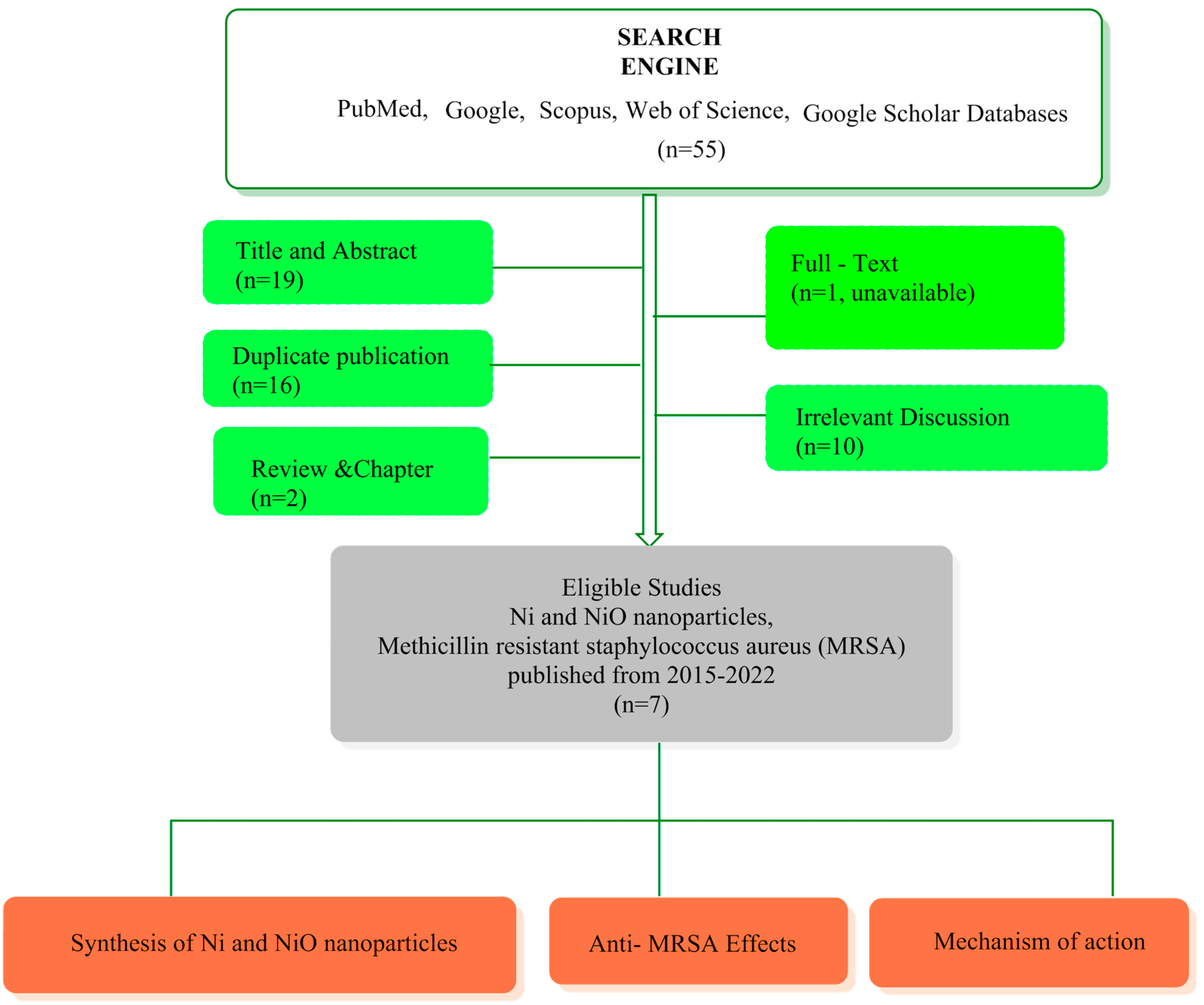
Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections
Abstract
:1. Background
2. Pathogenicity of MRSA
3. Nanoparticle Applications to Combat MRSA
4. Advantages and Disadvantages of Nanoparticles
5. Nanoparticle Features and Synthesis
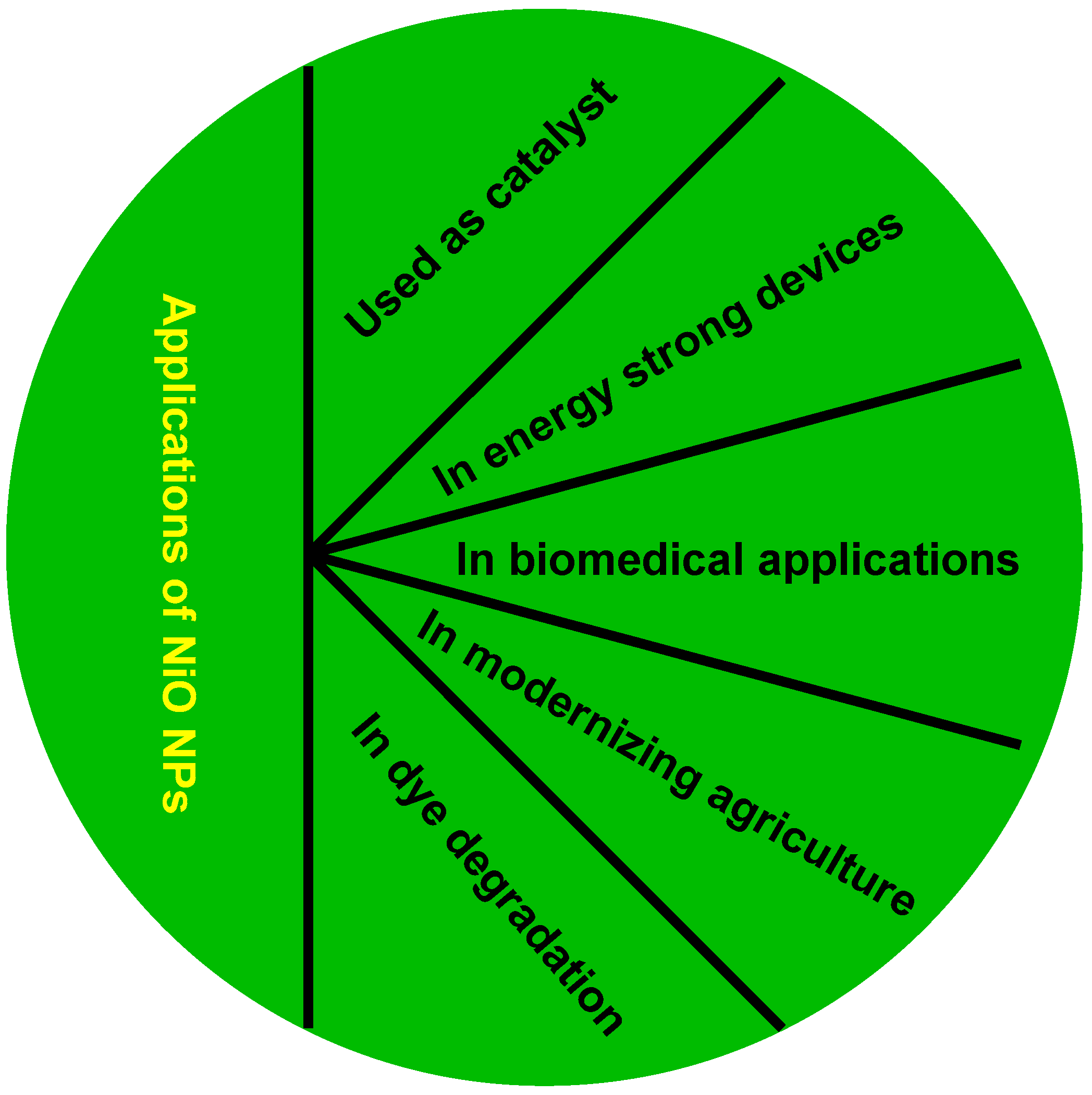
6. Importance of Nickel and Nickel-Oxide Nanoparticles
7. Mechanism of Action of Nickel Nanoparticles
8. Recent Data Regarding NiONP Effects against MRSA
9. Future Prospects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRSA | methicillin-resistant Staphylococcus aureus |
| HA-MRSA | healthcare-associated MRSA |
| CA-MRSA | community-associated MRSA |
| PBP | penicillin-binding protein |
| WHO | world health organization |
| VISA | vancomycin-intermediate S. aureus |
| VRSA | vancomycin-resistant S. aureus |
| MSNs | Mesoporous silica nanoparticles |
| ESβLs | Extended spectrum beta lactamases |
| FTIR | Fourier transform infrared spectroscopy |
| MB | Methylene blue |
| MIC | Minimum inhibitory concentration |
| NiONPs | Nickel oxide nanoparticles |
| RBCs | red blood cells |
| PI | Propidium iodide |
| RBCs | Red blood cells |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| UV-Vis | Ultra-violet visible spectroscopy |
| XRD | X-ray diffraction |
References
- Parente, D.M.; Cunha, C.; Mylonakis, E.; Timbrook, T.T. The Clinical Utility of Methicillin-Resistant Staphylococcus aureus (MRSA) Nasal Screening to Rule Out MRSA Pneumonia: A Diagnostic Meta-analysis With Antimicrobial Stewardship Implications. Clin. Infect. Dis. 2018, 67, 1–7. [Google Scholar] [CrossRef]
- Wong, J.W.; Ip, M.; Tang, A.; Wei, V.W.; Wong, S.Y.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Mosslemin, M.H.; Alborzi, A.; Anaraki-Ardakani, H.; Shams, N.; Khoshnood, M.M.; Zarenezhad, A. Efficient synthesis of 3, 4-dihydro-1 H-quinoxalin-2-ones and 1 H-quinolin-2-ones and evaluation of their anti-bacterial activity. J. Chem. Res. 2014, 38, 337–340. [Google Scholar] [CrossRef]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7, 18. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- Liu, W.-T.; Chen, E.-Z.; Yang, L.; Peng, C.; Wang, Q.; Xu, Z.; Chen, D.-Q. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: A comprehensive review. Microb. Pathog. 2021, 156, 104915. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Baede, V.O.; David, M.Z.; Andrasevic, A.T.; Blanc, D.S.; Borg, M.; Brennan, G.; Catry, B.; Chabaud, A.; Empel, J.; Enger, H.; et al. MRSA surveillance programmes worldwide: Moving towards a harmonised international approach. Int. J. Antimicrob. Agents 2022, 59, 106538. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, A.; Tenhagen, B.-A. Risk Factors for the Occurrence of Methicillin-Resistant Staphylococcus aureus in Dairy Herds: An Update. Foodborne Pathog. Dis. 2020, 17, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Krukowski, H.; Bakuła, Z.; Iskra, M.; Olender, A.; Bis-Wencel, H.; Jagielski, T. The first outbreak of methicillin-resistant Staphylococcus aureus in dairy cattle in Poland with evidence of on-farm and intrahousehold transmission. J. Dairy Sci. 2020, 103, 10577–10584. [Google Scholar] [CrossRef]
- Schnitt, A.; Lienen, T.; Wichmann-Schauer, H.; Cuny, C.; Tenhagen, B.-A. The occurrence and distribution of livestock-associated methicillin-resistant Staphylococcus aureus ST398 on German dairy farms. J. Dairy Sci. 2020, 103, 11806–11819. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Walther, S.; Leone, M.; Barie, P.S.; Rello, J.; Lipman, J.; Marshall, J.C.; Anzueto, A.; Sakr, Y.; Pickkers, P.; et al. Increased mortality associated with meticillin-resistant Staphylococcus aureus (MRSA) infection in the Intensive Care Unit: Results from the EPIC II study. Int. J. Antimicrob. Agents 2011, 38, 331–335. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Mahdally, N.H.; George, R.F.; Kashef, M.T.; Al-Ghobashy, M.; Murad, F.E.; Attia, A.S. Staquorsin: A Novel Staphylococcus aureus Agr-Mediated Quorum Sensing Inhibitor Impairing Virulence in vivo Without Notable Resistance Development. Front. Microbiol. 2021, 12, 700494. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Liu, R.; Hunt, R.L.; Zheng, Y.; Otto, M. Bacterial virulence plays a crucial role in MRSA sepsis. PLoS Pathog. 2021, 17, e1009369. [Google Scholar] [CrossRef]
- Ghasemian, A.; Peerayeh, S.N.; Bakhshi, B.; Mirzaee, M. The Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs) Genes among Clinical Isolates of Staphylococcus aureus from Hospitalized Children. Iran. J. Pathol. 2015, 10, 258–264. [Google Scholar]
- Ghasemian, A.; Peerayeh, S.N.; Bakhshi, B.; Mirzaee, M. Comparison of Biofilm Formation between Methicillin-Resistant and Methicillin-Susceptible Isolates of Staphylococcus aureus. Iran. Biomed. J. 2016, 20, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. MRSA virulence and spread. Cell. Microbiol. 2012, 14, 1513–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Kong, C.; Neoh, H.-M.; Nathan, S. Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy. Toxins 2016, 8, 72. [Google Scholar] [CrossRef]
- Salinas, N.; Colletier, J.-P.; Moshe, A.; Landau, M. Extreme amyloid polymorphism in Staphylococcus aureus virulent PSMα peptides. Nat. Commun. 2018, 9, 3512. [Google Scholar] [CrossRef]
- Bukowski, M.; Wladyka, B.; Dubin, G. Exfoliative toxins of Staphylococcus aureus. Toxins 2010, 2, 1148–1165. [Google Scholar] [CrossRef]
- Gaebler Vasconcelos, N.; de Lourdes Ribeiro de Souza da Cunha, M. Staphylococcal enterotoxins: Molecular aspects and detection methods. J. Public Health Epidemiol. 2010, 2, 29–42. [Google Scholar]
- Weese, J.S. Methicillin-Resistant Staphylococcus aureus in Animals. ILAR J. 2010, 51, 233–244. [Google Scholar] [CrossRef]
- Graveland, H.; Wagenaar, J.A.; Bergs, K.; Heesterbeek, H.; Heederik, D. Persistence of Livestock Associated MRSA CC398 in Humans Is Dependent on Intensity of Animal Contact. PLoS ONE 2011, 6, e16830. [Google Scholar] [CrossRef] [PubMed]
- Dorado-García, A.; Bos, M.E.; Graveland, H.; Van Cleef, B.A.; Verstappen, K.M.; Kluytmans, J.A.; Wagenaar, J.A.; Heederik, D.J. Risk factors for persistence of livestock-associated MRSA and environmental exposure in veal calf farmers and their family members: An observational longitudinal study. BMJ Open 2013, 3, e003272. [Google Scholar] [CrossRef] [PubMed]
- Van Loo, I.; Huijsdens, X.; Tiemersma, E.; De Neeling, A.; van de Sande-Bruinsma, N.; Beaujean, D.; Voss, A.; Kluytmans, J. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg. Infect. Dis. 2007, 13, 1834. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Moleman, M.; van Oldruitenborgh-Oosterbaan, M.S.; Multem, J.; Troelstra, A.; Fluit, A.; van Wamel, W.; Houwers, D.; de Neeling, A.; Wagenaar, J. Methicillin-resistant Staphylococcus aureus in horses and horse personnel: An investigation of several outbreaks. Veter. Microbiol. 2010, 141, 96–102. [Google Scholar] [CrossRef]
- Idelevich, E.A.; Lanckohr, C.; Horn, D.; Wieler, L.H.; Becker, K.; Koeck, R. Multidrug-resistant bacteria in Germany. The impact of sources outside healthcare facilities. Bundesgesundheitsblatt Gesundh. Gesundh. 2016, 59, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Köck, R.; Ballhausen, B.; Bischoff, M.; Cuny, C.; Eckmanns, T.; Fetsch, A.; Harmsen, D.; Goerge, T.; Oberheitmann, B.; Schwarz, S.; et al. The impact of zoonotic MRSA colonization and infection in Germany. Berl. Munch. Tierarztl. Wochenschr. 2015, 127, 384–398. [Google Scholar]
- Lienen, T.; Schnitt, A.; Hammerl, J.A.; Maurischat, S.; Tenhagen, B.-A. Genomic Distinctions of LA-MRSA ST398 on Dairy Farms From Different German Federal States With a Low Risk of Severe Human Infections. Front. Microbiol. 2021, 11, 575321. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Washer, P.; Joffe, H. The “hospital superbug”: Social representations of MRSA. Soc. Sci. Med. 2006, 63, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Du, Y.; Xia, Q.; Li, Y.; Song, S.; Huang, X. Role of linezolid combination therapy for serious infections: Review of the current evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1043–1052. [Google Scholar] [CrossRef]
- Kelesidis, T.; Humphries, R.; Ward, K.; Lewinski, M.A.; Yang, O.O. Combination therapy with daptomycin, linezolid, and rifampin as treatment option for MRSA meningitis and bacteremia. Diagn. Microbiol. Infect. Dis. 2011, 71, 286–290. [Google Scholar] [CrossRef]
- Jacqueline, C.; Navas, D.; Batard, E.; Miegeville, A.-F.; Le Mabecque, V.; Kergueris, M.-F.; Bugnon, D.; Potel, G.; Caillon, J. In Vitro and In Vivo Synergistic Activities of Linezolid Combined with Subinhibitory Concentrations of Imipenem against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 45–51. [Google Scholar] [CrossRef]
- Mehta, S.; Singh, C.; Plata, K.B.; Chanda, P.K.; Paul, A.; Riosa, S.; Rosato, R.R.; Rosato, A.E. β-Lactams Increase the Antibacterial Activity of Daptomycin against Clinical Methicillin-Resistant Staphylococcus aureus Strains and Prevent Selection of Daptomycin-Resistant Derivatives. Antimicrob. Agents Chemother. 2012, 56, 6192–6200. [Google Scholar] [CrossRef]
- Alosaimy, S.; Sabagha, N.L.; Lagnf, A.M.; Zasowski, E.J.; Morrisette, T.; Jorgensen, S.C.J.; Trinh, T.D.; Mynatt, R.P.; Rybak, M.J. Monotherapy with Vancomycin or Daptomycin versus Combination Therapy with β-Lactams in the Treatment of Methicillin-Resistant Staphylococcus Aureus Bloodstream Infections: A Retrospective Cohort Analysis. Infect. Dis. Ther. 2020, 9, 325–339. [Google Scholar] [CrossRef]
- Dilworth, T.J.; Ibrahim, O.; Hall, P.; Sliwinski, J.; Walraven, C.; Mercier, R.-C. β-Lactams Enhance Vancomycin Activity against Methicillin-Resistant Staphylococcus aureus Bacteremia Compared to Vancomycin Alone. Antimicrob. Agents Chemother. 2014, 58, 102–109. [Google Scholar] [CrossRef]
- Morrisette, T.; Alosaimy, S.; Abdul-Mutakabbir, J.; Kebriaei, R.; Rybak, M. The Evolving Reduction of Vancomycin and Daptomycin Susceptibility in MRSA—Salvaging the Gold Standards with Combination Therapy. Antibiotics 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Karageorgiou, V.; Pergialiotis, V.; Perrea, D. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: A network meta-analysis. Clin. Microbiol. Infect. 2020, 26, 696–705. [Google Scholar] [CrossRef]
- Tong, S.Y.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Davis, J. SEffect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: A randomized clinical trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef]
- Wang, C.; Ye, C.; Liao, L.; Wang, Z.; Hu, Y.; Deng, C.; Liu, L. Adjuvant β-Lactam Therapy Combined with Vancomycin or Daptomycin for Methicillin-Resistant Staphylococcus aureus Bacteremia: A Systematic Review and Meta-analysis. Antimicrob. Agents Chemother. 2020, 64, e01377-20. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Abdelkhalek, A.; Seleem, M. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 29707. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-J.; Li, Y.-L.; Pu, M.-J.; Luo, L.-H.; Xiong, Q.; Xie, F.-J.; Li, T.-L.; Feng-Jiao, X. Aminoglycosides with Anti-MRSA Activity: A Concise Review. Curr. Top. Med. Chem. 2021, 21, 2483–2499. [Google Scholar] [CrossRef]
- Verma, R.; Verma, S.K.; Rakesh, K.P.; Girish, Y.R.; Ashrafizadeh, M.; Kumar, K.S.S.; Rangappa, K.S. Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation. Eur. J. Med. Chem. 2021, 212, 113134. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Yan, G.; Li, T.; Hou, N. Vancomycin-induced reversible pancytopenia and rash in a 16-month-old boy with osteomyelitis: A case report. Int. J. Clin. Pharmacol. Ther. 2020, 58, 242–246. [Google Scholar] [CrossRef]
- Niu, H.; Yang, T.; Wang, J.; Wang, R.; Cai, Y. Immunomodulatory Effect of Colistin and its Protective Role in Rats with Methicillin-Resistant Staphylococcus aureus-induced Pneumonia. Front. Pharmacol. 2021, 11, 602054. [Google Scholar] [CrossRef] [PubMed]
- Parish, D.; Scheinfeld, N. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr. Opin. Investig. Drugs 2008, 9, 201–209. [Google Scholar] [PubMed]
- Shirley, D.-A.T.; Heil, E.L.; Johnson, J.K. Ceftaroline Fosamil: A Brief Clinical Review. Infect. Dis. Ther. 2013, 2, 95–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.; Soriano, A.; Rivolo, S.; Remak, E.; Peral, C.; Kantecki, M.; Ansari, W.; Charbonneau, C.; Hammond, J.; Grau, S.; et al. Ceftaroline Fosamil for the Empiric Treatment of Hospitalized Adults with cSSTI: An Economic Analysis from the Perspective of the Spanish National Health System. Clin. Econ. Outcomes Res. 2022, 14, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Bongiorno, D.; Mongelli, G.; Campanile, F. Linezolid Resistance in Staphylococci. Pharmaceuticals 2010, 3, 1988–2006. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Chegini, Z.; van Belkum, A.; Mirzaii, M.; Khoramrooz, S.S.; Darban-Sarokhalil, D. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase–negative staphylococci strains: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 1–20. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Gan, Y.; Jiang, F.; Zhao, H.; Tan, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Selective Capture, Separation, and Photothermal Inactivation of Methicillin-Resistant Staphylococcus aureus (MRSA) Using Functional Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 20566–20575. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.B.E.D.; El-Ela, F.I.A.; Mahmoud, R.K.; Farghali, A.A.; Gamil, S.; Aziz, S.A.A.A. Cefotax-magnetic nanoparticles as an alternative approach to control Methicillin-Resistant Staphylococcus aureus (MRSA) from different sources. Sci. Rep. 2022, 12, 624. [Google Scholar] [CrossRef]
- Zhang, L.; Bhatti, M.M.; Michaelides, E.E.; Marin, M.; Ellahi, R. Hybrid nanofluid flow towards an elastic surface with tantalum and nickel nanoparticles, under the influence of an induced magnetic field. Eur. Phys. J. Spéc. Top. 2022, 231, 521–533. [Google Scholar] [CrossRef]
- Hou, Y.; Kondoh, H.; Ohta, T.; Gao, S. Size-controlled synthesis of nickel nanoparticles. Appl. Surf. Sci. 2005, 241, 218–222. [Google Scholar] [CrossRef]
- Abdollahi, A.; Mirzaei, E.; Amoozegar, F.; Moemenbellah-Fard, M.D.; Zarenezhad, E.; Osanloo, M. High Antibacterial Effect of Impregnated Nanofiber Mats with a Green Nanogel Against Major Human Pathogens. BioNanoScience 2021, 11, 549–558. [Google Scholar] [CrossRef]
- Qasemi, H.; Fereidouni, Z.; Karimi, J.; Abdollahi, A.; Zarenezhad, E.; Rasti, F.; Osanloo, M. Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 66, 102844. [Google Scholar] [CrossRef]
- Hulme, J. Application of Nanomaterials in the Prevention, Detection, and Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA). Pharmaceutics 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Nisar, P.; Ali, N.; Rahman, L.; Ali, M.; Shinwari, Z.K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. JBIC J. Biol. Inorg. Chem. 2019, 24, 929–941. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Radzig, M.; Nadtochenko, V.; Koksharova, O.; Kiwi, J.; Lipasova, V.; Khmel, I. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef]
- Nunez, N.V.A.; Villegas, H.H.L.; Turrent, L.D.C.I.; Padilla, C.R. Silver Nanoparticles Toxicity and Bactericidal Effect Against Methicillin-Resistant Staphylococcus aureus: Nanoscale Does Matter. Nanobiotechnology 2009, 5, 2–9. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, Y.; Cho, H.; Lee, S.; Yang, S.-H.; Kim, S.-J.; Lee, D. Robust Copper Metal–Organic Framework-Embedded Polysiloxanes for Biomedical Applications: Its Antibacterial Effects on MRSA and In Vitro Cytotoxicity. Nanomaterials 2021, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Ahmad, A.; Sabir, A.; Iqbal, S.S.; Felemban, B.F.; Riaz, T.; Bahadar, A.; Hossain, N.; Khan, R.U.; Inam, F. Novel antibacterial polyurethane and cellulose acetate mixed matrix membrane modified with functionalized TiO2 nanoparticles for water treatment applications. Chemosphere 2022, 301, 134711. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Ain, N.U.; Gul, A.; Ahmad, M.A.; Guo, W.; Ao, Q.; Tian, S. Diverse biotechnological applications of multifunctional titanium dioxide nanoparticles: An up-to-date review. IET Nanobiotechnol. 2022, 16, 171–189. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Andreevna Alekhina, N.; Aravindhan, S.; Thangavelu, L.; Elena, A.; Viktorovna Kartamysheva, N.; Rafkatovna Zakieva, R. Synthesis of NiO nanoparticles and sulfur, and nitrogen co doped-graphene quantum dots/nio nanocomposites for antibacterial application. J. Nanostruct. 2021, 11, 181–188. [Google Scholar]
- Kannan, K.; Radhika, D.; Nesaraj, A.; Sadasivuni, K.K.; Reddy, K.R.; Kasai, D.; Raghu, A.V. Photocatalytic, antibacterial and electrochemical properties of novel rare earth metal oxides-based nanohybrids. Mater. Sci. Energy Technol. 2020, 3, 853–861. [Google Scholar] [CrossRef]
- Munawar, T.; Iqbal, F.; Yasmeen, S.; Mahmood, K.; Hussain, A. Multi metal oxide NiO-CdO-ZnO nanocomposite–synthesis, structural, optical, electrical properties and enhanced sunlight driven photocatalytic activity. Ceram. Int. 2020, 46, 2421–2437. [Google Scholar] [CrossRef]
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Li, Y.-F.; Fang, T.-L.; Zhou, J.; Li, X.-L.; Wang, Y.-C.; Dong, J. Vancomycin-loaded nano-hydroxyapatite pellets to treat MRSA-induced chronic osteomyelitis with bone defect in rabbits. Inflamm. Res. 2011, 61, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M. Effect of silver nanoparticles on the antibacterial activity of Levofloxacin against methicillin-resistant Staphylococcus aureus. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5507–5510. [Google Scholar] [PubMed]
- Wang, G.; Wang, J.-J.; Li, F.; To, S.-S.T. Development and Evaluation of a Novel Drug Delivery: Pluronics/SDS Mixed Micelle Loaded With Myricetin In Vitro and In Vivo. J. Pharm. Sci. 2016, 105, 1535–1543. [Google Scholar] [CrossRef]
- Adler-Moore, J.P.; Gangneux, J.-P.; Pappas, P.G. Comparison between liposomal formulations of amphotericin B. Sabouraudia 2016, 54, 223–231. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Jones, S.; Pramanik, A.; Kanchanapally, R.; Nellore, B.P.V.; Begum, S.; Sweet, C.; Ray, P.C. Multifunctional Three-Dimensional Chitosan/Gold Nanoparticle/Graphene Oxide Architecture for Separation, Label-Free SERS Identification of Pharmaceutical Contaminants, and Effective Killing of Superbugs. ACS Sustain. Chem. Eng. 2017, 5, 7175–7187. [Google Scholar] [CrossRef]
- Devlin, H.; Fulaz, S.; Hiebner, D.W.; O’Gara, J.P.; Casey, E. Enzyme-Functionalized Mesoporous Silica Nanoparticles to Target Staphylococcus aureus and Disperse Biofilms. Int. J. Nanomed. 2021, 16, 1929–1942. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Marzi, M.; Chijan, M.R.; Zarenezhad, E. Hydrogels as Promising Therapeutic Strategy for the Treatment of Skin Cancer. J. Mol. Struct. 2022, 1262, 133014. [Google Scholar] [CrossRef]
- Raza, S.; Matuła, K.; Karoń, S.; Paczesny, J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics 2021, 10, 435. [Google Scholar] [CrossRef]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef]
- Malik, A.; Alshehri, M.A.; Alamery, S.F.; Khan, J.M. Impact of metal nanoparticles on the structure and function of metabolic enzymes. Int. J. Biol. Macromol. 2021, 188, 576–585. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2021, 199, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef]
- Wang, Z.K.; Kuok, M.H.; Ng, S.C.; Lockwood, D.J.; Cottam, M.G.; Nielsch, K.; Wehrspohn, R.B.; Gösele, U. Spin-Wave Quantization in Ferromagnetic Nickel Nanowires. Phys. Rev. Lett. 2002, 89, 027201. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, C.Q. Electronic process of nitriding: Mechanism and applications. Prog. Solid State Chem. 2006, 34, 1–20. [Google Scholar] [CrossRef]
- Lee, K.-B.; Park, S.; Mirkin, C.A. Multicomponent Magnetic Nanorods for Biomolecular Separations. Angew. Chem. Int. Ed. 2004, 43, 3048–3050. [Google Scholar] [CrossRef]
- Hatamifard, A.; Nasrollahzadeh, M.; Sajadi, S.M. Biosynthesis, characterization and catalytic activity of an Ag/zeolite nanocomposite for base- and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of a variety of dyes at room temperature. New J. Chem. 2016, 40, 2501–2513. [Google Scholar] [CrossRef]
- Jaji, N.-D.; Lee, H.L.; Hussin, M.H.; Akil, H.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotechnol. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Berisio, R.; Grieco, P.; Morelli, G.; Galdiero, M. Antimicrobial Peptides as an Opportunity Against Bacterial Diseases. Curr. Med. Chem. 2015, 22, 1665–1677. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, R.G.; Tanna, J.A.; Gandhare, N.V.; Rai, A.R.; Juneja, H.D. Synthesis Of Nickel Nanoparticles: Microscopic Investigation, An Efficient Catalyst And Effective Antibacterial Activity. Adv. Mater. Lett. 2015, 6, 990–998. [Google Scholar] [CrossRef]
- Haghshenas, L.; Faraji, A. Evaluation of the effect of Gold and Nickel nanoparticles on Escherichia coliand Staphylococcus aurousbacteria in milk. J. Micro Nano Biomed. 2016, 1, 1–6. [Google Scholar]
- Haider, A.; Ijaz, M.; Ali, S.; Haider, J.; Imran, M.; Majeed, H.; Shahzadi, I.; Ali, M.M.; Khan, J.A.; Ikram, M. Green Synthesized Phytochemically (Zingiber officinale and Allium sativum) Reduced Nickel Oxide Nanoparticles Confirmed Bactericidal and Catalytic Potential. Nanoscale Res. Lett. 2020, 15, 50. [Google Scholar] [CrossRef]
- Rheima, A.M.; Al Marjani, M.F.; Aboksour, M.F.; Mohammed, S.H. Evaluation of Anti-Biofilm Formation Effect of Nickel Oxide Nanoparticles (NiO-NPs) Against Methicillin-Resistant Staphylococcus Aureus (MRSA). Int. J. Nanosci. Nanotechnol. 2021, 17, 221–230. [Google Scholar]
- Deotale, A.J.; Nandedkar, R.V.; Sinha, A.K.; Upadhyay, A.; Mondal, P.; Srivastava, A.K.; Deb, S.K. Effect of isochronal annealing on phase transformation studies of iron oxide nanoparticles. Bull. Mater. Sci. 2015, 38, 599–606. [Google Scholar] [CrossRef]
- Deotale, A.J.; Singh, U.; Golani, M.; Hajela, K.; Nandedkar, R.V. Raman spectroscopic study of nickel oxide nanoparticles and its antibacterial activity. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Ali, S.R.; De, M. Superparamagnetic Nickel Nanocluster-Embedded MoS2 Nanosheets for Gram-Selective Bacterial Adhesion and Antibacterial Activity. ACS Biomater. Sci. Eng. 2022, 8, 2932–2942. [Google Scholar] [CrossRef]
- Rashad, M.M.; El-Kemary, N.M.; Amer, S.; El-Kemary, M. Bovine serum albumin/chitosan-nanoparticle bio-complex; spectroscopic study and in vivo toxicological—Hypersensitivity evaluation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119582. [Google Scholar] [CrossRef]
- Scutera, S.; Argenziano, M.; Sparti, R.; Bessone, F.; Bianco, G.; Bastiancich, C.; Castagnoli, C.; Stella, M.; Musso, T.; Cavalli, R. Enhanced Antimicrobial and Antibiofilm Effect of New Colistin-Loaded Human Albumin Nanoparticles. Antibiotics 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.W.Y.; Maran, S.; Kong, A.S.-Y.; Cheng, W.-H.; Lim, S.-H.E.; Loh, J.-Y.; Lai, K.-S. A Metal-Containing NP Approach to Treat Methicillin-Resistant Staphylococcus aureus (MRSA): Prospects and Challenges. Materials 2022, 15, 5802. [Google Scholar] [CrossRef]
| Nanoparticle | MIC | Mechanism of Action | Conditions | Reference |
|---|---|---|---|---|
| NiNPs | 0.21 (µg/mL) | ND | In vitro | [106] |
| NiO NPs | 1 mg/50 µL | ND | In vitro | [107] |
| NiO NPs | 265 µg/mL | ND | In vitro | [108] |
| (S,N-GQDs/NiO) NPs | ND * | ND | In vitro | [74] |
| NiO NPs | ND ** | ND | In vitro | [110] |
| NiO NPs | 0.8 mg/mL *** | ND | In vitro | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarenezhad, E.; Abdulabbas, H.T.; Marzi, M.; Ghazy, E.; Ekrahi, M.; Pezeshki, B.; Ghasemian, A.; Moawad, A.A. Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics 2022, 11, 1208. https://doi.org/10.3390/antibiotics11091208
Zarenezhad E, Abdulabbas HT, Marzi M, Ghazy E, Ekrahi M, Pezeshki B, Ghasemian A, Moawad AA. Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics. 2022; 11(9):1208. https://doi.org/10.3390/antibiotics11091208
Chicago/Turabian StyleZarenezhad, Elham, Hussein T. Abdulabbas, Mahrokh Marzi, Esraa Ghazy, Mohammad Ekrahi, Babak Pezeshki, Abdolmajid Ghasemian, and Amira A. Moawad. 2022. "Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections" Antibiotics 11, no. 9: 1208. https://doi.org/10.3390/antibiotics11091208
APA StyleZarenezhad, E., Abdulabbas, H. T., Marzi, M., Ghazy, E., Ekrahi, M., Pezeshki, B., Ghasemian, A., & Moawad, A. A. (2022). Nickel Nanoparticles: Applications and Antimicrobial Role against Methicillin-Resistant Staphylococcus aureus Infections. Antibiotics, 11(9), 1208. https://doi.org/10.3390/antibiotics11091208