione, J. Coronavirus disease 2019 (COVID-19) pandemic, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect. Control. Hosp. Epidemiol. 2022, 43, 26–31. [Google Scholar] [CrossRef]
- Díaz, P.B.; Guedez, L.G.V.; García, C.P.M.; Jiménez, G.M.; García, B.S.; Gómez-Gil, M.M.R. Urinary Tract Infections in Hospitalized COVID-19 Patients, What’s Up, Doc? J. Clin. Med. 2022, 11, 1815. [Google Scholar] [CrossRef]
- Iqbal, Z.; Mumtaz, M.Z.; Malik, A. Extensive drug-resistance in strains of Escherichia coli and Klebsiella pneumoniae isolated from paediatric urinary tract infections. J. Taibah Univ. Med. Sci. 2021, 16, 565–574. [Google Scholar] [CrossRef]
- Goncalves, A.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy, R.; Bhargav, R.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2021, 93, 1489–1495. [Google Scholar] [CrossRef]
- Azekawa, S.; Namkoong, H.; Mitamura, K.; Kawaoka, Y.; Saito, F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases 2020, 20, e00775. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.L.; Buesa, J.; Colomina, J.; Forner, M.J.; Galindo, M.J.; Navarro, J.; Noceda, J.; Redón, J.; Signes-Costa, J.; Navarro, D. Co-detection of respiratory pathogens in patients hospitalized with Coronavirus viral disease-2019 pneumonia. J. Med. Virol. 2020, 92, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Payan, E.; Montagud-Marraha, E.; Torres-Elorza, M.; Bodro, M.; Blasco, M.; Poch, E.; Soriano, A.; Piñeiro, G.J. SARS-CoV-2 and influenza virus co-infection. Lancet 2020, 395, e84. [Google Scholar] [CrossRef]
- Ding, Q.; Lu, P.; Fan, Y.; Xia, Y.; Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 noval coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020, 92, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Lim, K.; Chong, V.; Chan, S.; Ong, K.; Kuperan, P. COVID-19 and mycoplasma pneumoniae coinfection. Am. J. Hematol. 2020, 95, 723–724. [Google Scholar] [CrossRef]
- Khodamoradi, Z.; Moghadami, M.; Lotfi, M. Co-infection of coronavirus disease 2019 and influenza A: A report from Iran. Arch. Iran. Med. 2020, 23, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Konala, V.; Adapa, S.; Gayam, V.; Naramala, S.; Daggubati, S.; Kammari, C.; Chenna, A. Co-infection with Influenza A and COVID-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 001656. [Google Scholar] [CrossRef]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Guo, Y.; Dai, Y.; Xu, Y.; Cai, Y.; et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020, 63, 606–609. [Google Scholar] [CrossRef] [Green Version]
- Luyt, C.E.; He’kimian, G.; Koulenti, D.; Chastre, J. Microbial cause of ICU-acquired pneumonia: Hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr. Opin. Crit. Care 2018, 24, 332–338. [Google Scholar] [CrossRef]
- Motta, J.; Gomez, C. Adenovirus and novel coronavirus (SARS-CoV2) coinfection: A case report. IDCases 2020, 22, e00936. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ozaras, R.; Cirpin, R.; Duran, A.; Duman, H.; Arslan, O.; Bakcan, Y.; Kaya, M.; Mutlu, H.; Isayeva, L.; Kebanlı, F.; et al. Influenza and COVID-19 coinfection: Report of six cases and review of the literature. J. Med. Virol. 2020, 92, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Touzard-Romo, F.; Tape, C.; Lonks, J. Co-infection with SARS-CoV-2 and human metapneumovirus. R. I. Med. J. 2020, 103, 75–76. Available online: http://rimed.org/rimedicaljournal/2020/03/2020-03-75-touzard-romo.pdf (accessed on 21 November 2021).
- Wee, L.; Ko, K.K.K.; Ho, W.Q.; Kwek, G.T.C.; Tan, T.T.; Wijaya, L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: Co-infection and clinical outcomes. J. Clin. Virol. 2020, 128, 104436. [Google Scholar] [CrossRef]
- Wu, X.; Cai, T.; Huang, X.; Yu, X.; Zhao, L.; Wang, F.; Li, Q.; Gu, S.; Xu, T.; Li, Y.; et al. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg. Infect. Dis. 2020, 6, 1324–1326. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Maddukuri, G.; Al-Aly, Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with COVID-19 and seasonal influenza: A cohort study. BMJ 2020, 371, m4677. [Google Scholar] [CrossRef]
- Ginsburg, A.S.; Klugman, K.P. COVID-19 pneumonia and the appropriate use of antibiotics. Lancet Glob. Health 2020, 8, e1453–e1454. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Zhu, X.; Ge, Y.; Wu, T.; Zhao, K.; Chen, Y.; Wu, B.; Zhu, F.; Zhu, B.; Cui, L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020, 285, 198005. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Man-Ling, T.; Li, Y.Q.; Chen, X.; Lin, H.; Jiang, Z.C.; Gu, D.L.; Tang, C.X.; Xie, Z.Q. Co-Infection with Common Respiratory Pathogens and SARS-CoV-2 in Patients with COVID-19 Pneumonia and Laboratory Biochemistry Findings: A Retrospective Cross-Sectional Study of 78 Patients from a Single Center in China. Med. Sci. Monit. 2021, 3, e929783. [Google Scholar] [CrossRef]
- Rothe, K.; Feihl, S.; Schneider, J.; Wallnöfer, F.; Wurst, M.; Lukas, M.; Treiber, M.; Lahmer, T.; Heim, M.; Dommasch, M.; et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: A retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team RStudio: Integrated Development for R. RStudio. Version 4.0.2, PBC, Boston, USA 2020. Available online: http://www.rstudio.com (accessed on 7 January 2022).
- StataCorp LLC. Stata Statistical Software. Version 16.0, College Station, Washington, DC, USA 2021. Available online: https://filecr.com/windows/stata-mp (accessed on 10 January 2022).
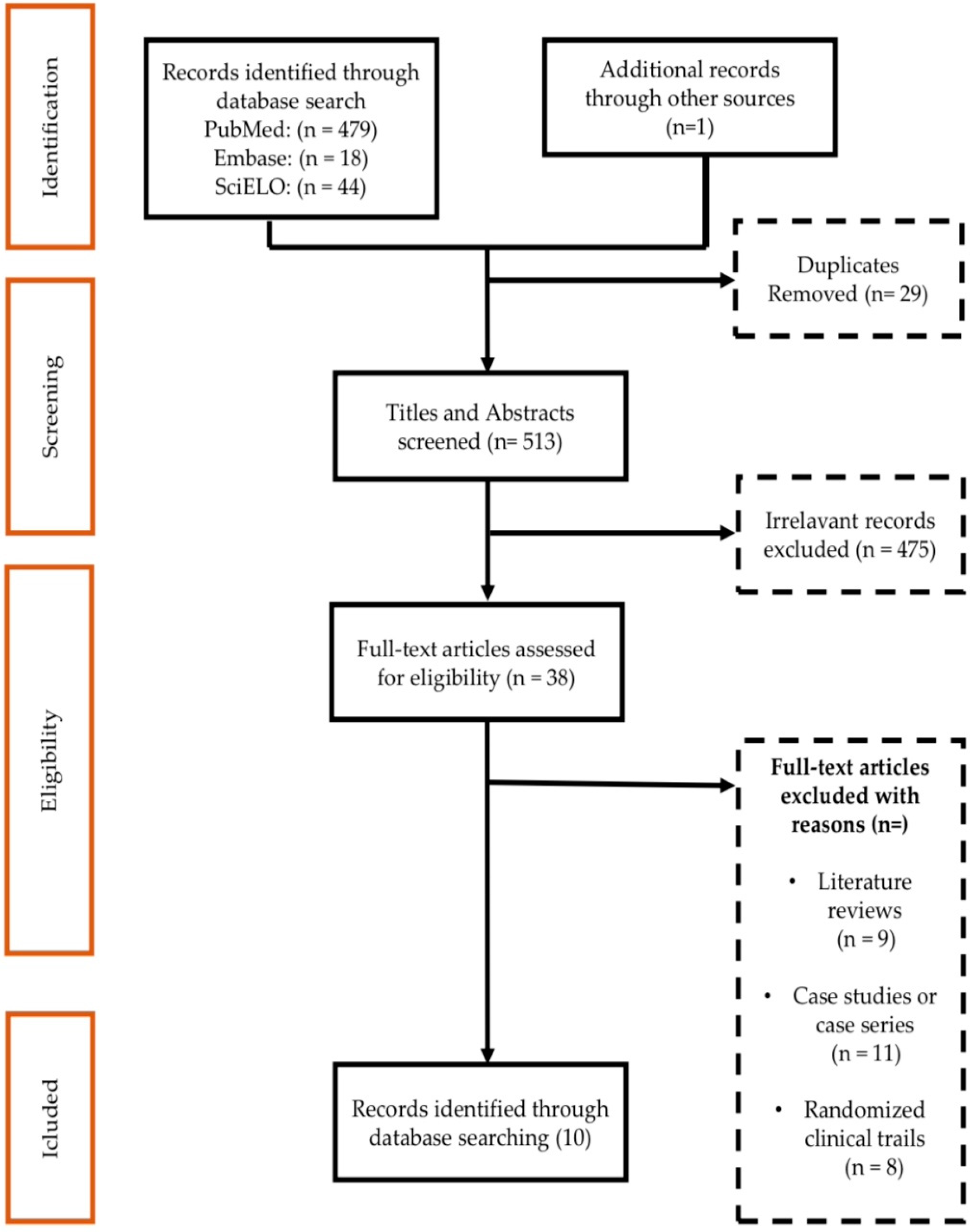

| Studies Selected for Meta-Analysis | |||
|---|---|---|---|
| Author | Year | Countries | Type of Study |
| Wang et al. [40] | 2021 | London | Retrospective observational study |
| Martinez-Guerra et al. [45] | 2021 | Mexico | Prospective cohort study |
| Contou et al. [46] | 2020 | France | Retrospective study |
| Hughes et al. [49] | 2020 | London | Retrospective observational analysis |
| Mahmoudi [50] | 2020 | Iran | Cross-sectional study |
| Neto et al. [54] | 2020 | USA | Retrospective analysis |
| Zhu et al. [73] | 2020 | China | Retrospective study |
| Garcia-Vidal et al. [74] | 2021 | Spain | Retrospective cohort study |
| Man-Ling et al. [75] | 2021 | China | Retrospective analysis |
| Rothe et al. [26] | 2021 | Germany | Retrospective cohort study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.P.; Gonçalves, L.C.; Oliveira, A.C.C.; Queiroz, P.H.P.; Ito, C.R.M.; Santos, M.O.; Carneiro, L.C. Bacterial Co-Infection in Patients with COVID-19 Hospitalized (ICU and Not ICU): Review and Meta-Analysis. Antibiotics 2022, 11, 894. https://doi.org/10.3390/antibiotics11070894
Santos AP, Gonçalves LC, Oliveira ACC, Queiroz PHP, Ito CRM, Santos MO, Carneiro LC. Bacterial Co-Infection in Patients with COVID-19 Hospitalized (ICU and Not ICU): Review and Meta-Analysis. Antibiotics. 2022; 11(7):894. https://doi.org/10.3390/antibiotics11070894
Chicago/Turabian StyleSantos, Adailton P., Lucas C. Gonçalves, Ana C. C. Oliveira, Pedro H. P. Queiroz, Célia R. M. Ito, Mônica O. Santos, and Lilian C. Carneiro. 2022. "Bacterial Co-Infection in Patients with COVID-19 Hospitalized (ICU and Not ICU): Review and Meta-Analysis" Antibiotics 11, no. 7: 894. https://doi.org/10.3390/antibiotics11070894







