Population Pharmacokinetics of Temocillin Administered by Continuous Infusion in Patients with Septic Shock Associated with Intra-Abdominal Infection and Ascitic Fluid Effusion
Abstract
:1. Introduction
2. Results
2.1. Study Population, Treatment Parameters, and Outcomes
2.2. Microbiological Data
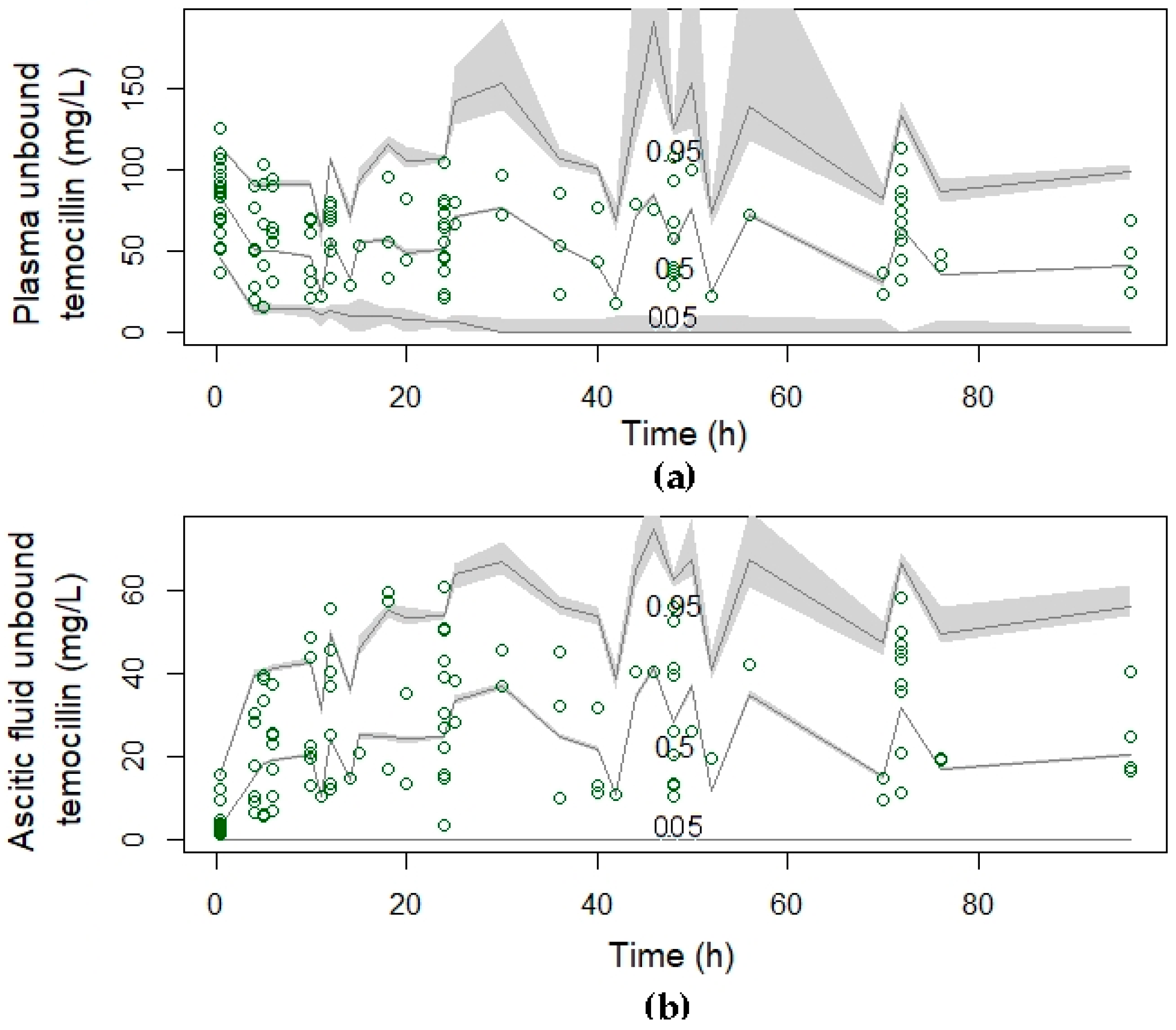
2.3. Pharmacokinetic Analysis
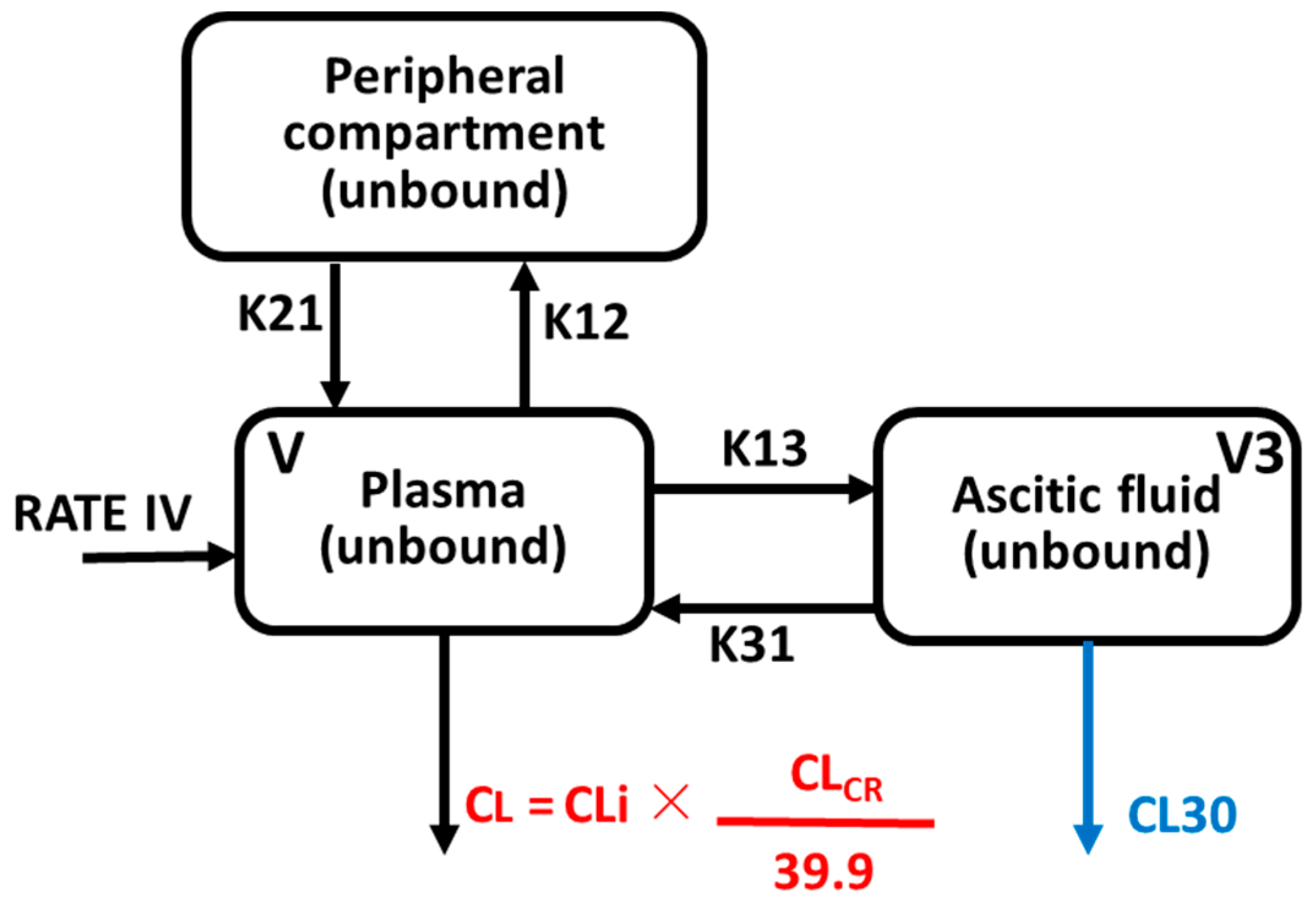
2.4. Population Pharmacokinetic Modelling
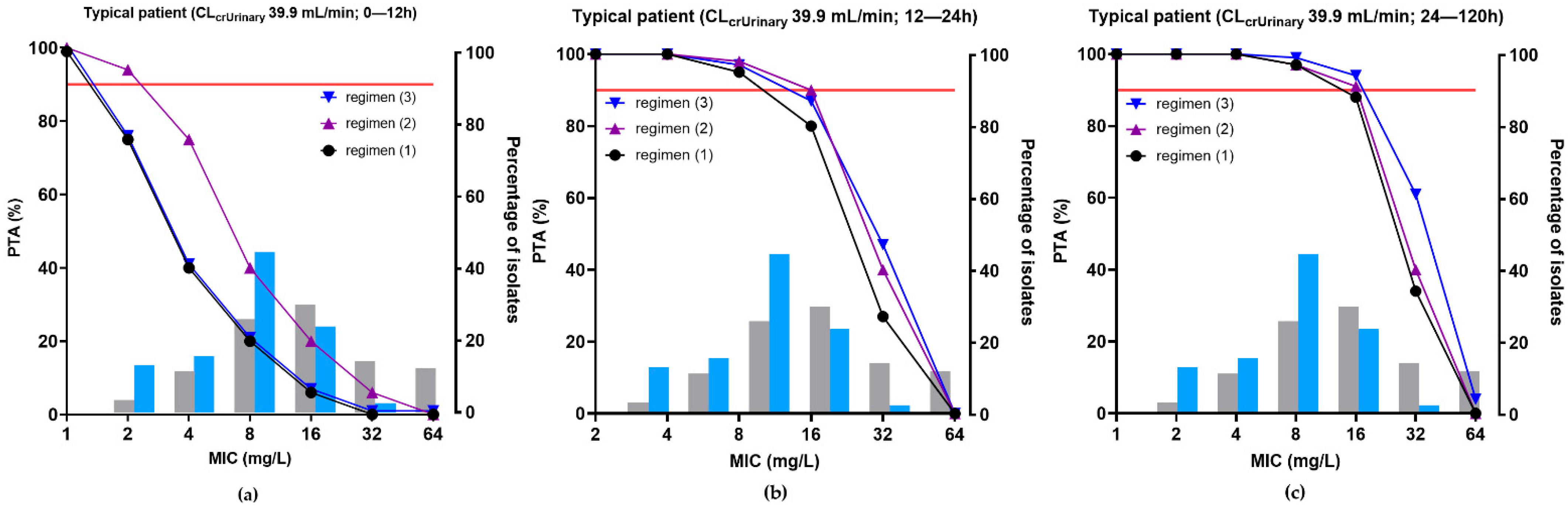
2.5. Probability of Target Attainment (PTA)
3. Discussion
4. Materials and Methods
4.1. Study Design, Patients, and Data Collection
4.2. Antibiotic Treatment and Sample Collection
4.3. Analytical Method
4.3.1. Chemicals and Reagents
4.3.2. Temocillin Assay
4.4. Microorganisms and Minimum Inhibitory Concentrations (MIC) Determinations
4.5. Pharmacokinetic Analysis
4.6. Population Pharmacokinetic Modeling
4.6.1. Model Building
4.6.2. Covariate Exploration and Model
4.6.3. Model Diagnostics and Selection
4.6.4. Model Validation
4.6.5. Dosing Simulations and Probability of Target Attainment
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartelli, M.; Catena, F.; Abu-Zidan, F.M.; Ansaloni, L.; Biffl, W.L.; Boermeester, M.A.; Ceresoli, M.; Chiara, O.; Coccolini, F.; De Waele, J.J.; et al. Management of intra-abdominal infections: Recommendations by the WSES 2016 consensus conference. World J. Emerg. Surg. 2017, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Adnan, S.; Paterson, D.L.; Lipman, J.; Kumar, S.; Li, J.; Rudd, M.; Roberts, J.A. Pharmacokinetics of beta-lactam antibiotics in patients with intra-abdominal disease: A structured review. Surg. Infect. 2012, 13, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.A.; Wood, G.C.; Swanson, J.M. Pharmacokinetic changes in critical illness. Crit. Care Clin. 2006, 22, 255–271. [Google Scholar] [CrossRef]
- De Waele, J.; Lipman, J.; Sakr, Y.; Marshall, J.C.; Vanhems, P.; Groba, C.B.; Leone, M.; Vincent, J.L. Abdominal infections in the intensive care unit: Characteristics, treatment and determinants of outcome. BMC. Infect. Dis. 2014, 14, 420. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.C. Principles of source control in the early management of sepsis. Curr. Infect. Dis. Rep. 2010, 12, 345–353. [Google Scholar] [CrossRef]
- Sartelli, M.; Weber, D.G.; Ruppe, E.; Bassetti, M.; Wright, B.J.; Ansaloni, L.; Catena, F.; Coccolini, F.; Abu-Zidan, F.M.; Coimbra, R.; et al. Antimicrobials: A global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J. Emerg. Surg. 2016, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Shirah, G.R.; O’Neill, P.J. Intra-abdominal Infections. Surg. Clin. N. Am. 2014, 94, 1319–1333. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Gutierrez-Pizarraya, A.; Escoresca-Ortega, A.; Corcia-Palomo, Y.; Fernandez-Delgado, E.; Herrera-Melero, I.; Ortiz-Leyba, C.; Marquez-Vacaro, J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Leone, M.; Bechis, C.; Baumstarck, K. De-escalation in severe sepsis: Still an important part of our armamentarium against antimicrobial resistance, of course! Intensive Care Med. 2014, 40, 1619. [Google Scholar] [CrossRef] [Green Version]
- Montravers, P.; Augustin, P.; Grall, N.; Desmard, M.; Allou, N.; Marmuse, J.P.; Guglielminotti, J. Characteristics and outcomes of anti-infective de-escalation during health care-associated intra-abdominal infections. Crit. Care 2016, 20, 83. [Google Scholar] [CrossRef] [Green Version]
- Kollef, M.H.; Shorr, A.F.; Bassetti, M.; Timsit, J.F.; Micek, S.T.; Michelson, A.P.; Garnacho-Montero, J. Timing of antibiotic therapy in the ICU. Crit. Care 2021, 25, 360. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Ansaloni, L.; Coccolini, F.; Corbella, D.; Moore, E.E.; Malangoni, M.; Velmahos, G.; Coimbra, R.; Koike, K.; et al. Complicated intra-abdominal infections worldwide: The definitive data of the CIAOW Study. World J. Emerg. Surg. 2014, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Burns, K.; Bano, R.J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef]
- Jules, K.; Neu, H.C. Antibacterial activity and beta-lactamase stability of temocillin. Antimicrob. Agents Chemother. 1982, 22, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Slocombe, B.; Basker, M.J.; Bentley, P.H.; Clayton, J.P.; Cole, M.; Comber, K.R.; Dixon, R.A.; Edmondson, R.A.; Jackson, D.; Merrikin, D.J.; et al. BRL 17421, a novel beta-lactam antibiotic, highly resistant to beta-lactamases, giving high and prolonged serum levels in humans. Antimicrob. Agents Chemother. 1981, 20, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Villalobos, H.; Bogaerts, P.; Berhin, C.; Bauraing, C.; Deplano, A.; Montesinos, I.; de Mendonca, R.; Jans, B.; Glupczynski, Y. Trends in production of extended-spectrum beta-lactamases among Enterobacteriaceae of clinical interest: Results of a nationwide survey in Belgian hospitals. J. Antimicrob. Chemother. 2011, 66, 37–47. [Google Scholar] [CrossRef]
- Alexandre, K.; Reveillon-Istin, M.; Fabre, R.; Delbos, V.; Etienne, M.; Pestel-Caron, M.; Dahyot, S.; Caron, F. Temocillin against Enterobacteriaceae isolates from community-acquired urinary tract infections: Low rate of resistance and good accuracy of routine susceptibility testing methods. J. Antimicrob. Chemother. 2018, 73, 1848–1853. [Google Scholar] [CrossRef]
- Kuch, A.; Zieniuk, B.; Zabicka, D.; Van de Velde, S.; Literacka, E.; Skoczynska, A.; Hryniewicz, W. Activity of temocillin against ESBL-, AmpC-, and/or KPC-producing Enterobacterales isolated in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1185–1191. [Google Scholar] [CrossRef]
- Farfour, E.; Si Larbi, A.G.; Cattoir, V.; Corvec, S.; Guillard, T.; Grillon, A.; Isnard, C.; Merens, A.; Degand, N.; Billard-Pomares, T.; et al. Temocillin susceptibility among Enterobacterales strains recovered from blood culture in France. Diagn. Microbiol. Infect. Dis. 2021, 100, 115368. [Google Scholar] [CrossRef]
- Mittermayer, H.W. Influence of temocillin on human bowel flora. Drugs 1985, 29 (Suppl S5), 43–48. [Google Scholar] [CrossRef]
- Edlund, C.; Ternhag, A.; Skoog Stahlgren, G.; Edquist, P.; Balkhed, O.A.; Athlin, S.; Mansson, E.; Tempe, M.; Bergstrom, J.; Giske, C.G.; et al. The clinical and microbiological efficacy of temocillin versus cefotaxime in adults with febrile urinary tract infection, and its effects on the intestinal microbiota: A randomised multicentre clinical trial in Sweden. Lancet Infect. Dis. 2021, 22, 390–400. [Google Scholar] [CrossRef]
- Balakrishnan, I.; Awad-El-Kariem, F.M.; Aali, A.; Kumari, P.; Mulla, R.; Tan, B.; Brudney, D.; Ladenheim, D.; Ghazy, A.; Khan, I.; et al. Temocillin use in England: Clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC beta-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2011, 66, 2628–2631. [Google Scholar] [CrossRef] [Green Version]
- Livermore, D.M.; Tulkens, P.M. Temocillin revived. J. Antimicrob. Chemother. 2009, 63, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Negaban (Temocillin) Belgium SmPC Negaban Powder for Solution for Injection/Infusion, Summary of Product Characteristics, (CBIP). 2021. Available online: https://www.cbip.be/fr/chapters/12?frag=9563&trade_family=18549 (accessed on 3 February 2022).
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [Green Version]
- Kunin, C.M. Clinical pharmacology of the new penicillins. 1. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin. Pharmacol. Ther. 1966, 7, 166–179. [Google Scholar] [CrossRef]
- Liu, P.; Muller, M.; Derendorf, H. Rational dosing of antibiotics: The use of plasma concentrations versus tissue concentrations. Int. J. Antimicrob. Agents 2002, 19, 285–290. [Google Scholar] [CrossRef]
- Onufrak, N.J.; Forrest, A.; Gonzalez, D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016, 38, 1930–1947. [Google Scholar] [CrossRef] [Green Version]
- Overbosch, D.; van Gulpen, C.; Mattie, H. Renal clearance of temocillin in volunteers. Drugs 1985, 29 (Suppl S5), 128–134. [Google Scholar] [CrossRef]
- Ngougni Pokem, P.; Matzneller, P.; Vervaeke, S.; Wittebole, X.; Goeman, L.; Coessen, M.; Cottone, E.; Capron, A.; Wulkersdorfer, B.; Wallemacq, P.; et al. Binding of temocillin to plasma proteins in-vitro and in-vivo: The importance of plasma protein levels in different populations and of comedications. 2022; submitted for publication. [Google Scholar]
- Matzneller, P.; Pokem, N.P.; Capron, A.; Lackner, E.; Wulkersdorfer, B.; Nussbaumer-Proll, A.; Osterreicher, Z.; Duchek, M.; Van de Velde, S.; Wallemacq, P.E.; et al. Single-dose pharmacokinetics of temocillin in plasma and soft tissues of healthy volunteers after intravenous and subcutaneous administration: A randomized crossover microdialysis trial. J. Antimicrob. Chemother. 2020, 75, 2650–2656. [Google Scholar] [CrossRef]
- Laterre, P.F.; Wittebole, X.; Van de Velde, S.; Muller, A.E.; Mouton, J.W.; Carryn, S.; Tulkens, P.M.; Dugernier, T. Temocillin (6 g daily) in critically ill patients: Continuous infusion versus three times daily administration. J. Antimicrob. Chemother. 2015, 70, 891–898. [Google Scholar] [CrossRef]
- Mouton, J.W.; Theuretzbacher, U.; Craig, W.A.; Tulkens, P.M.; Derendorf, H.; Cars, O. Tissue concentrations: Do we ever learn? J. Antimicrob. Chemother. 2008, 61, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, O.; Trautmann, M.; Borner, K. A study of the penetration of temocillin in the cerebrospinal fluid. Drugs 1985, 29 (Suppl. S5), 162–166. [Google Scholar] [CrossRef]
- Bergan, T.; Engeset, A.; Olszewski, W. Temocillin in peripheral lymph. J. Antimicrob. Chemother. 1983, 12, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.M.; Wise, R.; Andrews, J.M. Temocillin, in-vitro activity and the pharmacokinetics and tissue penetration in healthy volunteers. J. Antimicrob. Chemother. 1982, 10, 295–302. [Google Scholar] [CrossRef]
- Wise, R.; Donovan, I.A.; Drumm, J.; Dyas, A.; Cross, C. The intraperitoneal penetration of temocillin. J. Antimicrob. Chemother. 1983, 12, 93–96. [Google Scholar] [CrossRef]
- Layios, N.; Visee, C.; Mistretta, V.; Denooz, R.; Maes, N.; Descy, J.; Frippiat, F.; Marchand, S.; Gregoire, N. Modelled Target Attainment after Temocillin Treatment in Severe Pneumonia: Systemic and Epithelial Lining Fluid Pharmacokinetics of Continuous versus Intermittent Infusions. Antimicrob. Agents Chemother. 2022, 66, e0205221. [Google Scholar] [CrossRef]
- Poston, G.J.; Greengrass, A.; Moryson, C.J. Biliary concentrations of temocillin. Drugs 1985, 29 (Suppl. S5), 140–145. [Google Scholar] [CrossRef]
- Spelsberg, F.; Bauernfeind, A.; Wiest, W.; Hanser, P. Biliary concentrations of temocillin. Drugs 1985, 29 (Suppl. S5), 122–127. [Google Scholar] [CrossRef]
- Negaban (Temocillin) UK SmPC Negaban Powder for Solution for Injection/Infusion, SUMMARY of Product Characteristics, (emc). 2018. Available online: https://www.medicines.org.uk/emc/product/466/smpc (accessed on 3 February 2022).
- European Committee on Antimicrobial Susceptibility Testing Rationale for the EUCAST Clinical Breakpoints, Version 1.0. 2019. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Temocillin_rationale_document_v1.0_20200327.pdf (accessed on 3 February 2022).
- Arina, P.; Singer, M. Pathophysiology of sepsis. Curr. Opin. Anaesthesiol. 2021, 34, 77–84. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Sanz Codina, M.; Zeitlinger, M. Biomarkers Predicting Tissue Pharmacokinetics of Antimicrobials in Sepsis: A Review. Clin. Pharmacokinet. 2022. [Google Scholar] [CrossRef]
- Jager, N.G.L.; van Hest, R.M.; Xie, J.; Wong, G.; Ulldemolins, M.; Bruggemann, R.J.M.; Lipman, J.; Roberts, J.A. Optimization of flucloxacillin dosing regimens in critically ill patients using population pharmacokinetic modelling of total and unbound concentrations. J. Antimicrob. Chemother. 2020, 75, 2641–2649. [Google Scholar] [CrossRef]
- Schleibinger, M.; Steinbach, C.L.; Topper, C.; Kratzer, A.; Liebchen, U.; Kees, F.; Salzberger, B.; Kees, M.G. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br. J. Clin. Pharmacol. 2015, 80, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Benoni, G.; Arosio, E.; Raimondi, M.G.; Pancera, P.; Lechi, A.; Velo, G.P. Pharmacokinetics of ceftazidime and ceftriaxone and their penetration into the ascitic fluid. J. Antimicrob. Chemother. 1985, 16, 267–273. [Google Scholar] [CrossRef]
- McNamara, P.J.; Trueb, V.; Stoeckel, K. Protein binding of ceftriaxone in extravascular fluids. J. Pharm. Sci. 1988, 77, 401–404. [Google Scholar] [CrossRef]
- Salvioli, G.; Tata, C.; Panini, R.; Pellati, M.; Lugli, R.; Gaetti, E. Composition of ascitic fluid in liver cirrhosis: Bile acid and lipid content. Eur. J. Clin. Invest 1993, 23, 534–539. [Google Scholar] [CrossRef]
- Attali, P.; Turner, K.; Pelletier, G.; Ink, O.; Etienne, J.P. pH of ascitic fluid: Diagnostic and prognostic value in cirrhotic and noncirrhotic patients. Gastroenterology 1986, 90, 1255–1260. [Google Scholar] [CrossRef]
- Baert, L.; Aswarie, H.; Verbist, L.; Horton, R. Penetration of temocillin into prostatic tissue after intravenous dosing. Acta Clin. Belg. 1989, 44, 358–359. [Google Scholar] [CrossRef]
- Ngougni Pokem, P.; Capron, A.; Wallemacq, P.; Tulkens, P.M.; Van Bambeke, F.; Laterre, P.F. Temocillin plasma and pancreatic tissue concentrations in a critically ill patient with septic shock. J. Antimicrob. Chemother. 2019, 74, 1459–1461. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Roberts, J.A.; Carlier, M.; Verstraete, A.G.; Stove, V.; De Waele, J.J. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int. J. Antimicrob. Agents 2018, 51, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Leegwater, E.; Kraaijenbrink, B.V.C.; Moes, D.J.A.R.; Purmer, I.M.; Wilms, E.B. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 1554–1558. [Google Scholar] [CrossRef]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented renal clearance in critically ill patients: Etiology, definition and implications for beta-lactam dose optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Nunn, B.; Baird, A.; Chamberlain, P.D. Effect of temocillin and moxalactam on platelet responsiveness and bleeding time in normal volunteers. Antimicrob. Agents Chemother. 1985, 27, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Singal, A.K.; Kamath, P.S. Model for End-stage Liver Disease. J. Clin. Exp. Hepatol. 2013, 3, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Ngougni Pokem, P.; Miranda Bastos, A.C.; Tulkens, P.M.; Wallemacq, P.; Van Bambeke, F.; Capron, A. Validation of a HPLC-MS/MS assay for the determination of total and unbound concentration of temocillin in human serum. Clin. Biochem. 2015, 48, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Ngougni Pokem, P.; Stephenne, X.; Van der Linden, D.; Godet, M.L.; Wijnant, G.J.; Chatzis, O.; Houtekie, L.; Haenecour, A.; Wallemacq, P.E.; Tulkens, P.M.; et al. Population pharmacokinetics and dosing simulation of the b-lactam temocillin in liver transplanted paediatric patients. 2022; submitted for publication. [Google Scholar]
- Pai, M.P.; Russo, A.; Novelli, A.; Venditti, M.; Falcone, M. Simplified equations using two concentrations to calculate area under the curve for antimicrobials with concentration-dependent pharmacodynamics: Daptomycin as a motivating example. Antimicrob. Agents Chemother. 2014, 58, 3162–3167. [Google Scholar] [CrossRef] [Green Version]
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouton, J.W.; Brown, D.F.J.; Apfalter, P.; Canton, R.; Giske, C.G.; Ivanova, M.; MacGowan, A.P.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; et al. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: The EUCAST approach. Clin. Microbiol. Infect. 2012, 18, E37–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Value (Median (Range)) a | |
|---|---|---|
| Patients enrolled, n | 19 | |
| Demographic data | ||
| Males, n (%) | 6 (31.58%) | |
| Age (years) | 56 (21–74) | |
| Weight (kg) | 67 (45–95) | |
| Body mass index (kg/m2) | 23.87 (15.03–33.65) | |
| Biological and physiological parameters [local normal values] | ||
| C-reactive protein (mg/L) [<5 mg/L] | 114.2 (20.00–364.6) | |
| CLCRurinary (mL/min) [>78 mL/min] | 39.90 (20.55–149.3) | |
| Plasma | Total protein (g/L) [64–83 g/L] | 47.35 (29.59–58.74) |
| Albumin (g/L) [35–52 g/L] | 22.30 (13.70–30.80) | |
| Ascitic fluid | Total protein (g/L) | 11.64 (6.23–36.46) |
| Albumin (g/L) | 5.30 (2.12–12.45) | |
| Gamma-glutamyl-transferase (IU/L) [<40 UI/L] | 51.00 (13.0–205.0) | |
| Alanine aminotransferase (IU/L) [7–35 UI/L] | 34.00 (5.00–120.0) | |
| Aspartate aminotransferase (IU/L) [9–36 UI/L] | 49.00 (14.00–198.0) | |
| Alkaline phosphatase (IU/L) (40–130) | 119.0 (39.00–440.0) | |
| Bilirubin—total (mg/dL) [<1.2] | 3.3 (0.2–12.8) | |
| Bilirubin—conjugated (mg/dL) [<0.3] | 4.3 (0–11.9) | |
| INR [0.80–1.20] | 1.55 (1.09–4.7) | |
| Clinical scores at admission | ||
| SOFA score | 9 (4–14) | |
| APACHE II score | 18 (13–32) | |
| Type of infection | ||
| Spontaneous bacterial peritonitis, n (%) | 11 (58%) | |
| Secondary peritonitis, n (%) | 4 (21%) | |
| Pancreatic infected necrosis, n (%) | 3 (16%) | |
| Liver abscess, n (%) | 1 (5%) | |
| Temocillin treatment duration (days) | 5 (4–21) | |
| Outcome | ||
| Microbial eradication, n (%) | 16 (84.21%) | |
| Death, n (%) | 7 (36.84%) | |
| Type of Sample | Bacterial Species a | Number of Isolates with a MIC (mg/L) b | Detected β-Lactamase | |||||
|---|---|---|---|---|---|---|---|---|
| ≤2 | 3–4 | 6–8 | 12–16 | >16 | ESBL | Cephalos-Porinase | ||
| Ascitic fluid | E. coli | 2 | 4 | 2 | 1 | 2 | ||
| K. pneumoniae | 1 | 2 | 1 | 1 | 1 | |||
| E. cloacae | 1 | 1 | ||||||
| P. mirabilis | 1 | |||||||
| E. aerogenes | 1 | |||||||
| Abdominal pus/necrosis | E. coli | 1 | 1 | 2 | 2 | 3 | ||
| K. pneumoniae | 1 | |||||||
| S. marcescens | 1 | 1 | ||||||
| Hemoculture | E. coli | 1 | 3 | 2 | ||||
| K. pneumoniae | 2 | 1 | 1 | 2 | 1 | |||
| K. oxytoca | 1 | |||||||
| P. mirabilis | 1 | |||||||
| S. marcescens | 1 | |||||||
| Urine | E. coli | 1 | 1 | |||||
| K. pneumoniae | 1 | |||||||
| K. oxytoca | 1 | 1 | ||||||
| Total, n (%) | 5 (13.15) | 6 (15.78) | 17 (44.73) | 9 (23.84) | 1 (2.63) | 10 (26.31) | 6 (15.78) | |
| Model | -2LL a | AIC a | Sample | R2 b | Slope b (95% CI) | Intercept b (95% CI) | Bias b | Imprecision b |
|---|---|---|---|---|---|---|---|---|
| Simple two-compartment | 1860 | 1870 | Plasma | 0.812 | 0.891 (−0.99 to 1.13) | 16.7 (−8.64 to −1.06) | 0.54 | 1.46 |
| Ascitic fluid | 0.87 | 0.68 (0.81 to 0.95) | 5.64 (0.11 to 4.36) | 0.40 | 1.45 | |||
| Two-compartment + additional distribution compartment c | 1497 | 1514 | Plasma | 0.90 | 1.06 (−0.99 to 1.13) | −4.26 (−8.64 to −1.06) | 0.21 | 1.26 |
| Ascitic fluid | 0.84 | 0.88 (0.81 to 0.95) | 2.24 (0.11 to 4.36) | 0.20 | 1.21 | |||
| Two-compartment + additional elimination compartment d | 1487 | 1506 | Plasma | 0.86 | 1.01 (0.0.93 to 1.08) | −0.14 (−5.46 to 1.90) | 0.11 | 2.39 |
| Ascitic fluid | 0.91 | 1.00 (0.94 to 1.06) | 0.02 (−1.69 to 1.73) | 0.09 | 1.07 | |||
| Two-compartment + additional elimination compartment d + covariate e,f | 1485 | 1504 | Plasma | 0.92 | 1.02 (0.96 to 1.08) | 0.72 (−3.05 to 4.50) | −0.13 | 0.75 |
| Ascitic fluid | 0.93 | 0.99 (0.94 to 1.04) | 0.25 (−1.21 to 1.73) | 0.05 | 0.60 |
| Parameter a,b | Mean | SD | CV (%) | Median (95% CI) | Shrink (%) |
|---|---|---|---|---|---|
| V (L) | 14.36 | 4.18 | 29.15 | 13.90 (11.76–15.98) | 0.898 |
| CLi (L/h) | 2.45 | 0.91 | 37.33 | 2.56 (2.34–3.71) | 0.235 |
| K12 (h−1) | 4.95 | 2.89 | 58.47 | 4.62 (2.93–6.53) | 6.539 |
| K21 (h−1) | 5.38 | 3.62 | 67.20 | 5.85 (2.08–8.85) | 6.677 |
| K13 (h−1) | 0.42 | 0.47 | 110.67 | 0.24 (0.16–0.41) | 0.039 |
| K31 (h−1) | 0.33 | 0.46 | 137.94 | 0.15 (0.04–0.26) | 0.010 |
| V3 (L) | 30.00 | 16.76 | 55.87 | 28.93 (15.83–42.77) | 0.567 |
| CL30 (L/h) | 2.91 | 1.42 | 48.75 | 2.94 (2.11–3.60) | 1.98 |
| Target MIC (mg/L) | CLCRurinary (mL/min) | Temocillin Doses b | ||
|---|---|---|---|---|
| Studied | Simulated | |||
| Regimen (1) | Regimen (2) | Regimen (3) | ||
| 8 mg/L | 20 | 100 | 100 | 100 |
| 39.9 | 100 | 100 | 100 | |
| 60 | 100 | 100 | 100 | |
| 90 | 100 | 100 | 100 | |
| 120 | 100 | 100 | 100 | |
| 150 | 100 | 100 | 100 | |
| 16 mg/L | 20 | 98 | 99 | 98 |
| 39.9 | 98 | 99 | 98 | |
| 60 | 98 | 99 | 98 | |
| 90 | 98 | 99 | 98 | |
| 120 | 98 | 99 | 98 | |
| 150 | 90 | 94 | 98 | |
| Target MIC (mg/L) | CLCRurinary (mL/min) | Temocillin Doses b | ||
|---|---|---|---|---|
| Studied | Simulated | |||
| Regimen (1) | Regimen (2) | Regimen (3) | ||
| 8 mg/L | 20 | 98 | 98 | 99 |
| 39.9 | 97 | 97 | 99 | |
| 60 | 96 | 98 | 98 | |
| 90 | 96 | 96 | 98 | |
| 120 | 87 | 87 | 94 | |
| 150 | 73 | 73 | 90 | |
| 16 mg/L | 20 | 93 | 95 | 96 |
| 39.9 | 88 | 91 | 94 | |
| 60 | 76 | 80 | 90 | |
| 90 | 51 | 54 | 75 | |
| 120 | 32 | 33 | 56 | |
| 150 | 22 | 22 | 40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngougni Pokem, P.; Wittebole, X.; Collienne, C.; Rodriguez-Villalobos, H.; Tulkens, P.M.; Elens, L.; Van Bambeke, F.; Laterre, P.-F. Population Pharmacokinetics of Temocillin Administered by Continuous Infusion in Patients with Septic Shock Associated with Intra-Abdominal Infection and Ascitic Fluid Effusion. Antibiotics 2022, 11, 898. https://doi.org/10.3390/antibiotics11070898
Ngougni Pokem P, Wittebole X, Collienne C, Rodriguez-Villalobos H, Tulkens PM, Elens L, Van Bambeke F, Laterre P-F. Population Pharmacokinetics of Temocillin Administered by Continuous Infusion in Patients with Septic Shock Associated with Intra-Abdominal Infection and Ascitic Fluid Effusion. Antibiotics. 2022; 11(7):898. https://doi.org/10.3390/antibiotics11070898
Chicago/Turabian StyleNgougni Pokem, Perrin, Xavier Wittebole, Christine Collienne, Hector Rodriguez-Villalobos, Paul M. Tulkens, Laure Elens, Françoise Van Bambeke, and Pierre-François Laterre. 2022. "Population Pharmacokinetics of Temocillin Administered by Continuous Infusion in Patients with Septic Shock Associated with Intra-Abdominal Infection and Ascitic Fluid Effusion" Antibiotics 11, no. 7: 898. https://doi.org/10.3390/antibiotics11070898
APA StyleNgougni Pokem, P., Wittebole, X., Collienne, C., Rodriguez-Villalobos, H., Tulkens, P. M., Elens, L., Van Bambeke, F., & Laterre, P.-F. (2022). Population Pharmacokinetics of Temocillin Administered by Continuous Infusion in Patients with Septic Shock Associated with Intra-Abdominal Infection and Ascitic Fluid Effusion. Antibiotics, 11(7), 898. https://doi.org/10.3390/antibiotics11070898







