4.1. Chemistry
All the required chemicals were purchased from Merck, GLR, Sigma-Aldrich and were used without further purification. Pre-coated Merck Silica gel 60 F254 TLC aluminum sheets were used under UV light and I2 vapor staining was used for visualization of spots. Purification was performed by column chromatography using Merck 230–400 mesh silica gel. Digital BÜCHI melting point apparatus (M-560) was used for the measurement of melting points and were uncorrected. IR spectra were recorded, and only major peaks are reported in cm−1 using Agilent Cary 630 FT-IR spectrometer. DMSO-d6 (as solvent) with TMS as an internal standard on Bruker Spectrospin DPX-300 spectrometer at 300 MHz and 75 MHz were used for 1H and 13C NMR spectra respectively. s (singlet), d (doublet), t (triplet), m (multiplet) or brs (broad) were labelled as splitting pattern. The chemical shift values of 1H NMR were reported in parts per million (ppm) relative to residual solvent (DMSO-d6, δ 7.26) and 13C NMR chemical shifts (δ) of 13C NMR were reported in ppm relative to (DMSO-d6, δ 77.16) and Hertz (Hz) is the unit of coupling constants (J). Agilent Quadrupole-6150 LC/MS spectrometer was used for recording of mass spectra. Synthesis protocols and spectral characterization is discussed below:
4.1.1. Synthesis of Ethyl 4-(3-Bromophenyl)-2,4-Dioxobutanoate (3)
In a dried RB flask, Na metal (0.09 g, 3.70 mmol) was cautiously added portion-wise to anhyd. EtOH (4.4 mL) under stirring until all the Na had reacted. Ethyl oxalate 2 (0.60 g, 4.07 mmol) and then the ketone 1 (3.70 mmol) were added sequentially. The solution was stirred at room temperature for 1 h and then slowly poured into ice. To this cold mixture, 2 M HCl solution was added and the resulting suspension was filtered, and the collected solid was dried in air [
39].
4.1.2. Synthesis of Ethyl 3-(3-Bromophenyl)-1H-Pyrazole-3-Carboxylate (4)
To a mixture of diketoester 3 (1 g. 3.3 mmol) in acetic acid (10 mL), hydrazine hydrate (0.247 g. 4.95 mmol, 1.5 equiv.) was added and the reaction mixture was refluxed for 4 h. The reaction was confirmed by TLC, the mixture was concentrated, cooled and poured into crushed ice. The obtained solid was filtered and dried to afford a light-yellow solid material (0.75 g. 75%) [
39].
4.1.3. Synthesis of 3-(3-Bromophenyl)-1H-Pyrazole-3-Carbohydrazide (5)
In a dried RB flask, a solution of pyrazole ethyl ester (ethyl 3-(3-bromophenyl) isoxazole-5-carboxylate) 4 (1.0 equiv.) in ethanol was added hydrazine hydrate (5.0 equiv.) dropwise and the reaction mixture was refluxed overnight at 80 °C. After completion of the reaction, the reaction mixture was cooled to room temperature and added ice-cold water. The solid precipitate obtained was filtered and washed with cold ethanol. The solid product was dried under vacuo to afford the desired product [
40].
4.1.4. Synthesis of Substituted Isonitrosoacetanilides (7a–n)
To a solution of substituted aniline (6a–n) (4.29 mmol) in a dry round bottom flask was added water (23 mL) and 2 N HCl (1.72 mL). After complete dissolution of substituted aniline was then added in the order: anhyd. sodium sulphate (28.35 mmol), hydroxylamine hydrochloride (15.03 mmol) and chloral hydrate (5.58 mmol). The reaction mixture was then allowed to stir overnight at 55 °C and cooled to room temperature after completion of the reaction as monitored by TLC. The crude was filtered under vacuo, washed with distilled water to afford substituted isonitrosoacetanilides (7a–n) which were used to form Isatin derivatives without any further purification [
40].
4.1.5. Synthesis of Substituted Isatin (8a–n)
In a two-neck round bottom flask, 1.08 mL of concentrated sulfuric acid was warmed to 50 °C followed by addition of 1.52 mmol of dry isonitrosoacetanilide (7a–n) at such a rate to keep the temperature of the reaction vessel between 60–70 °C. The reaction mixture was heated to 80 °C for 15–20 min to complete the reaction as monitored by TLC. Finally, the reaction mixture was cooled to room temperature and poured onto ice water over three times its volume. The solid precipitate was filtered with suction, washed with water until sulfuric acid was completely removed, and dried to yield substituted Isatin (8a–n) with high purity [
40].
4.1.6. Synthesis of Substituted 3-(3-Bromophenyl)-N′-(2-Oxoindolin-3-Ylidene) -1H-Pyrazole-3-Carbohydrazide PS (1–14)
In a dried RB flask, pyrazole hydrazide (3-(3-bromophenyl)isoxazole-5-carbohydrazide) 5 (1.0 equiv.) dissolved in ethanol was added appropriate Isatin derivatives 8a–n followed by few drops of glacial acetic acid and the reaction mixture was refluxed for overnight at 80 °C. After completion of the reaction, the reaction mixture was cooled to room temperature and added ice-cold water. The solid precipitate obtained was filtered through a funnel and washed with ethanol. The solid product was dried under vacuo to afford the desired product (PS1–14) [
40].
4.1.7. (E)-3-(3-Bromophenyl)-N′-(2-Oxoindolin-3-YLIDENE)-1H-Pyrazole-5-Carbohydrazide (PS1)
Light yellow solid; yield 77%; Rf = 0.75 (Ethylacetate: Hexane = 50:50); mp: 218–221 °C; FT-IR (cm−1): 3298, 3033, 2842, 1610, 1546, 1499, 1320, 1152; 1H NMR (500 MHz, DMSO- d6) (δ, ppm): 13.88 (s, 1H, N-H, pyrazole ring), 11.32 (s, 1H, NH, hydrazone), 10.09 (s, 1H, N-H, Isatin ring), 7.57 (d, J = 7.4 Hz, 1H, Ar-H), 7.42 (d, J = 2.0 Hz, 1H, Ar-H), 7.34 (ddd, J = 16.8, 8.4, 1.6 Hz, 3H, Ar-H), 7.10–7.06 (m, 1H, Ar-H), 6.93 (dd, J = 8.0, 3.9 Hz, 2H, Ar-H),6.92 (s, 1H, Ar-H);13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.8, 163.6, 145.9, 132.73, 130.9, 130.1, 129.9, 129.2, 127.2, 124.5, 124.1, 123.7; ESI-MS (m/z) calcd. for C18H12BrN5O2: 409.01 Found: 410.25 [M+H]+
4.1.8. (E)-3-(3-Bromophenyl)-N′-(5-Methyl-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS2)
Yellow solid; yield 77%; Rf = 0.73 (Ethylacetate: Hexane = 50:50); mp: 209–213 °C; FT-IR (cm−1): 3349, 3212, 2953, 2853, 1548, 1473, 1335, 1160; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.93 (s, 1H, N-H, pyrazole ring), 11.34 (s, 1H, NH, hydrazone), 10.08 (s, 1H, N-H, Isatin ring), 7.57 (d, J = 7.4 Hz, 1H, Ar-H), 7.42 (d, J = 2.0 Hz, 1H, Ar-H), 7.34 (ddd, J = 16.8, 8.4, 1.6 Hz, 3H, Ar-H), 7.10–7.06 (m, 1H, Ar-H), 6.93 (dd, J = 8.0, 3.9 Hz, 2H, Ar-H), 2.70 (s, 3H, CH3). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.0, 162.1, 143.8, 140.9, 137.7, 134.0, 133.4, 132.9, 131.3, 130.1, 128.7, 125.6, 121.8, 121.4, 20.4; ESI-MS (m/z) calcd. for C19H14BrN5O2: 423.0331 Found: 424.10 [M+H]+.
4.1.9. (E)-3-(3-Bromophenyl)-N′-(6-Chloro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS3)
Light yellow solid; yield 77%; Rf = 0.71 (Ethylacetate: Hexane = 50:50); mp: 182–185 °C; FT-IR (cm−1): 3328, 3032, 1742, 1549, 1469,1328,1154; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.90 (s, 1H, N-H, pyrazole ring), 11.13 (s, 1H, NH, hydrazone), 10.10 (s, 1H, N-H, Isatin ring), 7.42 (d, J = 1.8 Hz, 1H, Ar-H), 7.32 (dd, J = 8.3, 1.8 Hz, 2H, Ar-H), 7.10 (d, J = 1.3 Hz, 1H, Ar-H), 6.92 (d, J = 8.2 Hz, 3H, Ar-H), 6.84 (d, J = 8.5 Hz, 1H, Ar-H);13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.0, 162.3, 146.0, 137.8, 133.4, 133.0, 131.3, 131.0, 130.3, 129.5, 128.7, 124.3, 124.2, 121.8; ESI-MS (m/z) calcd. for C18H11BrClN5O2:442.9785 Found: 443.60 [M+H]+.
4.1.10. (E)-3-(3-Bromophenyl)-N′-(5,7-Dimethyl-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS4)
Yellow solid; yield 76%; Rf = 0.69 (Ethylacetate: Hexane = 50:50); mp: 257–260 °C; FT-IR (cm−1): 3323, 3243, 3070, 2917, 1738 1490, 1332, 1170; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.93 (s, 1H, N-H, pyrazole ring,), 11.13 (s, 1H, NH, hydrazone), 10.10 (s, 1H, N-H, Isatin ring), 7.42 (d, J = 8.1 Hz, 1H, Ar-H), 7.41 (d, J = 2.0 Hz, 1H, Ar-H), 7.31 (dd, J = 8.3, 2.0 Hz, 1H, Ar-H), 7.10 (dd, J = 8.1, 1.8 Hz, 3H, Ar-H), 6.85 (d, J = 1.8 Hz, 1H, Ar-H), 2.82 (s, 3H, CH3), 2.74 (s, 3H, CH3). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.1, 163.7, 145.8, 137.4, 130.9, 130.2, 130.1, 129.3, 129.0, 124.4, 124.1, 122.6, 106.9, 105.2, 99.6, 56.6, 56.1; ESI-MS (m/z) calcd. for C20H16BrN5O2: 437.0487 Found: 438.01 [M+H]+.
4.1.11. (E)-N′-(5-Bromo-2-Oxoindolin-3-Ylidene)-3-(3-Bromophenyl)-1H-Pyrazole-5-Carbohydrazide (PS5)
Yellow solid; yield 79%; Rf = 0.70 (Ethylacetate: Hexane = 50:50); mp: 230–233 °C; FT-IR (cm−1): 3218, 2997, 1738, 1588, 1523, 1337, 1162; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.77 (s, 1H, N-H, pyrazole ring), 11.45 (s, 1H, NH, hydrazone), 10.11 (s, 1H, N-H, Isatin ring), 7.56 (d, J = 8.1 Hz, 2H, Ar-H), 7.41 (d, J = 2.0 Hz, 1H, Ar-H), 7.31 (dd, J = 8.3, 2.0 Hz, 2H, Ar-H), 7.10 (dd, J = 8.1, 1.8 Hz, 2H, Ar-H), 6.94 (d, J = 1.8 Hz, 1H, Ar-H); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 163.0, 161.2, 145.7, 132.4, 131.3, 130.9, 130.1, 129.3, 129.2, 124.5, 124.3, 124.0; ESI-MS (m/z) calcd. for C18H11Br2N5O2: 486.9279 Found: 487.20 [M+H]+.
4.1.12. (E)-3-(3-Bromophenyl)-N′-(6-Chloro-5-Fluoro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS6)
Light yellow solid; yield 79%; Rf = 0.71 (Ethylacetate: Hexane = 50:50); mp: 191–194 °C; FT-IR (cm−1): 3315, 2946, 2838, 1738, 1594, 1494, 1363, 1154; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.87 (s, 1H, N-H, pyrazole ring), 11.20 (s, 1H, NH, hydrazone), 10.08 (s, 1H, N-H, Isatin ring), 7.41 (d, J = 1.9 Hz, 1H, Ar-H), 7.38 (s, 1H, Ar-H), 7.31 (dd, J = 8.3, 1.8 Hz, 1H, Ar-H), 7.14 (d, J = 7.8 Hz, 1H, Ar-H), 6.92 (d, J = 8.2 Hz, 2H, Ar-H), 6.81 (d, J = 7.9 Hz, 1H, Ar-H);13C NMR (125 MHz, DMSO-d6) (δ, ppm): 163.50, 163.46, 153.5, 152.3, 145.9, 130.9, 130.1, 129.1, 124.5, 124.0, 119.8, 115.08, 115.07, 112.9; ESI-MS (m/z) calcd. for C18H10BrClFN5O2: 460.96 Found: 461.01 [M+H]+.
4.1.13. (E)-3-(3-Bromophenyl)-N′-(6-Fluoro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS7)
Yellow solid; yield 81%; Rf = 0.73 (Ethylacetate: Hexane = 50:50); mp: 228–230 °C; FT-IR (cm−1): 3388, 3272, 2950, 1738, 1548, 1484, 1315; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.81 (s, 1H, N-H, pyrazole ring), 11.25 (s, 1H, NH, hydrazone), 10.08 (s, 1H, N-H, Isatin ring), 7.41 (d, J = 1.9 Hz, 1H, Ar-H), 7.38 (s, 1H, Ar-H), 7.31 (dd, J = 8.3, 1.8 Hz, 2H, Ar-H), 7.14 (d, J = 7.8 Hz, 1H, Ar-H), 6.92 (d, J = 8.2 Hz, 2H, Ar-H), 6.81 (d, J = 7.9 Hz, 1H, Ar-H); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.4, 163.6, 159.1, 132.7, 132.7, 132.5, 129.9, 127.1, 126.6, 123.8, 115.5, 114.3,; ESI-MS (m/z) calcd. for C18H11BrFN5O2: 427.00 Found: 428.09 [M+H]+.
4.1.14. (E)-3-(3-Bromophenyl)-N′-(5-Chloro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS8)
Yellow solid; yield 76%; Rf = 0.70 (Ethylacetate: Hexane = 50:50); mp: 217–220 °C; FT-IR (cm−1): 3262, 3083, 1738, 1609, 1347, 1157; 1H NMR (500 MHz, DMSO- d6) (δ, ppm): δ 13.97 (s, 1H), 11.23 (s, 1H, N-H, pyrazole ring), 10.08 (s, 1H, NH, hydrazone), 7.42 (d, J = 2.0 Hz, 1H, N-H, Isatin ring), 7.32 (dd, J = 8.3, 1.9 Hz, 2H, Ar-H), 7.31 (s, 1H, Ar-H), 7.22 (d, J = 8.2 Hz, 2H, Ar-H), 6.93 (d, J = 7.4 Hz, 2H, Ar-H). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.0, 163.1, 162.9, 159.5, 158.8, 132.7, 132.5, 131.8, 126.3, 115.7, 114.3, 106.8, 105.2, 99.6; ESI-MS (m/z) calcd. for C18H11BrClN5O2: 442.9785 Found: 443.03 [M+H]+.
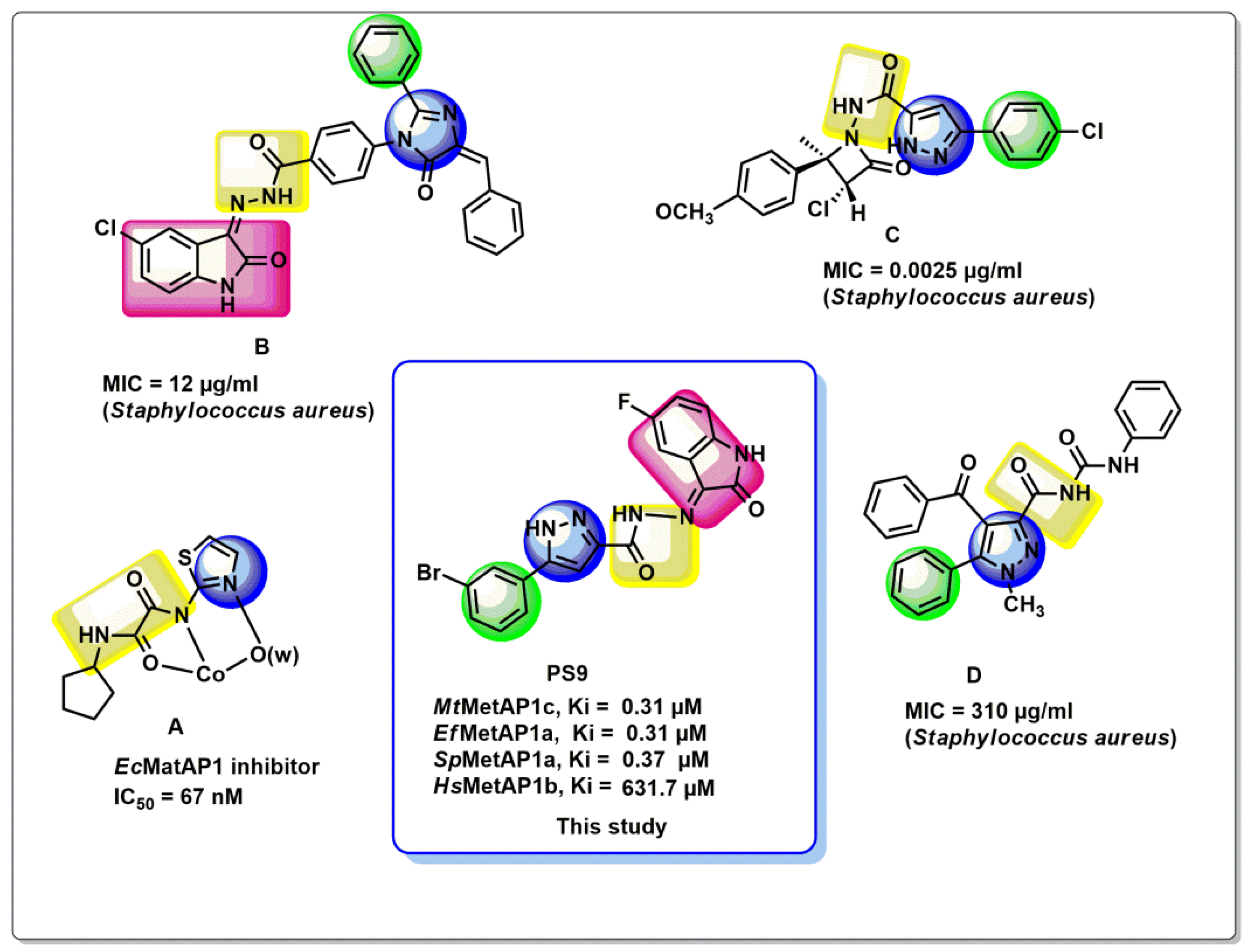
4.1.15. (E)-3-(3-Bromophenyl)-N′-(5-Fluoro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS9)
Dark yellow; yield 76%; Rf = 0.70 (Ethylacetate: Hexane = 50:50); mp: 229–232 °C; FT-IR (cm−1): 3152, 3033, 2891, 1582, 1457, 1325, 1164; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): δ 13.88 (s, 1H, N-H, pyrazole ring), 11.32 (s, 1H, NH, hydrazone), 10.09 (s, 1H, N-H, Isatin ring), 7.57 (d, J = 7.4 Hz, 1H, Ar-H), 7.42 (d, J = 2.0 Hz, 1H, Ar-H), 7.34 (ddd, J = 16.8, 8.4, 1.6 Hz, 3H, Ar-H), 7.10–7.06 (m, 1H, Ar-H), 6.93 (dd, J = 8.0, 3.9 Hz, 2H, Ar-H); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.2, 163.5, 162.9, 159.6, 143.7, 140.2, 133.9, 131.8, 129.7, 125.3, 122.0, 106.9, 105.2, 99.6; ESI-MS (m/z) calcd. for C18H11BrFN5O2: 427.0080 Found: 428.00 [M+H]+.
4.1.16. (E)-3-(3-Bromophenyl)-N′-(7-Nitro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS10)
Light yellow solid; Rf = 0.71 (Ethylacetate: Hexane = 50:50); yield 75%; mp: 215–218 °C; FT-IR (cm−1): 3362, 3255, 3079, 1738, 1563, 1456, 1158; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.92 (s, 1H, N-H, pyrazole ring), 11.20 (s, 1H, NH, hydrazone), 10.02 (s, 1H, N-H,. Isatin ring), 7.42 (d, J = 7.4 Hz, 1H, Ar-H), 7.38 (d, J = 2.0 Hz, 1H, Ar-H), 7.32 (d, J = 1.6 Hz, 2H, Ar-H), 7.15 (d, J = 2.1 Hz, 1H, Ar-H), 6.92 (d, J = 8.0 Hz, 2H Ar-H,), 6.80 (d, J = 2.8.0 Hz, 1H, Ar-H). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.2, 163.4, 148.8, 145.9, 133.3, 131.8, 130.9, 130.4, 129.4, 127.0, 125.2, 124.3, 124.2, 121.7; ESI-MS (m/z) calcd. for C18H11BrN6O4: 454.0025 Found: 455.01 [M+H]+.
4.1.17. (E)-3-(3-Bromophenyl)-N′-(5-Methoxy-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS11)
Reddish brown; yield 74%; Rf = 0.70 (Ethylacetate: Hexane = 50:50); mp: 245–248 °C; FT-IR (cm−1): 3379, 2971, 2839, 1738, 1607, 1585, 1460, 1330; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.81 (s, 1H, N-H, pyrazole ring), 11.20 (s, 1H, NH, hydrazone), 10.15 (s, 1H, N-H, Isatin ring), 7.42 (d, J = 7.4 Hz, 1H, Ar-H), 7.38 (d, J = 2.0 Hz, 1H, Ar-H), 7.32 (d, J = 1.6 Hz, 2H, Ar-H), 7.15 (d, J = 2.1 Hz, 1H, Ar-H), 6.92 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 2.8.0 Hz, 1H, Ar-H), 3.82 (s, 3H, OCH3). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.3, 162.9, 159.3, 148.8, 133.2, 132.9, 132.7, 131.8, 126.8, 125.3, 121.6, 115.2, 114.3; ESI-MS (m/z) calcd. for C19H14BrN5O3: 439.0280 Found: 440.20 [M+H]+.
4.1.18. (E)-3-(3-Bromophenyl)-N′-(6-Methoxy-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS12)
Dark brown solid; yield 81%; Rf = 0.60 (Ethylacetate: Hexane = 50:50);mp: 228–230 °C; FT-IR (cm−1): 3388, 3272, 2950, 1738, 1548, 1484, 1315; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.82 (s, 1H, N-H, pyrazole ring), 11.21 (s, 1H, NH, hydrazone), 10.02 (s, 1H, N-H, Isatin ring), 7.42 (d, J = 7.4 Hz, 1H, Ar-H), 7.38 (d, J = 2.0 Hz, 1H, Ar-H), 7.32 (d, J = 1.6 Hz, 2H, Ar-H), 7.15 (d, J = 2.1 Hz, 1H, Ar-H), 6.92 (d, J = 8.0 Hz, 2H, Ar-H), 6.80 (d, J = 2.8.0 Hz, 1H, Ar-H), 3.91 (s, 3H, OCH3). 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.4, 163.6, 159.1, 132.7, 132.7, 132.5, 129.9, 127.1,126.6, 123.8, 115.5, 114.3, 57.1; ESI-MS (m/z) calcd. for C19H14BrN5O3: 439.0280 Found: 440.10 [M+H]+.
4.1.19. (E)-3-(3-Bromophenyl)-N′-(7-Chloro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS13)
Yellow solid; yield 82%; Rf = 0.62 (Ethylacetate: Hexane = 50:50); mp: 205–208 °C; FT-IR (cm−1): 3332, 3241, 3082, 1605, 1483, 1341, 1167; 6.80; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.90 (s, 1H, N-H, pyrazole ring), 11.23 (s, 1H, NH, hydrazone), 10.08 (s, 1H, N-H,. Isatin ring), 7.42 (d, J = 7.4 Hz, 2H, Ar-H), 7.38 (d, J = 2.0 Hz, 2H, Ar-H), 7.32 (d, J = 1.6 Hz, 1H, Ar-H), 7.15 (d, J = 2.1 Hz, 2H, Ar-H), 6.92 (d, J = 8.0 Hz, 2H, Ar-H), 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.3, 163.5, 143.8, 140.9, 134.0, 133.9, 130.2, 127.9, 125.7, 121.5, 114.7; ESI-MS (m/z) calcd. for C18H11BrClN5O2: 442.9785 Found: 443.25 [M+H]+.
4.1.20. (E)-3-(3-Bromophenyl)-N′-(6-Nitro-2-Oxoindolin-3-Ylidene)-1H-Pyrazole-5-Carbohydrazide (PS14)
Light brown; yield 75%; Rf = 0.72 (Ethylacetate: Hexane = 50:50);mp: 225–228 °C; FT-IR (cm−1): 3325, 3253, 3087, 1741, 1536, 1463, 1343, 1169; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 13.88 (s, 1H, N-H, pyrazole ring), 11.32 (s, 1H, NH, hydrazone), 10.09 (s, 1H, N-H, Isatin ring), 7.42 (d, J = 7.4 Hz, 1H, Ar-H), 7.38 (d, J = 2.0 Hz, 1H, Ar-H), 7.32 (d, J = 1.6 Hz, 3H, Ar-H), 7.15 (d, J = 2.1 Hz, 1H, Ar-H), 6.92 (d, J = 8.0 Hz, 2H, Ar-H); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 164.2, 163.4, 148.8, 145.9, 133.3, 131.8, 130.9, 130.4, 129.4, 127.0, 125.2, 124.3, 124.2, 121.7; ESI-MS (m/z) calcd. for C18H11BrN6O4: 454.0025 Found: 455.08 [M+H]+.