Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus faecalis and Enterococcus faecium Clinical Isolates in Northern Japan: Identification of optrA in ST480 E. faecalis
Abstract
1. Introduction
2. Results
2.1. Bacterial Isolates
2.2. Prevalence of Virulence Factors
2.3. Prevalence and Profile of Antimicrobial Resistance and Resistance Determinants
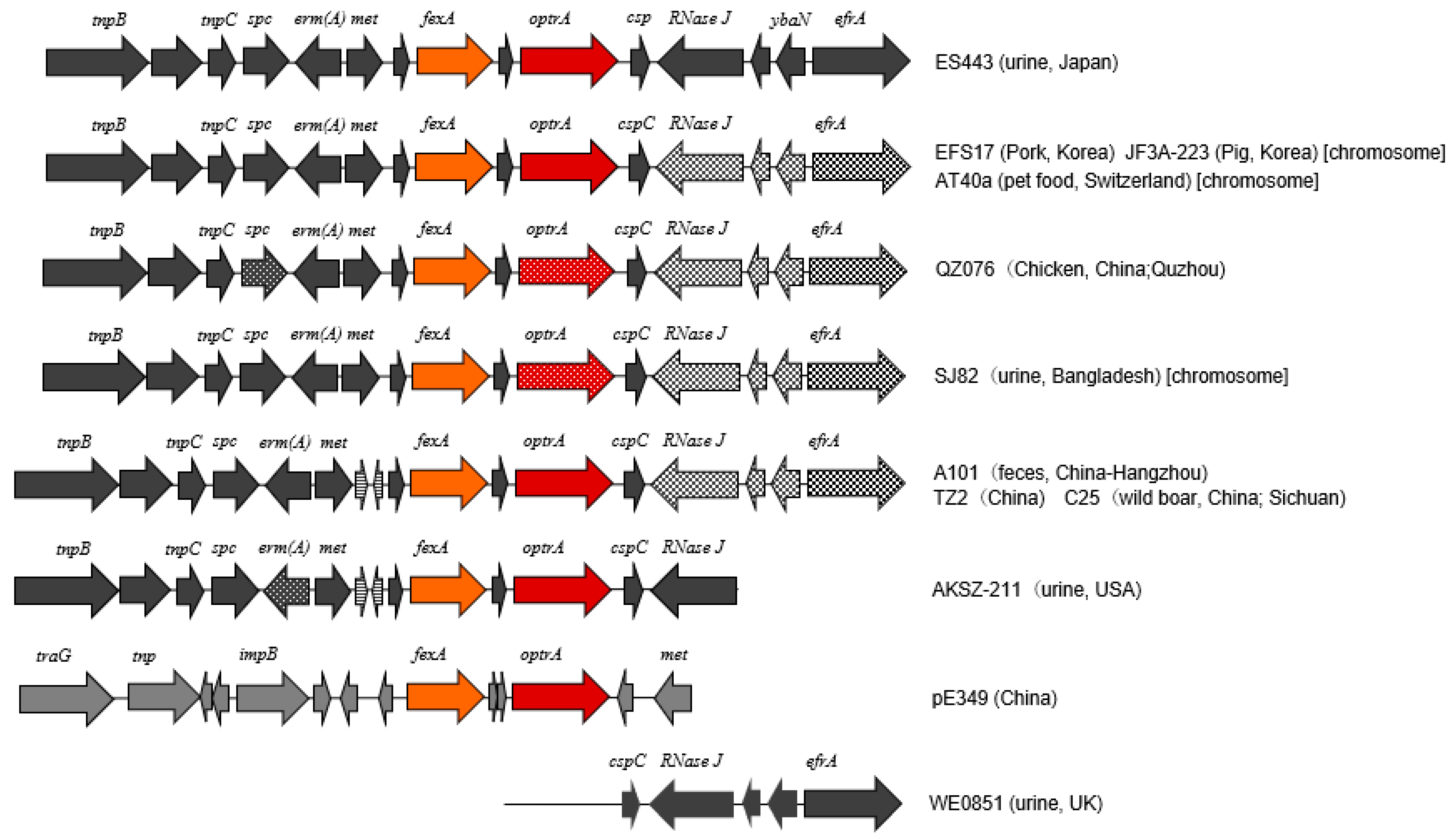
2.4. Characterization of optrA Gene and Its Cluster
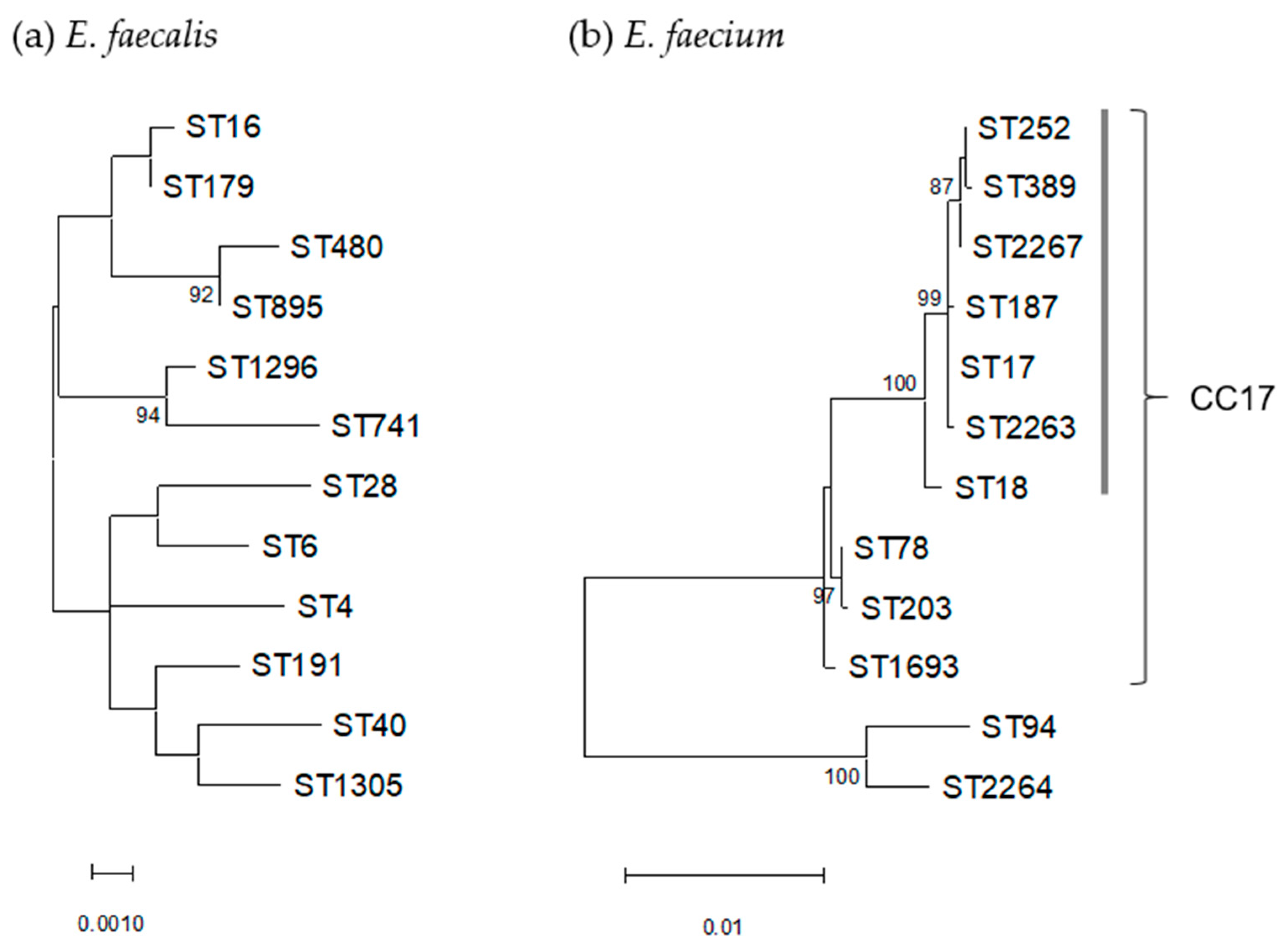
2.5. Genotypes of Isolates with Different Characteristics
3. Discussion
4. Materials and Methods
4.1. Clinical Isolates and Species Identification
4.2. Antibiotic Susceptibility Testing
4.3. Detection of Virulence Factors Genes
4.4. Detection of Drug Resistance Genes
4.5. Genetic Determinants of Oxazolidinone Resistance Isolate
4.6. Multilocus Sequence Typing (MLST), Phylogenetic Analysis
4.7. GenBank Accession Number
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. (Eds.) Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef] [PubMed]
- Sparo, M.; Delpech, G.; Allende, N.G. Impact on Public Health of the Spread of High-Level Resistance to Gentamicin and Vancomycin in Enterococci. Front. Microbiol. 2018, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Eurosurveillance 2008, 13, 19046. [Google Scholar] [CrossRef] [PubMed]
- Ament, P.W.; Jamshed, N.; Horne, J.P. Linezolid: Its role in the treatment of gram-positive, drug-resistant bacterial infections. Am. Fam. Physician 2002, 65, 663. [Google Scholar] [PubMed]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Dev. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Lentino, J.R.; Narita, M.; Yu, V.L. New antimicrobial agents as therapy for resistant gram-positive cocci. Eur. J. Clin. Microbiol. 2007, 27, 3–15. [Google Scholar] [CrossRef]
- Bi, R.; Qin, T.; Fan, W.; Ma, P.; Gu, B. The emerging problem of linezolid-resistant enterococci. J. Glob. Antimicrob. Resist. 2018, 13, 11–19. [Google Scholar] [CrossRef]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Sadowy, E.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: Towards a common nomenclature. Drug Resist. Updat. 2018, 40, 25–39. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Novais, C.; Lanza, V.F.; Peixe, L. Comparative genomics of global optrA-carrying Enterococcus faecalis uncovers a common chromosomal hotspot for optrA acquisition within a diversity of core and accessory genomes. Microb. Genom. 2020, 6, e000350. [Google Scholar] [CrossRef]
- Geraldes, C.; Tavares, L.; Gil, S.; Oliveira, M. Enterococcus Virulence and Resistant Traits Associated with Its Permanence in the Hospital Environment. Antibiotics 2022, 11, 857. [Google Scholar] [CrossRef]
- Billström, H.; Lund, B.; Sullivan, A.; Nord, C.E. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int. J. Antimicrob. Agents 2008, 32, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kobayashi, N.; Quiñones, D.; Nagashima, S.; Uehara, N.; Watanabe, N. Genetic diversity of enterococci harboring the high-level gentamicin resistance gene aac(6′)-Ie-aph(2″)-Ia or aph(2″)-Ie in a Japanese hospital. Microb. Drug Resist. 2009, 15, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Urushibara, N.; Kawaguchiya, M.; Ghosh, S.; Suzaki, K.; Watanabe, N.; Quiñones, D.; Kobayashi, N. Characterization of Enterococcus faecium with macrolide resistance and reduced susceptibility to quinupristin/dalfopristin in a Japanese hospital: Detection of extensive diversity in erm(B)-regulator regions. Microb. Drug Resist. 2013, 19, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Urushibara, N.; Suzaki, K.; Kawaguchiya, M.; Aung, M.S.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Contribution of Type II Topoisomerase Mutations to Fluoroquinolone Resistance in Enterococcus faecium from Japanese Clinical Setting. Microb. Drug Resist. 2018, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Shibue, Y.; Aoki, K.; Ishii, Y.; Tateda, K. Prevalence of High-Level Aminoglycoside Resistance and Genes Encoding Aminoglycoside-Modifying Enzymes in Enterococcus faecalis and Enterococcus faecium Isolated in a University Hospital in Tokyo. Jpn. J. Infect. Dis. 2020, 73, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Japan Nosocomial Infections Surveillance (JANIS). Annual Open Report 2019 (All Facilities). Clinical Laboratory Division. Available online: https://janis.mhlw.go.jp/english/report/open_report/2019/3/1/ken_Open_Report_Eng_201900_clsi2012.pdf (accessed on 20 December 2022).
- Matsushima, A.; Takakura, S.; Yamamoto, M.; Matsumura, Y.; Shirano, M.; Nagao, M.; Ito, Y.; Iinuma, Y.; Shimizu, T.; Fujita, N.; et al. Regional spread and control of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Kyoto, Japan. Eur. J. Clin. Microbiol. 2011, 31, 1095–1100. [Google Scholar] [CrossRef]
- Saito, N.; Kitazawa, J.; Horiuchi, H.; Yamamoto, T.; Kimura, M.; Inoue, F.; Matsui, M.; Minakawa, S.; Itoga, M.; Tsuchiya, J.; et al. Interhospital transmission of vancomycin-resistant Enterococcus faecium in Aomori, Japan. Antimicrob. Resist. Infect. Control 2022, 11, 99. [Google Scholar] [CrossRef]
- Goto, R.; Inose, R.; Kusama, Y.; Kawabe, A.; Ishii, S.; Ebisui, A.; Ishikane, M.; Yagi, T.; Ohmagari, N.; Muraki, Y. Trends of the Use of Anti-methicillin-Resistant Staphylococcus aureus Agents in Japan Based on Sales Data from 2006 to 2015. Biol. Pharm. Bull. 2020, 43, 1906–1910. [Google Scholar] [CrossRef]
- Nihonyanagi, S.; Adachi, Y.; Onuki, T.; Nakazaki, N.; Hirata, Y.; Fujiki, K.; Takayama, Y.; Kanoh, Y.; Bandoh, Y.; Dantsuji, Y.; et al. Emergence of linezolid-resistant Enterococcus faecalis strains from two inpatients in a pediatric ward. Kansenshogaku Zasshi 2012, 86, 555–562. [Google Scholar] [CrossRef]
- Kuroda, M.; Sekizuka, T.; Matsui, H.; Suzuki, K.; Seki, H.; Saito, M.; Hanaki, H. Complete Genome Sequence and Characterization of Linezolid-Resistant Enterococcus faecalis Clinical Isolate KUB3006 Carrying a cfr(B)-Transposon on Its Chromosome and optrA-Plasmid. Front. Microbiol. 2018, 9, 2576. [Google Scholar] [CrossRef]
- Iimura, M.; Hayashi, W.; Arai, E.; Natori, T.; Horiuchi, K.; Matsumoto, G.; Tanaka, H.; Soga, E.; Nagano, Y.; Arakawa, Y.; et al. Identification of a multiresistant mosaic plasmid carrying a new segment of IS1216E-flanked optrA with integrated Tn551-ermB element in linezolid-resistant Enterococcus faecalis human isolate. J. Glob. Antimicrob. Resist. 2020, 22, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.M.; Castanheira, M.; Flamm, R.K.; Mendes, R.E. Evolving oxazolidinone resistance mechanisms in a worldwide collection of enterococcal clinical isolates: Results from the SENTRY Antimicrobial Surveillance Program. J. Antimicrob. Chemother. 2018, 73, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Vankerckhoven, V.; Van Autgaerden, T.; Vael, C.; Lammens, C.; Chapelle, S.; Rossi, R.; Jabes, D.; Goossens, H. Development of a multiplex PCR for the detection of asa1, gelE, cylA, esp, and hyl genes in enterococci and survey for virulence determinants among European hospital isolates of Enterococcus faecium. J. Clin. Microbiol. 2004, 42, 4473–4479. [Google Scholar] [CrossRef]
- Creti, R.; Imperi, M.; Bertuccini, L.; Fabretti, F.; Orefici, G.; Di Rosa, R.; Baldassarri, L. Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J. Med. Microbiol. 2004, 53, 13–20. [Google Scholar] [CrossRef]
- Mannu, L.; Paba, A.; Daga, E.; Comunian, R.; Zanetti, S.; Duprè, I.; Sechi, L. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. Int. J. Food Microbiol. 2003, 88, 291–304. [Google Scholar] [CrossRef]
- Hammad, A.M.; Shimamoto, T.; Shimamoto, T. Genetic characterization of antibiotic resistance and virulence factors in Enterococcus spp. from Japanese retail ready-to-eat raw fish. Food Microbiol. 2014, 38, 62–66. [Google Scholar] [CrossRef]
- Todokoro, D.; Suzuki, T.; Kobayakawa, S.; Tomita, H.; Ohashi, Y.; Akiyama, H. Postoperative Enterococcus faecalis endophthalmitis: Virulence factors leading to poor visual outcome. Jpn. J. Ophthalmol. 2017, 61, 408–414. [Google Scholar] [CrossRef]
- Lins, R.X.; Junior, H.R.; Wilson, M.; Lewis, M.A.O.; Fidel, R.A.S.; Williams, D. Comparison of genotypes, antimicrobial resistance and virulence profiles of oral and non oral Enterococcus faecalis from Brazil, Japan and the United Kingdom. J. Dent. 2019, 84, 49–54. [Google Scholar] [CrossRef]
- El-Zamkan, M.A.; Mohamed, H.M.A. Antimicrobial resistance, virulence genes and biofilm formation in Enterococcus species isolated from milk of sheep and goat with subclinical mastitis. PLoS ONE 2021, 16, e0259584. [Google Scholar] [CrossRef]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Effects of osmotic and high pressure stress on expression of virulence factors among Enterococcus spp. isolated from food of animal origin. Food Microbiol. 2022, 102, 103900. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Evidence of Linezolid Resistance and Virulence Factors in Enterococcus spp. Isolates from Wild and Domestic Ruminants, Italy. Antibiotics 2022, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef]
- Taglialegna, A.; Matilla-Cuenca, L.; Dorado-Morales, P.; Navarro, S.; Ventura, S.; Garnett, J.A.; Lasa, I.; Valle, J. The biofilm-associated surface protein Esp of Enterococcus faecalis forms amyloid-like fibers. NPJ Biofilms Microbiomes 2020, 6, 15. [Google Scholar] [CrossRef]
- Rice, L.B.; Carias, L.; Rudin, S.; Vael, C.; Goossens, H.; Konstabel, C.; Klare, I.; Nallapareddy, S.R.; Huang, W.; Murray, B.E. A potential virulence gene, hylEfm, predominates in Enterococcus faecium of clinical origin. J. Infect. Dis. 2003, 187, 508–512. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Novais, C.; Ruiz-Garbajosa, P.; Werner, G.; Laverde-Gomez, J.A.; Cantón, R.; Peixe, L.; Baquero, F.; Coque, T.M. Global spread of the hylEfm colonization-virulence gene in megaplasmids of the Enterococcus faecium CC17 polyclonal subcluster. Antimicrob. Agents Chemother. 2010, 54, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Klare, I.; Konstabel, C.; Mueller-Bertling, S.; Werner, G.; Strommenger, B.; Kettlitz, C.; Borgmann, S.; Schulte, B.; Jonas, D.; Serr, A.; et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Mato, R.; Almeida, F.; Pires, R.; Rodrigues, P.; Ferreira, T.; Sanches, I.S. Assessment of high-level gentamicin and glycopeptide-resistant Enterococcus faecalis and E. faecium clonal structure in a Portuguese hospital over a 3-year period. Eur. J. Clin. Microbiol. 2009, 28, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Aung, M.S.; Paul, S.K.; Ahmed, S.; Haque, N.; Khan, E.R.; Barman, T.K.; Islam, A.; Abedin, S.; Sultana, C.; et al. Drug Resistance Determinants in Clinical Isolates of Enterococcus faecalis in Bangladesh: Identification of Oxazolidinone Resistance Gene optrA in ST59 and ST902 Lineages. Microorganisms 2020, 8, 1240. [Google Scholar] [CrossRef]
- Singh, K.V.; Malathum, K.; Murray, B.E. Disruption of an Enterococcus faecium species-specific gene, a homologue of acquired macrolide resistance genes of staphylococci, Is associated with an increase in macrolide susceptibility. Antimicrob. Agents Chemother. 2001, 45, 263–266. [Google Scholar] [CrossRef]
- Werner, G.; Hildebrandt, B.; Witte, W. The newly described msrC gene is not equally distributed among All Isolates of Enterococcus faecium. Antimicrob. Agents Chemother. 2001, 45, 3672–3673. [Google Scholar] [CrossRef] [PubMed]
- Muraki, Y.; Yagi, T.; Tsuji, Y.; Nishimura, N.; Tanabe, M.; Niwa, T.; Watanabe, T.; Fujimoto, S.; Takayama, K.; Murakami, N.; et al. Japanese antimicrobial consumption surveillance: First report on oral and parenteral antimicrobial consumption in Japan (2009–2013). J. Glob. Antimicrob. Resist. 2016, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Zhang, W.; Du, X.-D.; Krüger, H.; Feßler, A.T.; Ma, S.; Zhu, Y.; Wu, C.; Shen, J.; Wang, Y. Mobile Oxazolidinone Resistance Genes in Gram-Positive and Gram-Negative Bacteria. Clin. Microbiol. Rev. 2021, 34, e00188-20. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zou, J.; Zhao, J.; Tang, Y.; Yuan, Y.; Yang, B.; Huang, J.; Xia, P.; Xia, Y. Emergence of optrA-Mediated Linezolid Resistance in Enterococcus faecium: A Molecular Investigation in a Tertiary Hospital of Southwest China from 2014–2018. Infect. Drug Resist. 2022, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, Y.; Schwarz, S.; Lv, H.; Li, Y.; Liao, K.; Yu, S.; Zhao, K.; Gu, D.; Wang, X.; et al. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin. Microbiol. Infect. 2015, 21, 1095.e1–1095.e4. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Lv, Y.; Wang, S.; Song, Y.; Li, Y.; Liu, J.; Xue, F.; Yang, W.; Zhang, J. Nationwide Surveillance of Novel Oxazolidinone Resistance Gene optrA in Enterococcus Isolates in China from 2004 to 2014. Antimicrob. Agents Chemother. 2016, 60, 7490–7493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, G.; Li, J.; Chen, L.; Liu, H.; Bi, W.; Lu, H.; Zhou, T. A high incidence and coexistence of multiresistance genes cfr and optrA among linezolid-resistant enterococci isolated from a teaching hospital in Wenzhou, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1441–1448. [Google Scholar] [CrossRef]
- Kerschner, H.; Rosel, A.C.; Hartl, R.; Hyden, P.; Stoeger, A.; Ruppitsch, W.; Allerberger, F.; Apfalter, P. Oxazolidinone Resistance Mediated by optrA in Clinical Enterococcus faecalis Isolates in Upper Austria: First Report and Characterization by Whole Genome Sequencing. Microb. Drug Resist. 2021, 27, 685–690. [Google Scholar] [CrossRef]
- Rodríguez-Lucas, C.; Fernández, J.; Vázquez, X.; de Toro, M.; Ladero, V.; Fuster, C.; Rodicio, R.; Rodicio, M.R. Detection of the optrA Gene Among Polyclonal Linezolid-Susceptible Isolates of Enterococcus faecalis Recovered from Community Patients. Microb. Drug Resist. 2022, 28. [Google Scholar] [CrossRef]
- Mendes, R.E.; Deshpande, L.; Streit, J.M.; Sader, H.S.; Castanheira, M.; Hogan, P.A.; Flamm, R.K. ZAAPS programme results for 2016: An activity and spectrum analysis of linezolid using clinical isolates from medical centres in 42 countries. J. Antimicrob. Chemother. 2018, 73, 1880–1887. [Google Scholar] [CrossRef]
- Chen, M.; Pan, H.; Lou, Y.; Wu, Z.; Zhang, J.; Huang, Y.; Yu, W.; Qiu, Y. Epidemiological characteristics and genetic structure of linezolid-resistant Enterococcus faecalis. Infect. Drug Resist. 2018, 11, 2397–2409. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.K.; Fleige, C.; Lange, D.; Klare, I.; Werner, G. Rapid emergence of highly variable and transferable oxazolidinone and phenicol resistance gene optrA in German Enterococcus spp. clinical isolates. Int. J. Antimicrob. Agents 2018, 52, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Egan, S.; Shore, A.C.; O′Connell, B.; Brennan, G.I.; Coleman, D.C. Linezolid resistance in Enterococcus faecium and Enterococcus faecalis from hospitalized patients in Ireland: High prevalence of the MDR genes optrA and poxtA in isolates with diverse genetic backgrounds. J. Antimicrob. Chemother. 2020, 75, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Shen, Y.; Schwarz, S.; Cai, J.; Lv, Y.; Li, J.; Feßler, A.T.; Zhang, R.; Wu, C.; Shen, J.; et al. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J. Antimicrob. Chemother. 2016, 71, 1466–1473. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, S.; Xu, H.; Zhang, Z.; Chen, F.; Shen, H.; Zhang, C. Distribution of the optrA gene in Enterococcus isolates at a tertiary care hospital in China. J. Glob. Antimicrob. Resist. 2019, 17, 180–186. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Yin, Y.; Li, S.; Zhang, Y.; Wang, Q.; Wang, H. Molecular characteristics of oxazolidinone resistance in enterococci from a multicenter study in China. BMC Microbiol. 2019, 19, 162. [Google Scholar] [CrossRef]
- Park, K.; Jeong, Y.S.; Chang, J.; Sung, H.; Kim, M.N. Emergence of optrA-Mediated Linezolid-Nonsusceptible Enterococcus faecalis in a Tertiary Care Hospital. Ann. Lab. Med. 2020, 40, 321–325. [Google Scholar] [CrossRef]
- Morroni, G.; Brenciani, A.; Simoni, S.; Vignaroli, C.; Mingoia, M.; Giovanetti, E. Commentary: Nationwide Surveillance of Novel Oxazolidinone Resistance Gene optrA in Enterococcus Isolates in China from 2004 to 2014. Front. Microbiol. 2017, 8, 1631. [Google Scholar] [CrossRef]
- Argudín, M.A.; Youzaga, S.; Dodémont, M.; Heinrichs, A.; Roisin, S.; Deplano, A.; Nonhoff, C.; Hallin, M. Detection of optrA-positive enterococci clinical isolates in Belgium. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 985–987. [Google Scholar] [CrossRef]
- Cai, J.; Schwarz, S.; Chi, D.; Wang, Z.; Zhang, R.; Wang, Y. Faecal carriage of optrA-positive enterococci in asymptomatic healthy humans in Hangzhou, China. Clin. Microbiol. Infect. 2019, 25, 630.e1–630.e6. [Google Scholar] [CrossRef]
- Elghaieb, H.; Tedim, A.P.; Abbassi, M.S.; Novais, C.; Duarte, B.; Hassen, A.; Peixe, L.; Freitas, A.R. From farm to fork: Identical clones and Tn6674-like elements in linezolid-resistant Enterococcus faecalis from food-producing animals and retail meat. J. Antimicrob. Chemother. 2019, 75, 30–35. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Biggel, M.; Zurfluh, K.; Treier, A.; Stephan, R. Faecal carriage of enterococci harbouring oxazolidinone resistance genes among healthy humans in the community in Switzerland. J. Antimicrob. Chemother. 2022, 77, 2779–2783. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Dhawan, B.; Vishnubhatla, S.; Kapil, A.; Das, B.; Sood, S. Emergence of high-risk multidrug-resistant Enterococcus faecalis CC2 (ST181) and CC87 (ST28) causing healthcare-associated infections in India. Infect. Genet. Evol. 2020, 85, 104519. [Google Scholar] [CrossRef]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J. Glob. Antimicrob. Resist. 2018, 16, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, D.; Tuschak, C.; Werner, M.; Bogdan, C.; Bollinger, T.; Hossain, H.; Friedrich, P.; Hussein, Z.; Pöhlmann, C.; Würstl, B.; et al. Whole-genome analysis of vancomycin-resistant Enterococcus faecium causing nosocomial outbreaks suggests the occurrence of few endemic clonal lineages in Bavaria, Germany. J. Antimicrob. Chemother. 2020, 75, 1398–1404. [Google Scholar] [CrossRef]
- Sun, L.; Xu, J.; Wang, W.; He, F. Emergence of vanA-Type Vancomycin-Resistant Enterococcus faecium ST 78 Strain with a rep2-Type Plasmid Carrying a Tn1546-Like Element Isolated from a Urinary Tract Infection in China. Infect. Drug Resist. 2020, 13, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Alam, M.; Nishimoto, Y.; Urasawa, S.; Uehara, N.; Watanabe, N. Distribution of aminoglycoside resistance genes in recent clinical isolates of Enterococcus faecalis, Enterococcus faecium and Enterococcus avium. Epidemiol. Infect. 2001, 126, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mahbub Alam, M.; Kobayashi, N.; Ishino, M.; Sumi, A.; Kobayashi, K.; Uehara, N.; Watanabe, N. Detection of a novel aph(2″) allele (aph [2″]-Ie) conferring high-level gentamicin resistance and a spectinomycin resistance gene ant(9)-Ia (aad 9) in clinical isolates of enterococci. Microb. Drug Resist. 2005, 11, 239–247. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; M100-Ed32; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 23 June 2022).
- Tamang, M.D.; Moon, D.C.; Kim, S.-R.; Kang, H.Y.; Lee, K.; Nam, H.-M.; Jang, G.-C.; Lee, H.-S.; Jung, S.-C.; Lim, S.-K. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet. Microbiol. 2017, 201, 252–256. [Google Scholar] [CrossRef]
- Aung, M.; Urushibara, N.; Kawaguchiya, M.; Hirose, M.; Ike, M.; Ito, M.; Kobayashi, N. Distribution of Virulence Factors and Resistance Determinants in Three Genotypes of Staphylococcus argenteus Clinical Isolates in Japan. Pathogens 2021, 10, 163. [Google Scholar] [CrossRef]
- Nomura, T.; Hashimoto, Y.; Kurushima, J.; Hirakawa, H.; Tanimoto, K.; Zheng, B.; Ruan, G.; Xue, F.; Liu, J.; Hisatsune, J.; et al. New colony multiplex PCR assays for the detection and discrimination of vancomycin-resistant enterococcal species. J. Microbiol. Methods 2018, 145, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garbajosa, P.; Bonten, M.J.M.; Robinson, D.A.; Top, J.; Nallapareddy, S.R.; Torres, C.; Coque, T.M.; Cantón, R.; Baquero, F.; Murray, B.E.; et al. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J. Clin. Microbiol. 2006, 44, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Homan, W.L.; Tribe, D.; Poznanski, S.; Li, M.; Hogg, G.; Spalburg, E.; Van Embden, J.D.; Willems, R.J. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 2002, 40, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
| Virulence Factor Genes | E. faecalis n = 426 (%) | E. faecium n = 54 (%) |
|---|---|---|
| asa1 (Aggregation substance) | 252 (59.2) * | 0 (0) |
| efaA (endocarditis antigen) | 209 (49.1) * | 1 (1.9) |
| cylA (Cytolysin) | 147 (34.5) * | 0 (0) |
| gelE (Gelatinase) | 248 (58.2) * | 0 (0) |
| esp (Enterococcal surface protein) | 188 (44.1) | 26 (48.1) |
| ace (Collagen-binding protein) | 197 (46.2) * | 0 (0) |
| hyl (Hyaluronidase) | 0 (0) | 6 (11.1) * |
| Tested Antibiotics/Resistance Genes | E. faecalis n = 426 (%) | E. faecium n = 54 (%) |
|---|---|---|
| Penicillin (PEN) | 0 (0) | 50 (92.6) *1 |
| Ampicillin (AMP) | 0 (0) | 50 (92.6) *1 |
| Ampicillin-Sulbactam (SAM) | 0 (0) | 50 (92.6) *1 |
| Imipenem (IPM) | 0 (0) | 50 (92.6) *1 |
| Minocycline (MIN) | 27 (6.3) | 2 (3.7) |
| Erythromycin (ERY) | 212 (49.8) | 48 (88.9) *1 |
| Levofloxacin (LVX) | 29 (6.8) | 52 (96.3) *1 |
| High-level resistance to Gentamicin (GEN-HLR) | 54 (12.7) | 10 (18.5) |
| Linezolid (LZD) | 1 *2 (0.2) | 0 (0) |
| Rifampicin (RIF) | 54 (12.7) | 24 (44.4) *1 |
| erm(B) | 184 (43.2) | 21 (38.9) |
| msrC | 0 (0) | 30 (55.6) *1 |
| aac(6′)-Ie-aph(2″)-Ia | 98 (23.0) | 9 (16.7) |
| aph(3′)-IIIa | 93 (21.8) *1 | 2 (3.7) |
| ant(6)-Ia | 6 (1.4) | 0 (0) |
| ant(9)-Ia | 1 (0.2) | 0 (0) |
| optrA | 1 (0.2) | 0 (0) |
| fexA | 1 (0.2) | 0 (0) |
| erm(A), erm(C), erm(F), erm(G), erm(Q), erm(T), erm(X), erm(Y), lunA, lnuB, lsaA, mefA/E, msrA, msrB, vanA, vanB, vanD, vanM, poxtA, cfr | 0 (0) | 0 (0) |
| Drug Resistance Determinants *1 | E. faecalis | E. faecium |
|---|---|---|
| Macrolide resistance gene | n = 212 (%) | n = 48 (%) |
| erm(B) only | 184 (86.8) *2 | 18 (37.5) |
| erm(B) + msrC | 0 (0) | 3 (6.2) *2 |
| msrC only | 0 (0) | 27 (56.3) *2 |
| Aminoglycoside modifying enzyme gene | n = 170 *3 (%) | n = 10 (%) |
| aac(6′)-Ie-aph(2″)-Ia | 26 (15.3) | 8 (80.0) *2 |
| aph(3′)-IIIa | 19 (11.2) | 1 (10.0) |
| aph(3′)-IIIa, ant(6)-Ia | 2 (1.2) | 0 (0) |
| aac(6′)-Ie-aph(2″)-Ia+aph(3′)-IIIa | 67 (39.4) | 1 (10.0) |
| aac(6′)-Ie-aph(2″)-Ia+aph(3′)-IIIa+ant(6)-Ia | 4 (2.4) | 0 (0) |
| aac(6′)-Ie-aph(2″)-Ia+aph(3′)-IIIa+ant(9)-Ia | 1 (0.6) | 0 (0) |
| Mutation in QRDR (GyrA/ParC) | n = 29 (%) | n = 52 (%) |
| S84I/S82I | 18 (62.1) *2 | 9 (17.3) |
| S84Y/S82I | 7 (24.1) | 17 (32.7) |
| S84I/S82R | 0 (0) | 21 (40.4) *2 |
| S84Y/S82R | 0 (0) | 2 (3.8) |
| S84F/S82R | 0 (0) | 1 (1.9) |
| E88G/S82I | 1 (3.4) | 0 (0) |
| S84Y, E88G/S82I | 0 (0) | 1 (1.9) |
| NM/S82I | 2 (6.9) | 1 (1.9) |
| NM/E86K | 1 (3.4) | 0 (0) |
| Isolate ID | Specimen | Age/Sex | Patient Type | Virulence Factors | Antimicrobial Resistance Profile *1 | Antimicrobial Resistance Genes | QRDR Mutation *2 | ST (CC) | Allelic Profile | |
|---|---|---|---|---|---|---|---|---|---|---|
| GyrA | ParC | |||||||||
| ES 9 | sputum | 79/M | Inpatient | asa1, efaA, cylA, gelE, esp, ace | ERY | erm(B) | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 10 | urine | 52/F | Outpatient | asa1, efaA, cylA, esp, ace | ERY, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 12 | urine | 82/F | Outpatient | efaA, cylA, gelE | LVX | NM | S82I | ST741 | 15-2-3-88-17-15-11 | |
| ES 14 | urine | 28/F | Inpatient | asa1, efaA, cylA, gelE, ace | ERY, MIN, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST16 (CC16) | 5-1-1-3-7-7-6 |
| ES 16 | urine | 96/M | Inpatient | asa1, efaA, cylA, gelE, ace | All susceptible | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 | |
| ES 27 | urine | 69/M | Outpatient | ERY, LVX | erm(B) | S84Y | S82I | ST895 (ST480 SLV *1) | 5-1-22-22-7-17-6 | |
| ES 36 | urine | 83/F | Outpatient | asa1, cylA, gelE | ERY, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 86 | urine | 95/M | Inpatient | asa1, efaA, cylA, esp, ace | ERY | erm(B) | E88G | S82I | ST16 (CC16) | 5-1-1-3-7-7-6 |
| ES 94 | urine | 86/F | Outpatient | asa1, efaA, gelE, esp, ace | ERY, LVX, MIN, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa, ant(6)-Ia | S84I | S82I | ST1296 *3 | 5-2-3-6-17-1-11 |
| ES 101 | urine | 83/M | Outpatient | asa1, efaA, gelE, ace | ERY, LVX | aph(3′)-IIIa | NM | E86K | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 103 | pus | 82/F | Inpatient | ERY, LVX, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa, ant(6)-Ia | S84I | S82I | ST4 (CC4) | 8-7-7-5-4-4-1 | |
| ES 116 | venous blood | 73/M | Inpatient | ERY, LVX, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | S84Y | S82I | ST16 (CC16) | 5-1-1-3-7-7-6 | |
| ES 120 | urine | 48/F | Outpatient | All susceptible | NM | NM | ST16 (CC16) | 5-1-1-3-7-7-6 | ||
| ES 135 | Vaginal discharge | 32/F | Outpatient | asa1, efaA, cylA, gelE, esp, ace | ERY | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 141 | urine | 54/M | Outpatient | asa1, efaA, cylA, gelE, esp, ace | ERY, LVX | erm(B) | S84I | S84I | ST1296 *3 | 5-2-3-6-17-1-11 |
| ES 151 | urine | 39/F | Inpatient | efaA, gelE, ace | ERY | erm(B) | NM | NM | ST40 (CC40) | 3-6-23-12-9-10-7 |
| ES 153 | urine | 96/F | Outpatient | asa1, efaA, cylA, gelE, esp, ace | ERY, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 172 | urine | 80/F | Inpatient | asa1, gelE, esp, ace | All susceptible | NM | NM | ST40 (CC40) | 3-6-23-12-9-10-7 | |
| ES 174 | urine | 11/M | Outpatient | gelE, esp, ace | ERY, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 179 | urine | 80/F | Outpatient | gelE, esp | LVX | S84I | S82I | ST1296 *3 | 5-2-3-6-17-1-11 | |
| ES 180 | urine | 87/F | Inpatient | ERY, LVX, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | S84I | S82I | ST1296 *3 | 5-2-3-6-17-1-11 | |
| ES 203 | urine | 94/F | Inpatient | asa1, efaA, gelE, esp, ace | LVX | S84I | S82I | ST28 (CC28) | 4-4-8-3-8-1-3 | |
| ES 218 | urine | 82/F | Inpatient | asa1, efaA, cylA, gelE, esp | ERY, LVX | erm(B) | S84I | S82I | ST1296 *3 | 5-2-3-6-17-1-11 |
| ES 223 | catheter tip | 72/M | Inpatient | asa1, efaA, cylA, gelE, esp, ace | ERY | erm(B) | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 |
| ES 236 | urine | 80/M | Inpatient | asa1, efaA, cylA, gelE, esp, ace | LVX | S84I | S82I | ST28 (CC28) | 4-4-8-3-8-1-3 | |
| ES 239 | urine | 68/M | Outpatient | asa1, efaA, cylA | ERY, GEN-HLR | aac(6′)-Ie-aph(2″)-Ia | NM | NM | ST6 (CC6) | 12-7-3-7-6-1-5 |
| ES 290 | urine | 94/F | Inpatient | efaA | All susceptible | NM | NM | ST1305 *3 | 9-6-11-72-78-1-22 | |
| ES 297 | Vaginal discharge | 44/F | Outpatient | asa1, efaA, cylA, gelE, esp, ace | ERY | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 | |
| ES 334 | Vaginal discharge | 15/F | Outpatient | asa1, efaA, cylA, gelE, esp | ERY | NM | NM | ST179 (CC16) | 5-1-1-3-7-1-6 | |
| ES 360 | pus | 25/F | Outpatient | efaA, gelE, esp | RIF | NM | NM | ST191 | 27-1-11-1-21-1-2 | |
| ES443 | urine | 74/F | Outpatient | asa1, efaA, esp | ERY, LVX, LZD, CHL, FFC | erm(B), aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa,ant(9)-Ia, optrA, fexA | S84Y | S82I | ST480 | 1-1-22-22-7-17-6 |
| Isolate ID | Specimen | Age/Sex | Patient Type | Virulence Factors | Antimicrobial Resistance Profile *1 | Antimicrobial Resistance Genes | QRDR Mutation *2 | ST (CC) | Allelic Profile | |
|---|---|---|---|---|---|---|---|---|---|---|
| GyrA | ParC | |||||||||
| ES 11 | bile | 92/M | Inpatient | esp | ERY, LVX | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 13 | urine | 93/F | Inpatient | esp | ERY, LVX, RIF | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 17 | urine | 55/F | Outpatient | hyl | ERY, LVX, RIF | msrC | S84Y | S82I | ST389 (CC17) | 1-5-1-1-1-1-3 |
| ES 31 | CVC *1 | 77/M | Inpatient | hyl | ERY, LVX, RIF | msrC | S84Y | S82I | ST18 (CC17) | 7-1-1-1-5-1-1 |
| ES 32 | urine | 80/F | Inpatient | esp | ERY, LVX, RIF | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 35 | urine | 88/F | Inpatient | esp | ERY, LVX, RIF | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 39 | urine | 90/M | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 56 | urine | 80/F | Inpatient | esp | ERY, LVX, GEN-HLR, RIF | aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 61 | urine | 91/F | Inpatient | esp | GEN-HLR, RIF | aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 64 | urine | 90/F | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 66 | urine | 83/M | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 98 | venous blood | 77/M | Inpatient | esp | ERY, LVX, RIF | erm(B) | S84I | S82R | ST2263 *3 (CC17) | 1-1-1-1-1-1-4 |
| ES 102 | urine | 85/M | Outpatient | esp | ERY, LVX | msrC | S84I | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 |
| ES 106 | urine | 82/F | Inpatient | ERY, LVX | msrC | S84I | S82I | ST18 (CC17) | 7-1-1-1-5-1-1 | |
| ES 109 | urine | 90/M | Inpatient | ERY, LVX, GEN-HLR, RIF | erm(B), aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 117 | bile | 86/F | Inpatient | ERY, LVX | msrC | S84I | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 132 | urine | 81/F | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 140 | bile | 77/M | Inpatient | RIF | NM | NM | ST2264 *3 | 25-15-9-33-10-19-6 | ||
| ES 144 | urine | 75/F | Inpatient | ERY, LVX, MIN, RIF | erm(B), msrC | S84Y | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 146 | urine | 82/M | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 148 | urine | 85/M | Inpatient | esp | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 150 | urine | 91/F | Inpatient | esp | ERY, LVX, GEN-HLR, RIF | erm(B), aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 166 | urine | 90/F | Inpatient | ERY, LVX, RIF | erm(B) | S84Y | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 170 | venous blood | 77/M | Inpatient | ERY, LVX, RIF | msrC | S84Y | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 171 | oral cavity | 77/F | Inpatient | esp | LVX, GEN-HLR, RIF | aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 195 | urine | 87/F | Outpatient | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 196 | urine | 78/M | Inpatient | LVX | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | ||
| ES 209 | bile | 83/M | Inpatient | ERY, LVX | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 211 | urine | 92/M | Inpatient | LVX, MIN, GEN-HLR, RIF | aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 215 | drain fluid | 76/M | Inpatient | esp | ERY, LVX, RIF | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 217 | urine | 77/M | Inpatient | esp | ERY, LVX, RIF | erm(B) | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 225 | urine | 89/F | Inpatient | ERY, LVX, SXT | erm(B) | S84Y, E88G | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 231 | urine | 90/F | Outpatient | ERY, LVX, GEN-HLR, RIF | msrC, aac(6′)-Ie-aph(2″)-Ia | S84Y | S82I | ST2267 *3 (CC17) | 1-158-1-1-1-1-1 | |
| ES 240 | pus | 75/F | Inpatient | ERY, LVX, RIF | erm(B) | S84I | S82R | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 246 | urine | 80/F | Inpatient | esp, hyl | ERY, LVX | erm(B), msrC | S84I | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 |
| ES 254 | urine | 89/F | Inpatient | ERY, LVX, GEN-HLR, RIF | erm(B), msrC, aph(3′)-IIIa | S84Y | S82I | ST252 (CC17) | 1-5-1-1-1-1-1 | |
| ES 264 | urine | 89/F | Inpatient | esp | ERY, LVX | msrC | S84I | S82R | ST203 (CC17) | 15-1-1-1-1-20-1 |
| ES 279 | urine | 96/F | Inpatient | ERY, LVX | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 280 | urine | 73/M | Inpatient | hyl | ERY, LVX | msrC | S84I | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 |
| ES 288 | urine | 82/F | Inpatient | esp | ERY, LVX | msrC | S84Y | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 289 | urine | 83/F | Inpatient | ERY, LVX | msrC | S84Y | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 302 | urine | 74/M | Inpatient | esp | ERY, LVX | msrC | S84Y | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 303 | IVH tube | 92/F | Inpatient | esp | ERY, LVX | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 319 | urine | 63/M | Inpatient | ERY, LVX | msrC | S84Y | S82I | ST1693 (CC17) | 9-1-1-1-5-7-1 | |
| ES 339 | urine | 77/F | Inpatient | esp | ERY, LVX | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 348 | urine | 92/F | Inpatient | ERY, LVX, RIF | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 359 | urine | 50/F | Outpatient | RIF | NM | NM | ST94 (CC94) | 13-8-8-8-6-10-6 | ||
| ES 393 | urine | 77/F | Inpatient | ERY, LVX | msrC | S84I | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 | |
| ES 407 | urine | 78/M | Inpatient | esp | ERY, LVX | msrC | S84F | S82R | ST187 (CC17) | 31-1-1-1-1-1-1 |
| ES 410 | urine | 86/F | Inpatient | esp | ERY, LVX | msrC | S84I | S82R | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 434 | urine | 91/F | Inpatient | hyl | ERY, LVX | erm(B) | S84I | S82I | ST18 (CC17) | 7-1-1-1-5-1-1 |
| ES 421 | urine | 90/M | Inpatient | efaA | ERY, LVX, GEN-HLR | erm(B), aac(6′)-Ie-aph(2″)-Ia | NM | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 |
| ES 441 | urine | 75/M | Inpatient | ERY, LVX, RIF | msrC | S84Y | S82I | ST17 (CC17) | 1-1-1-1-1-1-1 | |
| ES 450 | urine | 78/M | Outpatient | hyl | ERY, LVX, GEN-HLR | msrC, aac(6′)-Ie-aph(2″)-Ia, aph(3′)-IIIa | S84I | S82I | ST78 (CC17) | 15-1-1-1-1-1-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ohashi, N.; Hirose, M.; Kudo, K.; Tsukamoto, N.; Ito, M.; Kobayashi, N. Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus faecalis and Enterococcus faecium Clinical Isolates in Northern Japan: Identification of optrA in ST480 E. faecalis. Antibiotics 2023, 12, 108. https://doi.org/10.3390/antibiotics12010108
Aung MS, Urushibara N, Kawaguchiya M, Ohashi N, Hirose M, Kudo K, Tsukamoto N, Ito M, Kobayashi N. Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus faecalis and Enterococcus faecium Clinical Isolates in Northern Japan: Identification of optrA in ST480 E. faecalis. Antibiotics. 2023; 12(1):108. https://doi.org/10.3390/antibiotics12010108
Chicago/Turabian StyleAung, Meiji Soe, Noriko Urushibara, Mitsuyo Kawaguchiya, Nobuhide Ohashi, Mina Hirose, Kenji Kudo, Naoyuki Tsukamoto, Masahiko Ito, and Nobumichi Kobayashi. 2023. "Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus faecalis and Enterococcus faecium Clinical Isolates in Northern Japan: Identification of optrA in ST480 E. faecalis" Antibiotics 12, no. 1: 108. https://doi.org/10.3390/antibiotics12010108
APA StyleAung, M. S., Urushibara, N., Kawaguchiya, M., Ohashi, N., Hirose, M., Kudo, K., Tsukamoto, N., Ito, M., & Kobayashi, N. (2023). Antimicrobial Resistance, Virulence Factors, and Genotypes of Enterococcus faecalis and Enterococcus faecium Clinical Isolates in Northern Japan: Identification of optrA in ST480 E. faecalis. Antibiotics, 12(1), 108. https://doi.org/10.3390/antibiotics12010108





