In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii
Abstract
:1. Introduction
2. Results
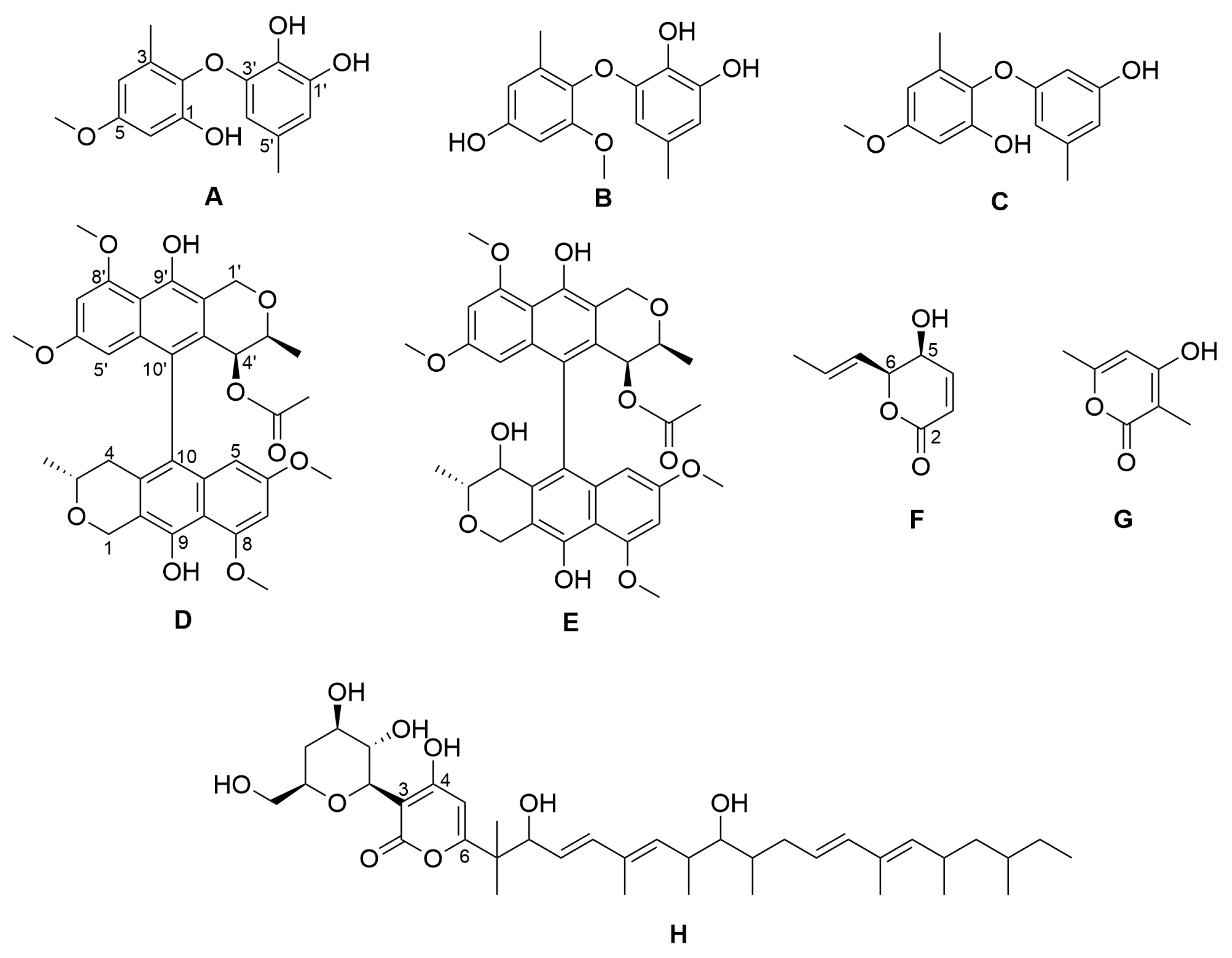
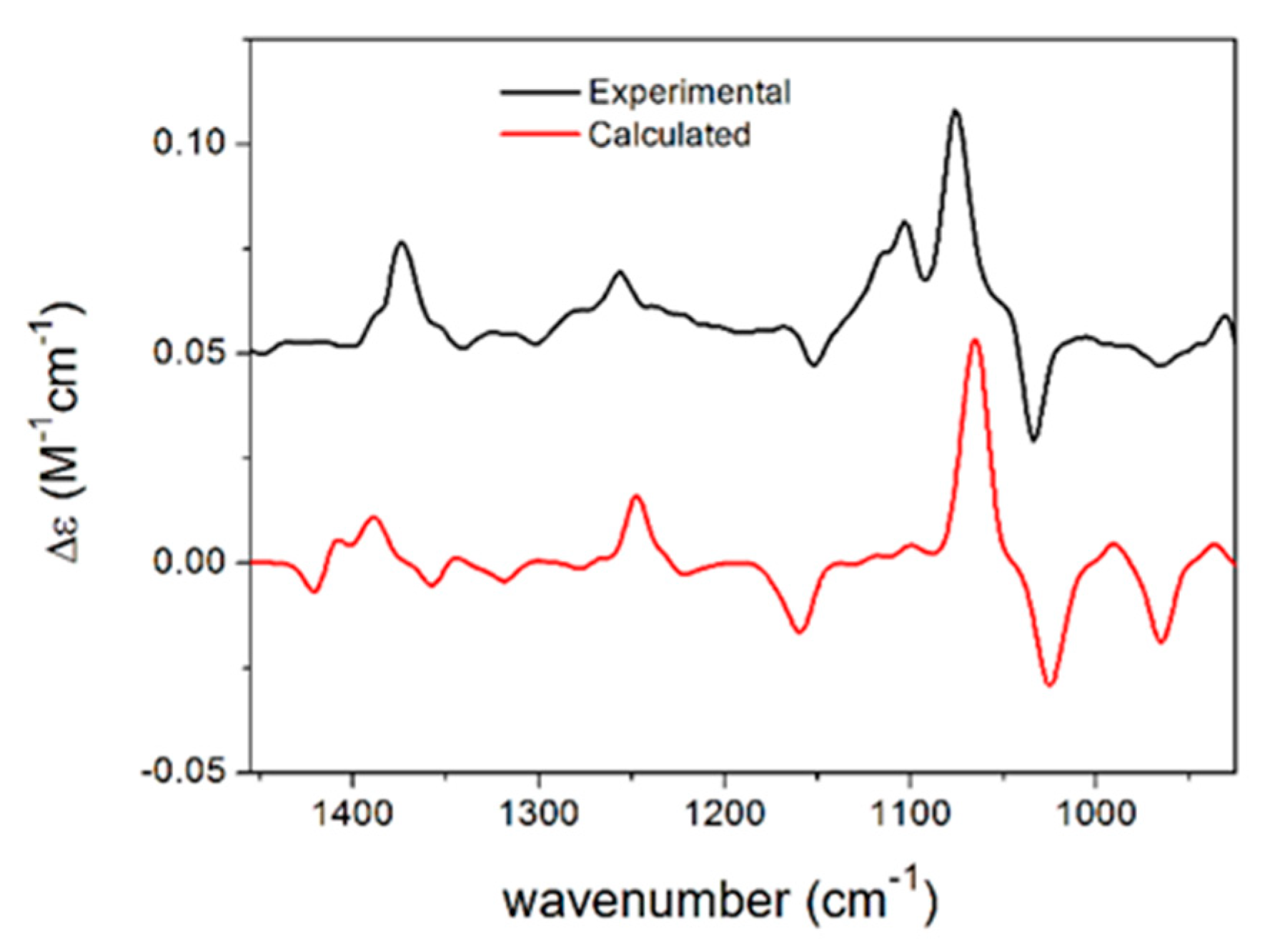
2.1. Isolation of Compounds from Paraboeremia selaginella
2.2. Anti-T. gondii Activity
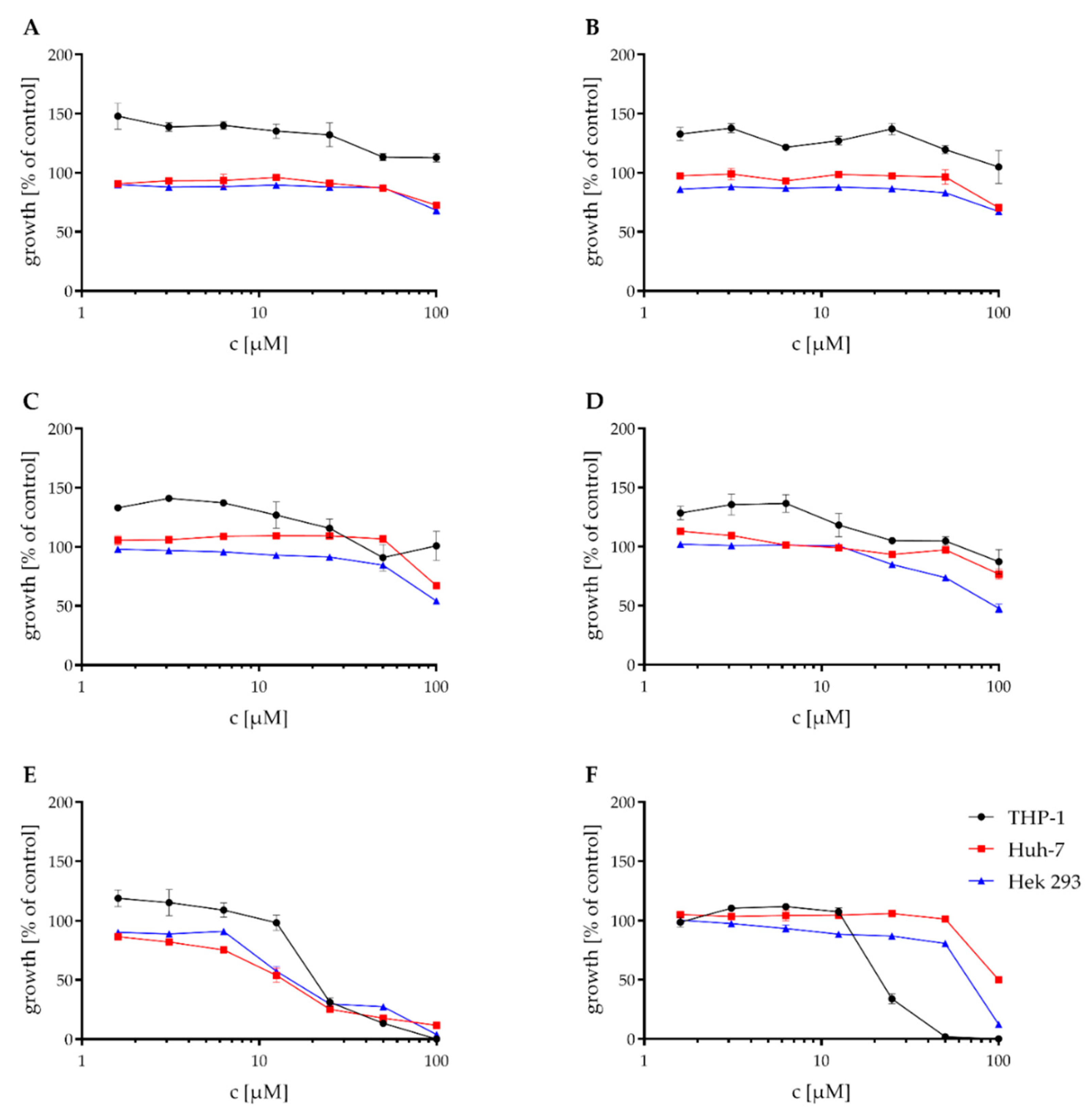
2.3. Cytotoxicity Assays
2.4. Determination of Anti-Bacterial Activity
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Fermentation and Extraction
4.4. Isolation
4.5. Preparation of Compounds for T. gondii Proliferation Assay
4.6. Parasites and Cell Culture for T. gondii Proliferation Assay
4.7. T. gondii Proliferation Assay
4.8. Cell Viability Assay against Hs27 Cells
4.9. Determination of the Minimal Inhibitory Concentration against Different Pathogenic Bacteria
4.10. Cytotoxicity Assay against Different Human Cell Lines
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frolich, S.; Entzeroth, R.; Wallach, M. Comparison of protective immune responses to apicomplexan parasites. J. Parasitol. Res. 2012, 2012, 852591. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Weiss, L.M. Toxoplasma gondii: The model apicomplexan. Int. J. Parasitol. 2004, 34, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Toxoplasmosis of Animals and Man. By J.P. Dubey and C. P. Beattie. 220 pages. ISBN 0 8493 4618 5. CRC Press, Boca Raton, 1988. £108.00. Parasitology 2009, 100, 500–501. [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Saadatnia, G.; Golkar, M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012, 44, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Furtado, J.M.; Smith, J.R.; Belfort, R., Jr.; Gattey, D.; Winthrop, K.L. Toxoplasmosis: A global threat. J. Glob. Infect. Dis. 2011, 3, 281–284. [Google Scholar] [CrossRef]
- de Jong, P.T. Ocular toxoplasmosis; common and rare symptoms and signs. Int. Ophthalmol. 1989, 13, 391–397. [Google Scholar] [CrossRef]
- Elbez-Rubinstein, A.; Ajzenberg, D.; Dardé, M.L.; Cohen, R.; Dumètre, A.; Yera, H.; Gondon, E.; Janaud, J.C.; Thulliez, P. Congenital toxoplasmosis and reinfection during pregnancy: Case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 2009, 199, 280–285. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.M.; López-Estraño, C. Comparative analysis of stage specific gene regulation of apicomplexan parasites: Plasmodium falciparum and Toxoplasma gondii. Infect. Disord. Drug Targets 2010, 10, 303–311. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, K.; Masoori, L.; Ezzatpour, B.; Roozbehani, M.; Sheikhian, A.; Malekara, V.; Niazi, M.; Mardanshah, O.; Moradpour, K.; Mahmoudvand, H. The Experimental Role of Medicinal Plants in Treatment of Toxoplasma gondii Infection: A Systematic Review. Acta Parasitol. 2021, 66, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Costa, T.M.; Alberton, M.D.; Goulart, J.A.G.; Tavares, L.B.B. Medicinal fungi: A source of antiparasitic secondary metabolites. Appl. Microbiol. Biotechnol. 2018, 102, 5791–5810. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Tanaka, M.; Gohbara, M.; Fujimori, T. Phytotoxicity of three lactones from Nigrospora sacchari. Phytochemistry 1998, 48, 625–630. [Google Scholar] [CrossRef]
- Evans, R.H.; Ellestad, G.A.; Kunstmann, M.P. Two new metabolites from an unidentified nigrospora species. Tetrahedron Lett. 1969, 10, 1791–1794. [Google Scholar] [CrossRef]
- Gusmao, A.S.; Abreu, L.S.; Tavares, J.F.; de Freitas, H.F.; Silva da Rocha Pita, S.; Dos Santos, E.G.; Caldas, I.S.; Vieira, A.A.; Silva, E.O. Computer-Guided Trypanocidal Activity of Natural Lactones Produced by Endophytic Fungus of Euphorbia umbellata. Chem. Biodivers 2021, 18, e2100493. [Google Scholar] [CrossRef]
- Hussain, H.; Kock, I.; Al-Harrasi, A.; Al-Rawahi, A.; Abbas, G.; Green, I.R.; Shah, A.; Badshah, A.; Saleem, M.; Draeger, S.; et al. Antimicrobial chemical constituents from endophytic fungus Phoma sp. Asian Pac. J. Trop. Med. 2014, 7, 699–702. [Google Scholar] [CrossRef]
- Khambay, B.P.S.; Bourne, J.M.; Cameron, S.; Kerry, B.R.; Zaki, M.J. A nematicidal metabolite from Verticillium chlamydosporium. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 1098–1099. [Google Scholar] [CrossRef]
- Meepagala, K.M.; Johnson, R.D.; Techen, N.; Wedge, D.E.; Duke, S.O. Phomalactone from a Phytopathogenic Fungus Infecting ZINNIA elegans (ASTERACEAE) Leaves. J. Chem. Ecol. 2015, 41, 602–612. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Preedanon, S.; Phongpaichit, S.; Sakayaroj, J. Pyrone derivatives from the marine-derived fungus Nigrospora sp. PSU-F18. Phytochemistry 2009, 70, 554–557. [Google Scholar] [CrossRef]
- Wu, S.H.; Chen, Y.W.; Shao, S.C.; Wang, L.D.; Yu, Y.; Li, Z.Y.; Yang, L.Y.; Li, S.L.; Huang, R. Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem. Biodivers 2009, 6, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Mandi, A.; Kurtan, T. Applications of OR/ECD/VCD to the structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 889–918. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Paczal, A.; Kovacs, T.; Mandi, A.; Kotschy, A.; Kurtan, T. Synthesis and Vibrational Circular Dichroism Analysis of N-Heterocyclic Carbene Precursors Containing Remote Chirality Centers. Int. J. Mol. Sci 2022, 23, 3471. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-Y.; Jin, C.; Zhang, H.-M.; Jin, C.-M.; Shen, Q.-K.; Quan, Z.-S. Synthesis and Biological Evaluation of (+)-Usnic Acid Derivatives as Potential Anti-Toxoplasma gondii Agents. J. Agric. Food Chem. 2019, 67, 9630–9642. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Romero, C.; Ortega-Barría, E.; Arnold, A.E.; Cubilla-Rios, L. Activity against Plasmodium falciparum of Lactones Isolated from the Endophytic Fungus Xylaria sp. Pharm. Biol. 2008, 46, 700–703. [Google Scholar] [CrossRef]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef]
- McLeod, R.; Muench, S.P.; Rafferty, J.B.; Kyle, D.E.; Mui, E.J.; Kirisits, M.J.; Mack, D.G.; Roberts, C.W.; Samuel, B.U.; Lyons, R.E.; et al. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 2001, 31, 109–113. [Google Scholar] [CrossRef]
- Tipparaju, S.K.; Muench, S.P.; Mui, E.J.; Ruzheinikov, S.N.; Lu, J.Z.; Hutson, S.L.; Kirisits, M.J.; Prigge, S.T.; Roberts, C.W.; Henriquez, F.L.; et al. Identification and development of novel inhibitors of Toxoplasma gondii enoyl reductase. J. Med. Chem. 2010, 53, 6287–6300. [Google Scholar] [CrossRef]
- Toki, S.; Ando, K.; Kawamoto, I.; Sano, H.; Yoshida, M.; Matsuda, Y. ES-242-2, -3, -4, -5, -6, -7, and -8, novel bioxanthracenes produced by Verticillium sp., which act on the N-methyl-D-aspartate receptor. J. Antibiot. 1992, 45, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Jaturapat, A.; Isaka, M.; Hywel-Jones, N.L.; Lertwerawat, Y.; Kamchonwongpaisan, S.; Kirtikara, K.; Tanticharoen, M.; Thebtaranonth, Y. Bioxanthracenes from the insect pathogenic fungus. Cordyceps pseudomilitaris BCC 1620. I. Taxonomy, fermentation, isolation and antimalarial activity. J. Antibiot. 2001, 54, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.-I.; Hosoe, T.; Nozawa, K.; Okada, K.; de Campos Takaki, G.M.; Fukushima, K.; Miyaji, M.; Horie, Y.; Kawai, K.-I. Antifungal activity of pyranone and furanone derivatives, isolated from Aspergillus sp. IFM51759, against Aspergillus fumigatus. MYCOTOXINS-TOKYO- 2003, 53, 11–18. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Gupta, S. Identification of the antibiotic phomalactone from the entomopathogenic fungusHirsutella thompsonii var. synnematosa. J. Chem. Ecol. 1994, 20, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Krivobok, S.; Thomasson, F.; Seigle-Murandi, F.; Steiman, R.; Bottex-Gauthier, C. 6-Allyl-5, 6-dihydro-5-hydroxypyran-2-one, a lactone produced by a new Drechslera species: Specified 1H and 13C NMR assignments, mutagenic and immunomodulating testings. Die Pharm. 1994, 49, 605–607. [Google Scholar]
- Kumarihamy, M.; Ferreira, D.; Croom, E.M., Jr.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and Cytotoxic Cytochalasins from an Endophytic Fungus, Nemania sp. UM10M, Isolated from a Diseased Torreya taxifolia Leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef]
- Meier, D.; Hernandez, M.V.; van Geelen, L.; Muharini, R.; Proksch, P.; Bandow, J.E.; Kalscheuer, R. The plant-derived chalcone Xanthoangelol targets the membrane of Gram-positive bacteria. Bioorg Med. Chem. 2019, 27, 115151. [Google Scholar] [CrossRef]
- Pfefferkorn, E.R.; Pfefferkorn, L.C. Specific Labeling of Intracellular Toxoplasma gondii with Uracil. J. Protozool. 1977, 24, 449–453. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.; Kato, Y.; Morimoto, M.; Tomita, F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397–402. [Google Scholar] [CrossRef]
- Rehberg, N.; Akone, H.S.; Ioerger, T.R.; Erlenkamp, G.; Daletos, G.; Gohlke, H.; Proksch, P.; Kalscheuer, R. Chlorflavonin Targets Acetohydroxyacid Synthase Catalytic Subunit IlvB1 for Synergistic Killing of Mycobacterium tuberculosis. ACS Infect. Dis. 2018, 4, 123–134. [Google Scholar] [CrossRef]
- MacroModel; Schrödinger LLC. 2015. Available online: http://www.schrodinger.com/MacroModel (accessed on 31 July 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, V.; Barone, G.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision E.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Stephens, P.J.; Harada, N. ECD cotton effect approximated by the Gaussian curve and other methods. Chirality 2009, 22, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Varetto, U. Molekel 5.4; Swiss National Supercomputing Centre: Manno, Switzerland, 2009. [Google Scholar]
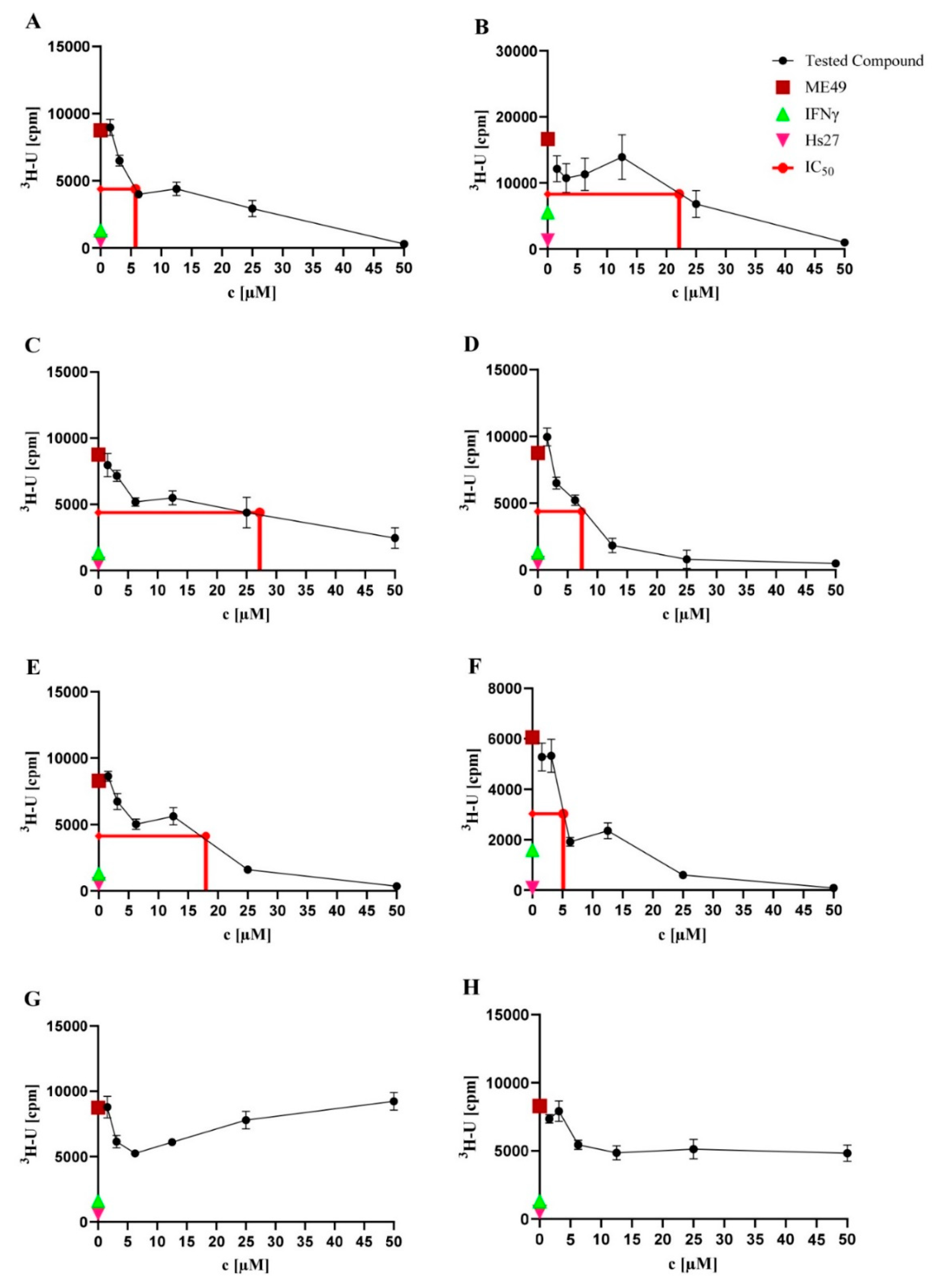

| Compound | IC50 (µM) |
|---|---|
| NK-A 17e233 (A) | 5.75 |
| 1,2-benzenediol, 3-(4-hydroxy-2-methoxy-6-methylphenoxy)-5-methyl-(ACI) (B) | 22.16 |
| cyperin (C) | 27.22 |
| ES-242-1 (D) | 7.38 |
| ES-242-3 (E) | 17.99 |
| 5S,6S-phomalactone (F) | 5.13 |
| methyltriaceticlactone (G) | Not active |
| S 39163/F-1 (H) | Not active |
| pyrimethamine | 0.06 |
| Compound | CC50 (µM) |
|---|---|
| A | >100 |
| B | >100 |
| C | >100 |
| D | >100 |
| E | >100 |
| F | 81 |
| Pyrimethamine | 44 |
| Compound | Mean IC50 [μM] | ||
|---|---|---|---|
| THP1 | Huh-7 | HEK-293 | |
| A | >100 | >100 | >100 |
| B | >100 | >100 | >100 |
| C | >100 | >100 | >100 |
| D | >100 | >100 | 93.8 |
| E | 21.9 | 13 | 16.95 |
| F | 24.3 | 100 | 66.9 |
| Compound | MIC90 [μM] | ||
|---|---|---|---|
| S. aureus ATCC 700699 | P. aeruginosa ATCC 87110 | M. tuberculosis H37Rv | |
| A | >100 | >100 | >100 |
| B | >100 | >100 | >100 |
| C | >100 | >100 | >100 |
| D | >100 | >100 | 50 |
| E | >100 | >100 | 100 |
| F | >100 | 100 | >100 |
| G | >100 | >100 | >100 |
| H | >100 | >100 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzone, F.; Simons, V.E.; van Geelen, L.; Frank, M.; Mándi, A.; Kurtán, T.; Pfeffer, K.; Kalscheuer, R. In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii. Antibiotics 2022, 11, 1176. https://doi.org/10.3390/antibiotics11091176
Mazzone F, Simons VE, van Geelen L, Frank M, Mándi A, Kurtán T, Pfeffer K, Kalscheuer R. In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii. Antibiotics. 2022; 11(9):1176. https://doi.org/10.3390/antibiotics11091176
Chicago/Turabian StyleMazzone, Flaminia, Viktor E. Simons, Lasse van Geelen, Marian Frank, Attila Mándi, Tibor Kurtán, Klaus Pfeffer, and Rainer Kalscheuer. 2022. "In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii" Antibiotics 11, no. 9: 1176. https://doi.org/10.3390/antibiotics11091176
APA StyleMazzone, F., Simons, V. E., van Geelen, L., Frank, M., Mándi, A., Kurtán, T., Pfeffer, K., & Kalscheuer, R. (2022). In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii. Antibiotics, 11(9), 1176. https://doi.org/10.3390/antibiotics11091176