Genetic Diversity of Virulent Polymyxin-Resistant Klebsiella aerogenes Isolated from Intensive Care Units
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Patient Characteristics
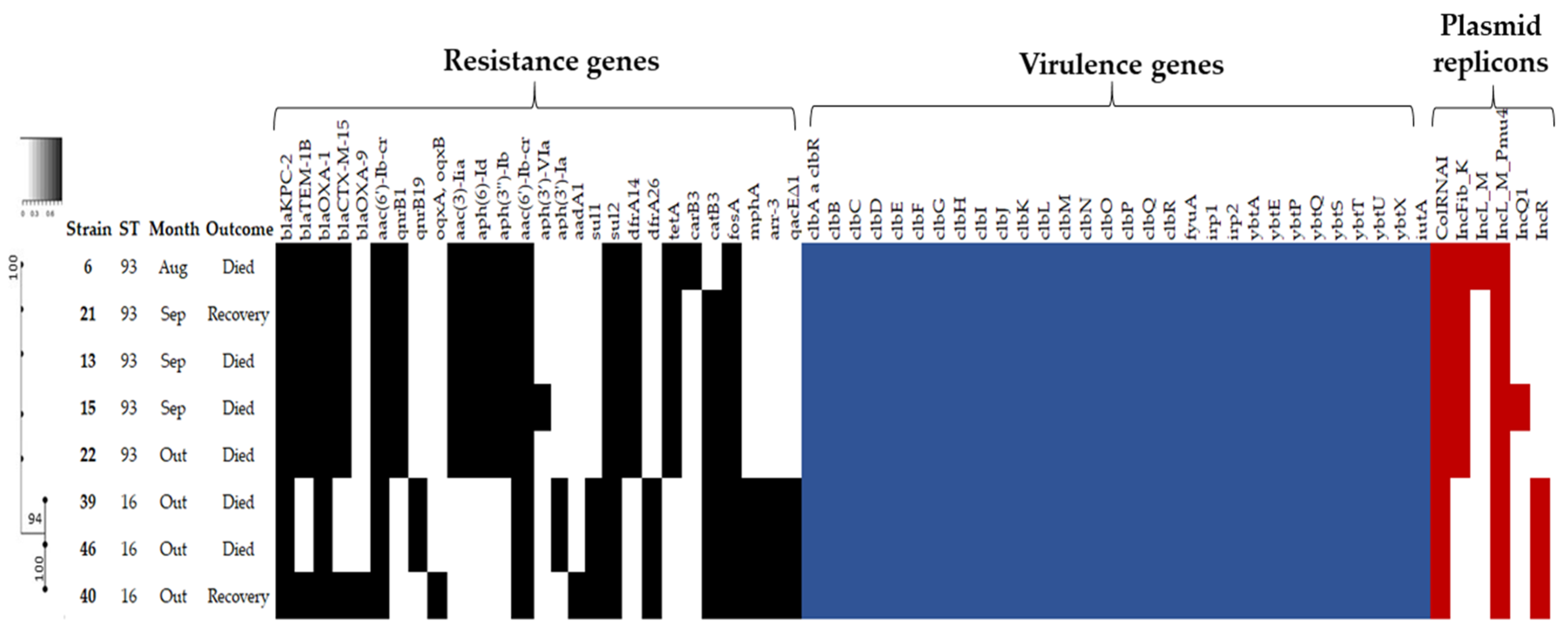
2.2. Antimicrobial Susceptibility Profile, Resistome and Plasmid Incompatibility Groups
3. Materials and Methods
3.1. Bacterial Strains
3.2. Bacterial Identification and Antimicrobial Susceptibility Testing
3.3. Whole-Genome Sequencing (WGS)
3.4. Bioinformatics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strain | Age/Sex | Clinical Isolates | Data of Isolation | Length of Stay (Days) | Place Prior to Admission | Clinical Signs of Sepsis | Outcome | Exposure to Antibiotics | Prior PMB (Days) |
|---|---|---|---|---|---|---|---|---|---|
| 6 | 31/F | Blood | 08/09/2016 | 29 | Home | Yes | Death | Carbapenems/Cephalosporins/ Polymyxin B | 8 |
| 13 | 48/F | Tracheal aspirates | 09/02/2016 | 87 | Another hospital | No | Recovery | Carbapenems/Cephalosporins | - |
| 15 | 50/M | Urine | 09/12/2016 | 84 | Another hospital | No | Recovery | Aminoglycosides/ Carbapenems | - |
| 21 | 38/M | Tracheal aspirates | 09/18/2016 | 49 | Another hospital | Yes | Death | Aminoglycosides/ Carbapenems | - |
| 22 | 76/M | Blood | 10/03/2016 | 19 | Another hospital | Yes | Death | Aminoglycosides/ Carbapenems | - |
| 39 | 48/F | Blood | 10/05/2016 | 16 | Another hospital | Yes | Death | Carbapenems/ Cephalosporins/ Polymyxin B | 6 |
| 40 | 36/F | Blood | 10/11/2016 | 29 | Home | Yes | Death | Carbapenems/Glycycycline/ Glycopeptide | - |
| 46 | 33/F | Urine | 10/23/2016 | 35 | Home | No | Recovery | Aminoglycosides/Cephalosporins/ Penicillins | - |
| Strain | ST | MICs (mg/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CTX | CRO | FEP | ATM | IPM | MEM | ETP | AMK | GEN | CIP | LEV | PMB | ||
| 6 | 93 | >256 (R) | >256 (R) | >256 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 64 (R) | >32 (R) | 16 (R) | 8 (R) | 16 (R) |
| 13 | 93 | >256 (R) | >256 (R) | >256 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 64 (R) | >32 (R) | 16 (R) | 8 (R) | 32 (R) |
| 15 | 93 | >256 (R) | >256 (R) | >256 (R) | 128 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 32 (I) | >32 (R) | 16 (R) | 8 (R) | 16 (R) |
| 21 | 93 | >256 (R) | >256 (R) | 128 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 32 (I) | >32 (R) | 16 (R) | 8 (R) | 32 (R) |
| 22 | 93 | 128 (R) | >256 (R) | >256 (R) | 128 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 32 (I) | >32 (R) | 16 (R) | 8 (R) | 32 (R) |
| 39 | 16 | >256 (R) | >256 (R) | >256 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 64 (R) | >32 (R) | 16 (R) | 8 (R) | 8 (R) |
| 40 | 16 | >256 (R) | >256 (R) | 128 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 64 (R) | >32 (R) | 16 (R) | 8 (R) | 8 (R) |
| 46 | 16 | 128 (R) | >256 (R) | >256 (R) | >256 (R) | >32 (R) | >16 (R) | >16 (R) | >32 (R) | 64 (R) | >32 (R) | 16 (R) | 8 (R) | 8 (R) |
| Strains | ST | Genes | ENA Run Accession | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mgrB | phoP | phoQ | pmrA | pmrB | eptA | arnT | |||
| 6 | 93 | M1V, G37S | - | - | - | - | T296S | - | ERR4298505 |
| 13 | 93 | M1V, G37S | - | - | - | - | T296S | - | ERR4298507 |
| 15 | 93 | M1V, G37S | - | - | - | - | T296S | - | ERR4298508 |
| 21 | 93 | M1V | - | - | - | - | T296S | - | ERR4298509 |
| 22 | 93 | M1V | - | - | - | - | T296S | - | ERR4298510 |
| 39 | 16 | M1V | - | - | - | T157P | - | - | ERR4298511 |
| 40 | 16 | M1V | - | - | Q140L, G145E | R256G | L252A, P257A | G127A, F353V, A462G | ERR4298512 |
| 46 | 16 | M1V | - | - | - | T157P | - | - | ERR4298513 |
References
- Davin-Regli, A.; Pages, J.-M. Enterobacter Aerogenes and Enterobacter cloacae; Versatile Bacterial Pathogens Confronting Antibiotic Treatment. Front. Microbiol. 2015, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- McCusker, M.P.; Alves Ferreira, D.; Cooney, D.; Martins Alves, B.; Fanning, S.; Pagès, J.-M.; Martins, M.; Davin-Regli, A. Modulation of Antimicrobial Resistance in Clinical Isolates of Enterobacter aerogenes: A Strategy Combining Antibiotics and Chemosensitisers. J. Glob. Antimicrob. Resist. 2019, 16, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Xu, Q.; Zhang, H. Emergence of NDM-5 Producing Carbapenem-Resistant Klebsiella aerogenes in a Pediatric Hospital in Shanghai, China. Front. Public Health 2021, 9, 621527. [Google Scholar] [CrossRef]
- Azevedo, P.A.A.; Furlan, J.P.R.; Oliveira-Silva, M.; Nakamura-Silva, R.; Gomes, C.N.; Costa, K.R.C.; Stehling, E.G.; Pitondo-Silva, A. Detection of Virulence and β-Lactamase Encoding Genes in Enterobacter aerogenes and Enterobacter Cloacae Clinical Isolates from Brazil. Braz. J. Microbiol. 2018, 49, 224–228. [Google Scholar] [CrossRef]
- WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The Global Distribution and Spread of the Mobilized Colistin Resistance Gene Mcr-1. Nat Commun 2018, 9, 1179. [Google Scholar] [CrossRef]
- Jeannot, K.; Bolard, A.; Plésiat, P. Resistance to Polymyxins in Gram-Negative Organisms. Int. J. Antimicrob. Agents 2017, 49, 526–535. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Wyres, K.L.; Gorrie, C.L.; Judd, L.M.; Jenney, A.W.J.; Brisse, S.; Holt, K.E. Genetic Diversity, Mobilisation and Spread of the Yersiniabactin-Encoding Mobile Element ICEKp in Klebsiella pneumoniae Populations. Microb. Genom. 2018, 4, e000196. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wyres, K.L.; Duchêne, S.; Wick, R.R.; Judd, L.M.; Gan, Y.-H.; Hoh, C.-H.; Archuleta, S.; Molton, J.S.; Kalimuddin, S.; et al. Population Genomics of Hypervirulent Klebsiella pneumoniae Clonal-Group 23 Reveals Early Emergence and Rapid Global Dissemination. Nat. Commun. 2018, 9, 2703. [Google Scholar] [CrossRef]
- Malek, A.; McGlynn, K.; Taffner, S.; Fine, L.; Tesini, B.; Wang, J.; Mostafa, H.; Petry, S.; Perkins, A.; Graman, P.; et al. Next-Generation-Sequencing-Based Hospital Outbreak Investigation Yields Insight into Klebsiella aerogenes Population Structure and Determinants of Carbapenem Resistance and Pathogenicity. Antimicrob. Agents Chemother. 2019, 63, e02577-18. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Chen, L.; Du, H. Virulence Factors in Hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 642484. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-K.; Ko, K.S. PmrAB and PhoPQ Variants in Colistin-Resistant Enterobacter Spp. Isolates in Korea. Curr. Microbiol. 2019, 76, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; Giani, T.; D’Andrea, M.M.; Di Pilato, V.; Arena, F.; Conte, V.; Tryfinopoulou, K.; Vatopoulos, A.; Rossolini, G.M. MgrB Inactivation Is a Common Mechanism of Colistin Resistance in KPC-Producing Klebsiella pneumoniae of Clinical Origin. Antimicrob. Agents Chemother. 2014, 58, 5696–5703. [Google Scholar] [CrossRef]
- Da Silva, K.E.; Thi Nguyen, T.N.; Boinett, C.J.; Baker, S.; Simionatto, S. Molecular and Epidemiological Surveillance of Polymyxin-Resistant Klebsiella pneumoniae Strains Isolated from Brazil with Multiple MgrB Gene Mutations. Int. J. Med. Microbiol. 2020, 310, 151448. [Google Scholar] [CrossRef]
- Gerson, S.; Betts, J.W.; Lucaßen, K.; Nodari, C.S.; Wille, J.; Josten, M.; Göttig, S.; Nowak, J.; Stefanik, D.; Roca, I.; et al. Investigation of Novel PmrB and EptA Mutations in Isogenic Acinetobacter baumannii Isolates Associated with Colistin Resistance and Increased Virulence In Vivo. Antimicrob. Agents Chemother. 2019, 63, e01586-18. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Song, J.; Velkov, T.; Wang, L.; Zhu, Y.; Li, J. Regulating Polymyxin Resistance in Gram-Negative Bacteria: Roles of Two-Component Systems PhoPQ and PmrAB. Future Microbiol. 2020, 15, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, A.; Marshall, S.H.; Perez, F.; Tomas, M.; Jacobs, M.R.; Hujer, A.M.; Domitrovic, T.N.; Rudin, S.D.; Rojas, L.J.; Kreiswirth, B.N.; et al. Emergence of Resistance to Colistin During the Treatment of Bloodstream Infection Caused by Klebsiella pneumoniae Carbapenemase–Producing Klebsiella pneumoniae. Open Forum Infect. Dis. 2018, 5, ofy054. [Google Scholar] [CrossRef]
- Liao, W.; Quan, J.; Liu, L.; Zhao, D.; Jiang, Y.; Du, X.; Zhao, F.; Yu, Y.; Zhou, Z. New Insights into the Mechanisms of Colistin Resistance in Klebsiella aerogenes of Clinical Origin. Int. J. Antimicrob. Agents 2020, 55, 105990. [Google Scholar] [CrossRef]
- Matheeussen, V.; Xavier, B.B.; Mermans, I.; De Weerdt, A.; Lammens, C.; Goossens, H.; Jansens, H.; Malhotra-Kumar, S. Emergence of Colistin Resistance during Treatment of Recurrent Pneumonia Caused by Carbapenemase Producing Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2019, 94, 407–409. [Google Scholar] [CrossRef]
- Mhaya, A.; Bégu, D.; Tounsi, S.; Arpin, C. MgrB Inactivation Is Responsible for Acquired Resistance to Colistin in Enterobacter Hormaechei Subsp. Steigerwaltii. Antimicrob. Agents Chemother. 2020, 64, e00128-20. [Google Scholar] [CrossRef]
- Nang, S.C.; Han, M.-L.; Yu, H.H.; Wang, J.; Torres, V.V.L.; Dai, C.; Velkov, T.; Harper, M.; Li, J. Polymyxin Resistance in Klebsiella pneumoniae: Multifaceted Mechanisms Utilized in the Presence and Absence of the Plasmid-Encoded Phosphoethanolamine Transferase Gene Mcr-1. J. Antimicrob. Chemother. 2019, 74, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Wesevich, A.; Sutton, G.; Ruffin, F.; Park, L.P.; Fouts, D.E.; Fowler, V.G.; Thaden, J.T. Newly Named Klebsiella Aerogenes (Formerly Enterobacter aerogenes) Is Associated with Poor Clinical Outcomes Relative to Other Enterobacter Species in Patients with Bloodstream Infection. J. Clin. Microbiol. 2020, 58, e00582-20. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Scharf, C.; Rocha, J.L.; Cieslinsk, J.; Becker, G.N.; Arend, L.N. KPC-Producing Enterobacter Aerogenes Infection. Braz. J. Infect. Dis. 2015, 19, 324–327. [Google Scholar] [CrossRef]
- Álvarez-Marín, R.; Navarro-Amuedo, D.; Gasch-Blasi, O.; Rodríguez-Martínez, J.M.; Calvo-Montes, J.; Lara-Contreras, R.; Lepe-Jiménez, J.A.; Tubau-Quintano, F.; Cano-García, M.E.; Rodríguez-López, F.; et al. A Prospective, Multicenter Case Control Study of Risk Factors for Acquisition and Mortality in Enterobacter Species Bacteremia. J. Infect. 2020, 80, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Passarelli-Araujo, H.; Palmeiro, J.K.; Moharana, K.C.; Pedrosa-Silva, F.; Dalla-Costa, L.M.; Venancio, T.M. Genomic Analysis Unveils Important Aspects of Population Structure, Virulence, and Antimicrobial Resistance in Klebsiella aerogenes. FEBS J. 2019, 286, 3797–3810. [Google Scholar] [CrossRef] [PubMed]
- De Florio, L.; Riva, E.; Giona, A.; Dedej, E.; Fogolari, M.; Cella, E.; Spoto, S.; Lai, A.; Zehender, G.; Ciccozzi, M.; et al. MALDI-TOF MS Identification and Clustering Applied to Enterobacter Species in Nosocomial Setting. Front. Microbiol. 2018, 9, 1885. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids Carrying Antimicrobial Resistance Genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef]
- Haeili, M.; Javani, A.; Moradi, J.; Jafari, Z.; Feizabadi, M.M.; Babaei, E. MgrB Alterations Mediate Colistin Resistance in Klebsiella pneumoniae Isolates from Iran. Front. Microbiol. 2017, 8, 2470. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Y.; Yi, L.; Liu, J.-H.; Yang, Q. Emergent Polymyxin Resistance: End of an Era? Open Forum Infect. Dis. 2019, 6, ofz368. [Google Scholar] [CrossRef]
- Uechi, K.; Tada, T.; Shimada, K.; Nakasone, I.; Kirikae, T.; Fujita, J. Emergence of a Carbapenem-Resistant and Colistin-Heteroresistant Enterobacter cloacae Clinical Isolate in Japan. J. Infect. Chemother. 2019, 25, 285–288. [Google Scholar] [CrossRef]
- De Araújo Longo, L.G.; Fontana, H.; Santos de Sousa, V.; Chilinque Zambão da Silva, N.; Souto Martins, I.; Meurer Moreira, B. Emergence of MgrB Locus Deletion Mediating Polymyxin Resistance in Pandemic KPC-Producing Klebsiella pneumoniae ST15 Lineage. J. Med. Microbiol. 2021, 70, 001309. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, V.; Conzemius, R.; Varda-Brkić, D.; Bogdan, M.; Grisold, A.; Gyssens, I.C.; Bedenić, B.; Barišić, I. Epidemiology of Colistin-Resistant, Carbapenemase-Producing Enterobacteriaceae and Acinetobacter baumannii in Croatia. Infect. Genet. Evol. 2020, 81, 104263. [Google Scholar] [CrossRef] [PubMed]
- Hamel, M.; Chatzipanagiotou, S.; Hadjadj, L.; Petinaki, E.; Papagianni, S.; Charalampaki, N.; Tsiplakou, S.; Papaioannou, V.; Skarmoutsou, N.; Spiliopoulou, I.; et al. Inactivation of MgrB Gene Regulator and Resistance to Colistin Is Becoming Endemic in Carbapenem-Resistant Klebsiella pneumoniae in Greece: A Nationwide Study from 2014 to 2017. Int. J. Antimicrob. Agents 2020, 55, 105930. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.E.; Rossato, L.; Leite, A.F.; Simionatto, S. Overview of Polymyxin Resistance in Enterobacteriaceae. Rev. Soc. Bras. Med. Trop. 2022, 55, e0349-2021. [Google Scholar] [CrossRef]
- Bedenić, B.; Vranić-Ladavac, M.; Venditti, C.; Tambić-Andrašević, A.; Barišić, N.; Gužvinec, M.; Karčić, N.; Petrosillo, N.; Ladavac, R.; di Caro, A. Emergence of Colistin Resistance in Enterobacter aerogenes from Croatia. J. Chemother. 2018, 30, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.-Y.; Lee, H.; Park, M.; Kang, K.; Lim, S.-K.; Shin, D.; Ko, K.S. Comparison of Fitness Cost and Virulence in Chromosome- and Plasmid-Mediated Colistin-Resistant Escherichia coli. Front. Microbiol. 2020, 11, 798. [Google Scholar] [CrossRef]
- Diene, S.M.; Merhej, V.; Henry, M.; El Filali, A.; Roux, V.; Robert, C.; Azza, S.; Gavory, F.; Barbe, V.; La Scola, B.; et al. The Rhizome of the Multidrug-Resistant Enterobacter aerogenes Genome Reveals How New “Killer Bugs” Are Created Because of a Sympatric Lifestyle. Mol. Biol. Evol. 2013, 30, 369–383. [Google Scholar] [CrossRef]
- Sampaio, J.L.M.; Gales, A.C. Antimicrobial Resistance in Enterobacteriaceae in Brazil: Focus on β-Lactams and Polymyxins. Braz. J. Microbiol. 2016, 47, 31–37. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing (M100); CLSI: Wayne, PA, USA, 2020. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Dung, T.T.N.; Duy, P.T.; Sessions, O.M.; Sangumathi, U.K.; Phat, V.V.; Tam, P.T.T.; To, N.T.N.; Phuc, T.M.; Hong Chau, T.T.; Chau, N.N.M.; et al. A Universal Genome Sequencing Method for Rotavirus a from Human Fecal Samples Which Identifies Segment Reassortment and Multi-Genotype Mixed Infection. BMC Genom. 2017, 18, 324. [Google Scholar] [CrossRef]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC Results into a Web-Based, Interactive and Extensible FASTQ Quality Control Tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid Large-Scale Prokaryote Pan Genome Analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Inouye, M.; Dashnow, H.; Raven, L.-A.; Schultz, M.B.; Pope, B.J.; Tomita, T.; Zobel, J.; Holt, K.E. SRST2: Rapid Genomic Surveillance for Public Health and Hospital Microbiology Labs. Genome Med. 2014, 6, 90. [Google Scholar] [CrossRef]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic Analysis of Diversity, Population Structure, Virulence and Antimicrobial Resistance in Klebsiella pneumoniae, an Urgent Threat to Public Health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chan, A.P. PROVEAN Web Server: A Tool to Predict the Functional Effect of Amino Acid Substitutions and Indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, K.E.; de Almeida de Souza, G.H.; Moura, Q.; Rossato, L.; Limiere, L.C.; Vasconcelos, N.G.; Simionatto, S. Genetic Diversity of Virulent Polymyxin-Resistant Klebsiella aerogenes Isolated from Intensive Care Units. Antibiotics 2022, 11, 1127. https://doi.org/10.3390/antibiotics11081127
da Silva KE, de Almeida de Souza GH, Moura Q, Rossato L, Limiere LC, Vasconcelos NG, Simionatto S. Genetic Diversity of Virulent Polymyxin-Resistant Klebsiella aerogenes Isolated from Intensive Care Units. Antibiotics. 2022; 11(8):1127. https://doi.org/10.3390/antibiotics11081127
Chicago/Turabian Styleda Silva, Kesia Esther, Gleyce Hellen de Almeida de Souza, Quézia Moura, Luana Rossato, Letícia Cristina Limiere, Nathalie Gaebler Vasconcelos, and Simone Simionatto. 2022. "Genetic Diversity of Virulent Polymyxin-Resistant Klebsiella aerogenes Isolated from Intensive Care Units" Antibiotics 11, no. 8: 1127. https://doi.org/10.3390/antibiotics11081127






