Vaccination against Community-Acquired Pneumonia in Spanish Adults: Practical Recommendations by the NeumoExperts Prevention Group
Abstract
:1. Introduction
2. Materials and Methods
3. Results
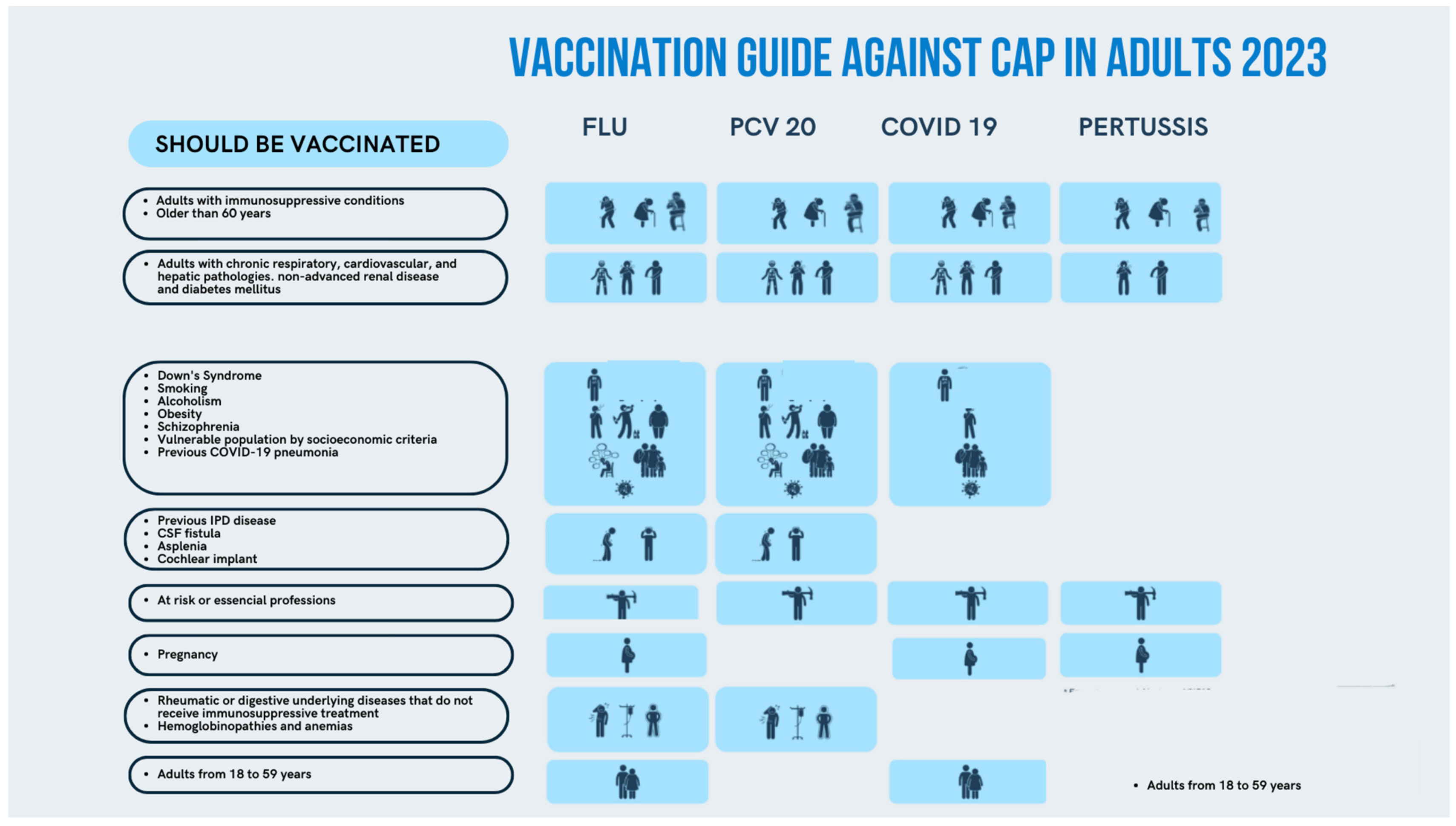
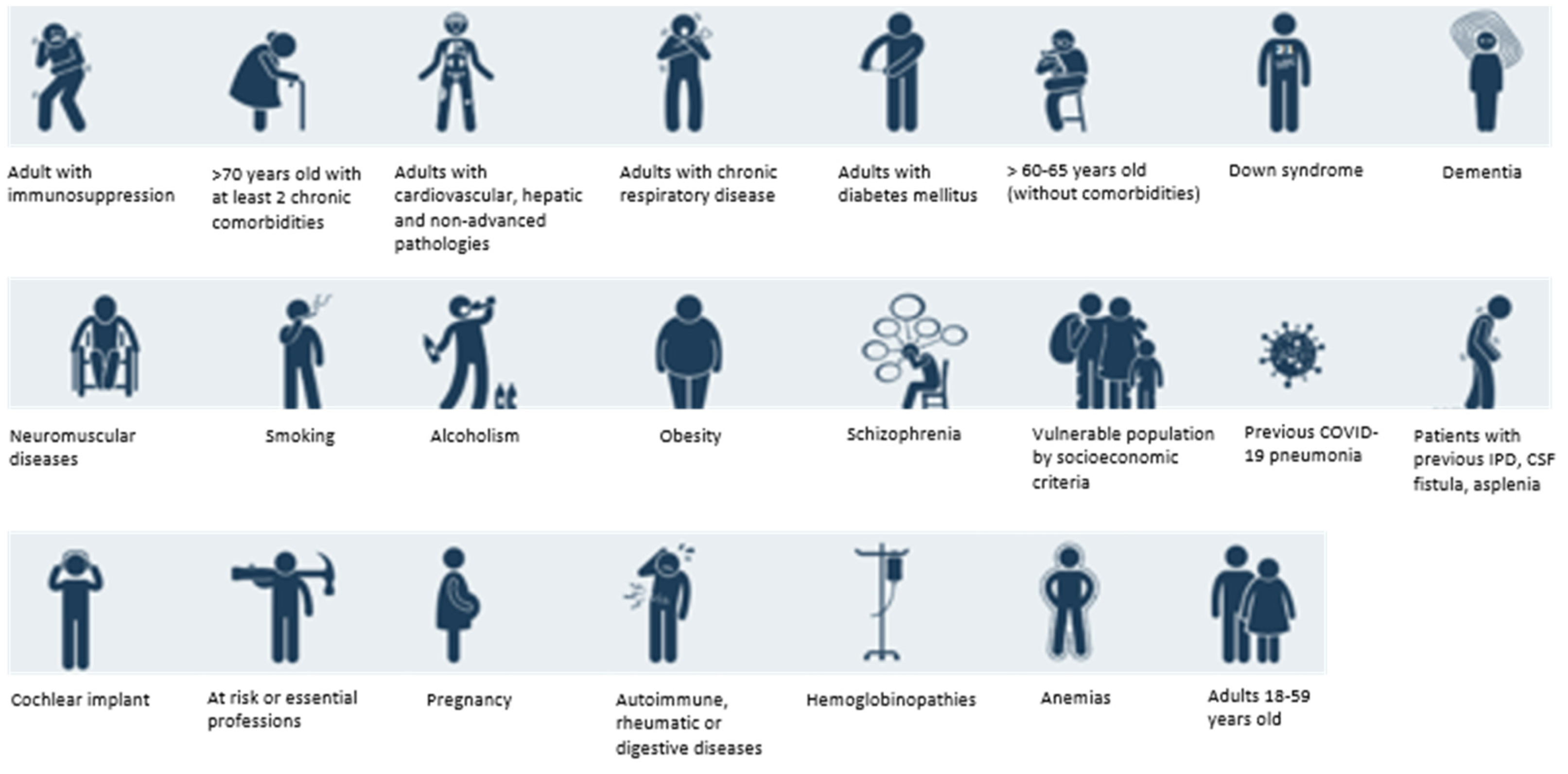
3.1. Vaccination against Streptococcus pneumoniae
3.1.1. Burden of Pneumococcal Disease in Spain
3.1.2. Position of NEP Group on Pneumococcal Vaccination in Adults
- 1.
- PCV13 has been demonstrated to be effective against pneumonia, IPD and antibiotic-resistant strains in adults.
- 2.
- The use of pediatric vaccination with PCVs is not enough to eliminate the burden of disease in adults, especially against mucosal infections, despite the indirect effects of these vaccines.
- 3.
- The polysaccharide vaccine (PPSV23), after being routinely used for many years, has not controlled the epidemiology of specific and unique serotypes contained in this vaccine, probably due to the different immune response elicited in comparison to PCVs.
- 4.
- Previous administration of polysaccharide vaccines can interfere with the immune response of subsequent conjugate vaccines.
- 5.
- Sequential vaccination strategies have the disadvantage of poor compliance by the targeted population. This approach also increases the attendance rates at primary care centers and hospitals for the next doses, including more human resources costs and the possibility of inducing mistakes by health care professionals regarding the correct sequential posology and dose intervals.
- 6.
- The commercialization of new PCVs of broader spectrum (PCV15 and PCV20) and the development of new vaccines (PCV21, PCV24 and PCV26) confirm our initial recommendation of using PCVs instead of PPSV23 for adults and reinforce the natural strategy of replacing plain polysaccharide vaccines with PCVs with greater coverage, as has happened with other infections such as meningococcal disease.
- 7.
- The new generation of PCVs (PCV15 and PCV20) has been approved exclusively on the basis of non-inferiority immunological criteria, and there are no studies directly comparing these new PCVs.
- 8.
- Policy decisions regarding the ideal vaccination strategy should include the following considerations:
- Epidemiological context at national level and not only using extrapolated data from other populations and countries with different burden of disease, circulating serotypes and vaccine calendars;
- The potential coverage of the different PCVs and their potential impact against the major forms of pneumococcal disease in our area;
- The relevance of specific serotypes based on their prevalence, clinical phenotype and/or antibiotic resistance in our geographic area (i.e., serotypes 3, 8, 11A or 22F);
- The lack of studies directly comparing the new PCVs and evaluating the clinical impact of the immunogenic differences between these vaccines against shared serotypes. These data are difficult to interpret in terms of clinical effectiveness, especially the prevention of mucosal disease that is, indeed, the most prevalent in adults;
- The degree of compliance in the sequential schedule;
- The intervention efficiency.
- Vaccination with a single dose of PCV20
- Sequential schedule using PCV15 followed by PPSV23
3.2. Vaccination against Influenza
3.2.1. Burden of Disease and Vaccine Coverage in Spain
3.2.2. Recommendations for Influenza Vaccination
3.3. Vaccination against COVID-19
- According to official Spanish recommendations, it is necessary to administer a new booster vaccine against COVID-19 (4th dose) to the most vulnerable groups (adults > 60 years old or regardless of age, all patients with high-risk factors for severe COVID-19) and to essential professional groups.
- The timing for administration of the booster dose should be ideally a period of at least 3 months after receiving the last vaccine dose (5 months with Comirnaty).
- There are other prevention interventions (non-pharmacology measures, early antiviral treatment, monoclonal antibodies), that are complementary to vaccination, and they are, indeed, recommended to many of the high-risk groups for COVID-19. These measures should be followed with the same fidelity and intensity to achieve the optimal protection coverage for the most vulnerable groups since viral evolution could also affect their effectiveness.
- All new and/or updated vaccines that have been approved by EMA have met all of the requirements for use in the target population following the package insert.
- Regarding the booster dose, there is not enough scientific evidence demonstrating the general superiority of a particular booster vaccine over the others. It is expected that the booster using bivalent vaccines would elicit a more specific and longer duration of immune response against infection by the Omicron variant. However, both booster doses using monovalent vaccines with the original strain or with multivariant vaccines (including the original strain and other variants such as beta, Omicron BA1/2 or Omicron BA4/5) are initially useful vaccines in order to reinforce the immune response against severe COVID-19 irrespective of the predominant circulating variant that is causing the disease.
- There are currently more than 400 vaccine candidates against COVID-19 in different clinical trials, which will increase the availability of new vaccines in the future. This aspect will be important to the evaluation of features such as the impact against transmission and/or the cross-protection activity against other SARS-CoV-2 viruses. These new generations of vaccines might modify the current vaccine strategies.
- The vaccine coverage using booster doses has notably decreased in comparison to the prime-boost vaccination, and in this sense, is essential to fulfill the recommended vaccination instructions against COVID-19, no matter if the patient has previously suffered the infection or not.
- We are probably looking towards a COVID-19 vaccination strategy based on booster doses only, aiming for specific high-risk populations. The type and composition of vaccine and interval between doses have not been established yet.
3.4. Burden of Disease and Future Preventive Strategies against RSV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schluger, N.W.; Koppaka, R. Lung disease in a global context. A call for public health action. Ann. Am. Thorac. Soc. 2014, 11, 407–416. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, T.; Salama, P.; Johansson, E.W.; Mason, E. Pneumonia: The leading killer of children. Lancet 2006, 368, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collaborators, G.B.D.L.R.I. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, C.-E.M. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Tanne, J.H. COVID-19: Antimicrobial resistance rose dangerously in US during pandemic, CDC says. BMJ 2022, 378, o1755. [Google Scholar] [CrossRef]
- Thomas, G.R.; Corso, A.; Pasteran, F.; Shal, J.; Sosa, A.; Pillonetto, M.; de Souza Peral, R.T.; Hormazabal, J.C.; Araya, P.; Saavedra, S.Y.; et al. Increased Detection of Carbapenemase-Producing Enterobacterales Bacteria in Latin America and the Caribbean during the COVID-19 Pandemic. Emerg. Infect. Dis. 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Sempere, J.; Llamosi, M.; Lopez Ruiz, B.; Del Rio, I.; Perez-Garcia, C.; Lago, D.; Gimeno, M.; Coronel, P.; Gonzalez-Camacho, F.; Domenech, M.; et al. Effect of pneumococcal conjugate vaccines and SARS-CoV-2 on antimicrobial resistance and the emergence of Streptococcus pneumoniae serotypes with reduced susceptibility in Spain, 2004–2020: A national surveillance study. Lancet Microbe 2022, 3, e744–e752. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N.; European Society of Clinical Microbiology Infectious Diseases Vaccine Study, G. Influenza vaccination and prevention of antimicrobial resistance. Expert Rev. Vaccines 2018, 17, 881–888. [Google Scholar] [CrossRef]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Lipsitch, M.; Siber, G.R. How Can Vaccines Contribute to Solving the Antimicrobial Resistance Problem? mBio 2016, 7, e00428-16. [Google Scholar] [CrossRef] [Green Version]
- Ginsburg, A.S.; Klugman, K.P. Vaccination to reduce antimicrobial resistance. Lancet Glob. Health 2017, 5, e1176–e1177. [Google Scholar] [CrossRef] [Green Version]
- Atkins, K.E.; Flasche, S. Vaccination to reduce antimicrobial resistance. Lancet Glob. Health 2018, 6, e252. [Google Scholar] [CrossRef] [Green Version]
- Atkins, K.E.; Lafferty, E.I.; Deeny, S.R.; Davies, N.G.; Robotham, J.V.; Jit, M. Use of mathematical modelling to assess the impact of vaccines on antibiotic resistance. Lancet Infect. Dis. 2018, 18, e204–e213. [Google Scholar] [CrossRef]
- Sempere, J.; Gonzalez-Camacho, F.; Domenech, M.; Llamosi, M.; Del Rio, I.; Lopez-Ruiz, B.; Gimeno, M.; Coronel, P.; Yuste, J. A national longitudinal study evaluating the activity of cefditoren and other antibiotics against non-susceptible Streptococcus pneumoniae strains during the period 2004-20 in Spain. J. Antimicrob. Chemother. 2022, 77, 1045–1051. [Google Scholar] [CrossRef]
- Giufre, M.; Cardines, R.; Caporali, M.G.; Accogli, M.; D’Ancona, F.; Cerquetti, M. Ten years of Hib vaccination in Italy: Prevalence of non-encapsulated Haemophilus influenzae among invasive isolates and the possible impact on antibiotic resistance. Vaccine 2011, 29, 3857–3862. [Google Scholar] [CrossRef]
- Domenech, M.; Sempere, J.; de Miguel, S.; Yuste, J. Combination of Antibodies and Antibiotics as a Promising Strategy Against Multidrug-Resistant Pathogens of the Respiratory Tract. Front. Immunol. 2018, 9, 2700. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.A.; D’Agnillo, F.; Sheng, Z.M.; Kindrachuk, J.; Schwartzman, L.M.; Kuestner, R.E.; Chertow, D.S.; Golding, B.T.; Taubenberger, J.K.; Kash, J.C. 1918 pandemic influenza virus and Streptococcus pneumoniae co-infection results in activation of coagulation and widespread pulmonary thrombosis in mice and humans. J. Pathol. 2016, 238, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Bosch, A.A.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Opatowski, L.; Baguelin, M.; Eggo, R.M. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: A key role for mathematical modelling. PLoS Pathog. 2018, 14, e1006770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rameix-Welti, M.A.; Zarantonelli, M.L.; Giorgini, D.; Ruckly, C.; Marasescu, M.; van der Werf, S.; Alonso, J.M.; Naffakh, N.; Taha, M.K. Influenza A virus neuraminidase enhances meningococcal adhesion to epithelial cells through interaction with sialic acid-containing meningococcal capsules. Infect. Immun. 2009, 77, 3588–3595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramegna, A.; Sotgiu, G.; Di Pasquale, M.; Radovanovic, D.; Terraneo, S.; Reyes, L.F.; Vendrell, E.; Neves, J.; Menzella, F.; Blasi, F.; et al. Atypical pathogens in hospitalized patients with community-acquired pneumonia: A worldwide perspective. BMC Infect. Dis. 2018, 18, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchiari, D.; Pericas, J.M.; Riera, J.; Gumucio, R.; Md, E.C.; Nicolas, D.; Hospital Clinic, H.T. Pneumococcal superinfection in COVID-19 patients: A series of 5 cases. Med. Clin. 2020, 155, 502–505. [Google Scholar] [CrossRef]
- Nieto-Moro, M.; Ecclesia, F.G.; Tome-Masa, I.; De Lama Caro-Paton, G.; Leoz-Gordillo, I.; Cabrero-Hernandez, M.; Garcia-Salido, A. SARS-CoV-2 and Streptococcus pneumoniae coinfection as a cause of severe pneumonia in an infant. Pediatr. Pulmonol. 2020, 55, 2198–2200. [Google Scholar] [CrossRef]
- Lopez-Cuadrado, T.; Llacer, A.; Palmera-Suarez, R.; Gomez-Barroso, D.; Savulescu, C.; Gonzalez-Yuste, P.; Fernandez-Cuenca, R. Trends in infectious disease mortality rates, Spain, 1980–2011. Emerg. Infect. Dis. 2014, 20, 782–789. [Google Scholar] [CrossRef]
- Redondo, E.; Rivero, I.; Vargas, D.A.; Mascaros, E.; Diaz-Maroto, J.L.; Linares, M.; Valdeperez, J.; Gil, A.; Molina, J.; Jimeno, I.; et al. Vaccination against community acquired pneumonia in adult patients. A position paper by Neumoexpertos en Prevencion. Semergen 2016, 42, 464–475. [Google Scholar] [CrossRef]
- Redondo, E.; Rivero-Calle, I.; Vargas, D.A.; Mascaros, E.; Diaz-Maroto, J.L.; Linares, M.; Gil, A.; Molina, J.; Jimeno, I.; Ocana, D.; et al. Adult community acquired pneumonia vaccination: 2018 Update of the positioning of the Pneumonia Prevention Expert Group. Semergen 2018, 44, 590–597. [Google Scholar] [CrossRef]
- Redondo, E.; Rivero-Calle, I.; Mascaros, E.; Yuste, J.E.; Fernandez-Prada, M.; Ocana, D.; Jimeno, I.; Gil, A.; Molina, J.; Diaz-Maroto, J.L.; et al. Vaccination against community acquired pneumonia in adults. Update 2021 of the position paper by Neumoexpertos en Prevencion Group. Semergen 2021, 47, 411–425. [Google Scholar] [CrossRef]
- OCEBM Levels of Evidence. The 2011 Oxford CEBM Levels of Evidence (Introductory Document). 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 29 October 2022).
- Yang, X.; Zhang, D.; Ou, W. Pneumococcal vaccination patterns among persons aged 65 years or older in the United States: A retrospective database analysis. Vaccine 2018, 36, 7574–7579. [Google Scholar] [CrossRef]
- Morga, A.; Kimura, T.; Feng, Q.; Rozario, N.; Schwartz, J. Compliance to Advisory Committee on Immunization Practices recommendations for pneumococcal vaccination. Vaccine 2022, 40, 2274–2281. [Google Scholar] [CrossRef]
- de Miguel, S.; Domenech, M.; Gonzalez-Camacho, F.; Sempere, J.; Vicioso, D.; Sanz, J.C.; Comas, L.G.; Ardanuy, C.; Fenoll, A.; Yuste, J. Nationwide Trends of Invasive Pneumococcal Disease in Spain From 2009 Through 2019 in Children and Adults During the Pneumococcal Conjugate Vaccine Era. Clin. Infect. Dis. 2021, 73, e3778–e3787. [Google Scholar] [CrossRef]
- Torres, A.; Menendez, R.; Espana, P.P.; Fernandez-Villar, J.A.; Marimon, J.M.; Cilloniz, C.; Mendez, R.; Egurrola, M.; Botana-Rial, M.; Ercibengoa, M.; et al. The Evolution and Distribution of Pneumococcal Serotypes in Adults Hospitalized With Community-Acquired Pneumonia in Spain Using a Serotype-Specific Urinary Antigen Detection Test: The CAPA Study, 2011–2018. Clin. Infect. Dis. 2021, 73, 1075–1085. [Google Scholar] [CrossRef]
- De Miguel, S.; Latasa, P.; Yuste, J.; Garcia, L.; Ordobas, M.; Ramos, B.; Perez, M.; Ortiz, M.A.; Sanz, J.C. Age-Dependent Serotype-Associated Case-Fatality Rate in Invasive Pneumococcal Disease in the Autonomous Community of Madrid between 2007 and 2020. Microorganisms 2021, 9, 2286. [Google Scholar] [CrossRef]
- Aguinagalde, L.; Corsini, B.; Domenech, A.; Domenech, M.; Camara, J.; Ardanuy, C.; Garcia, E.; Linares, J.; Fenoll, A.; Yuste, J. Emergence of Amoxicillin-Resistant Variants of Spain9V-ST156 Pneumococci Expressing Serotype 11A Correlates with Their Ability to Evade the Host Immune Response. PLoS ONE 2015, 10, e0137565. [Google Scholar] [CrossRef]
- Kobayashi, M.; Farrar, J.L.; Gierke, R.; Britton, A.; Childs, L.; Leidner, A.J.; Campos-Outcalt, D.; Morgan, R.L.; Long, S.S.; Talbot, H.K.; et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 109–117. [Google Scholar] [CrossRef]
- WHO. Influenza Update N° 432. 2022. Available online: https://cdn.who.int/media/docs/default-source/influenza/influenza-updates/2022/2022_11_14_surveillance_update_432.pdf?sfvrsn=19cda949_1&download=true (accessed on 29 October 2022).
- SVGE. Vigilancia Centinela de Infección Respiratoria Aguda en Atención Primaria (IRAs) y en Hospitales (IRAG) Gripe, COVID-19 y Otros Virus Respiratorio. 2021. Available online: https://vgripe.isciii.es/documentos/20202021/boletines/Informe%20semanal%20SVGE%20y%20otros%20virus%20respiratorios_2020-2021_202021.pdf (accessed on 29 October 2022).
- SIVIRA. Vigilancia Centinela de Infección Respiratoria Aguda en Atención Primaria (IRAs) y en Hospitales (IRAG) Gripe, COVID-19 y Otros Virus Respiratorio. 2022. Available online: https://vgripe.isciii.es/documentos/20212022/boletines/Informe%20semanal_SiVIRA_392022.pdf (accessed on 29 October 2022).
- WEP; Global Infrastructure Partners. Review of Global Influenza Circulation, Late 2019 to 2020, and the Impact of the COVID-19 Pandemic on Influenza Circulation. 2021. Available online: https://www.who.int/publications/i/item/who-wer-9625-241-264 (accessed on 29 October 2022).
- GISRS-WHO. Virus Detection by Subtype Reported to FLUNET. 2022. Available online: https://app.powerbi.com/view?r=eyJrIjoiZTkyODcyOTEtZjA5YS00ZmI0LWFkZGUtODIxNGI5OTE3YjM0IiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9 (accessed on 29 October 2022).
- Assembly, W. Prevention and Control of Influenza Pandemics and Annual Epidemics. 2003. Available online: https://apps.who.int/gb/archive/pdf_files/WHA56/ea56r19.pdf (accessed on 29 October 2022).
- SIVAMIN. Sistema de Información de Vacunaciones. 2022. Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S/sivamin (accessed on 29 October 2022).
- Diez-Domingo, J.; Redondo Marguello, E.; Ortiz de Lejarazu Leonardo, R.; Gil de Miguel, A.; Guillen Ortega, J.M.; Rincon Mora, J.; Martinon-Torres, F. A tool for early estimation of influenza vaccination coverage in Spanish general population and healthcare workers in the 2018-19 season: The Gripometro. BMC Public Health 2022, 22, 825. [Google Scholar] [CrossRef]
- Gil-de-Miguel, Á.; Martinón-Torres, F.; Díez-Domingo, J.; de Lejarazu Leonardo, R.O.; Pumarola, T.; Carmo, M.; Drago, G.; López-Belmonte, J.L.; Bricout, H.; de Courville, C.; et al. Clinical and economic burden of physician-diagnosed influenza in adults during the 2017/2018 epidemic season in Spain. BMC Public Health. 2022, 22, 2369. [Google Scholar] [CrossRef]
- Gil de Miguel, A.; Eiros Bouza, J.M.; Martinez Alcorta, L.I.; Callejo, D.; Minarro, C.; Vallejo-Aparicio, L.A.; Garcia, A.; Tafalla, M.; Cambronero, M.D.R.; Rodriguez, R.; et al. Direct Medical Costs of Four Vaccine-Preventable Infectious Diseases in Older Adults in Spain. Pharmacoecon. Open 2022, 6, 509–518. [Google Scholar] [CrossRef]
- Frobert, O.; Gotberg, M.; Erlinge, D.; Akhtar, Z.; Christiansen, E.H.; MacIntyre, C.R.; Oldroyd, K.G.; Motovska, Z.; Erglis, A.; Moer, R.; et al. Influenza Vaccination After Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Circulation 2021, 144, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, B.; Bhatt, D.L.; Cannon, C.P.; Vardeny, O.; Lee, D.S.; Solomon, S.D.; Udell, J.A. Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis. JAMA Netw. Open 2022, 5, e228873. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, S.; Barreira-Hernandez, D.; Gil, M.; Garcia-Lledo, A.; Izquierdo-Esteban, L.; De Abajo, F.J. Influenza Vaccination and Risk of Ischemic Stroke: A Population-Based Case-Control Study. Neurology 2022, 99, e2149–e2160. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chou, K.T.; Liu, J.C.; Chiu, C.C.; Yang, T.Y.; Lin, C.H.; Fang, Y.A.; Jian, W.; Lei, M.H.; Yeh, H.T.; et al. Association between Stroke Risk and Influenza Vaccination in Patients with Gout: A Nationwide Population-Based Study. Vaccines 2022, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mena, M.; Mandania, R.A.; Ghosh, A.; Dodoo, C.; Dwivedi, A.K.; Mukherjee, D. Associations between Combined Influenza and Pneumococcal Pneumonia Vaccination and Cardiovascular Outcomes. Cardiology 2021, 146, 772–780. [Google Scholar] [CrossRef]
- Martinez-Baz, I.; Navascues, A.; Portillo, M.E.; Casado, I.; Fresan, U.; Ezpeleta, C.; Castilla, J. Effect of Influenza Vaccination in Preventing Laboratory-Confirmed Influenza Hospitalization in Patients With Diabetes Mellitus. Clin. Infect. Dis. 2021, 73, 107–114. [Google Scholar] [CrossRef]
- Samson, S.I.; Konty, K.; Lee, W.N.; Quisel, T.; Foschini, L.; Kerr, D.; Liska, J.; Mills, H.; Hollingsworth, R.; Greenberg, M.; et al. Quantifying the Impact of Influenza Among Persons With Type 2 Diabetes Mellitus: A New Approach to Determine Medical and Physical Activity Impact. J. Diabetes Sci. Technol. 2021, 15, 44–52. [Google Scholar] [CrossRef]
- AEP. Vacunación Frente a la Gripe Estacional en la Infancia y la Adolescencia, 2022–2023. 2022. Available online: https://vacunasaep.org/sites/vacunasaep.org/files/gripe_recomendaciones-vacunacion-antigripal_2022-2023_v.2_22sep2022_0.pdf (accessed on 29 October 2022).
- CIMA. Ficha Tecnica Fluenz Tetra Suspension Para Pulverizacion Nasal. 2022. Available online: https://cima.aemps.es/cima/dochtml/ft/113887003/FT_113887003.html (accessed on 29 October 2022).
- Sanidad, M.D. Recomendaciones de Vacunación Frente a la Gripe en Población Infantil de 6 a 59 Meses. 2022. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/Recomendaciones_vacunacion_gripe_PoblacionInfantil.pdf (accessed on 29 October 2022).
- Swets, M.C.; Russell, C.D.; Harrison, E.M.; Docherty, A.B.; Lone, N.; Girvan, M.; Hardwick, H.E.; Investigators, I.C.; Visser, L.G.; Openshaw, P.J.M.; et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022, 399, 1463–1464. [Google Scholar] [CrossRef]
- Tang, C.Y.; Boftsi, M.; Staudt, L.; McElroy, J.A.; Li, T.; Duong, S.; Ohler, A.; Ritter, D.; Hammer, R.; Hang, J.; et al. SARS-CoV-2 and influenza co-infection: A cross-sectional study in central Missouri during the 2021–2022 influenza season. Virology 2022, 576, 105–110. [Google Scholar] [CrossRef]
- National Centre for Immunisation Research and Surveillance. High-Dose Influenza Vaccine (HD-IV) Compared with Standard Dose Influenza Vaccine (sIV) for Older Adults ≥65 Years to Prevent Influenza, Influenza-Related Complications or Mortality. 2022. Available online: https://www.ncirs.org.au/sites/default/files/2022-05/HD%20vs%20sIV%20SoF%20EP%20E2D_March%202022_Final.pdf (accessed on 29 October 2022).
- National Centre for Immunisation Research and Surveillance. MDCK Cell-Derived Influenza Vaccine Compared to Standard Dose Egg-Based Influenza Vaccine in People Aged ≥18 Years. 2021. Available online: https://www.ncirs.org.au/sites/default/files/2022-05/cIIV%20vs%20eIIV%20adults%20SoF%20EP%20E2D%20tables_March%202022_Final.pdf (accessed on 29 October 2022).
- National Centre for Immunisation Research and Surveillance. Summary of Findings: MF-59 Adjuvanted Influenza Vaccine Compared with Standard Dose Influenza Vaccine for People Aged ≥65 Years. 2020. Available online: https://www.ncirs.org.au/sites/default/files/2020-11/Grade%20table%20-%20Adjuvanted%20influenza%20vaccine%20Vs%20standard%20dose%20influenza%20vaccine%20SoF%20EP%20E2D%20tables_Final.pdf (accessed on 29 October 2022).
- ACIP. GRADE: Higher Dose and Adjuvanted Influenza Vaccines for Persons Aged ≥65 Years. 2022. Available online: https://www.cdc.gov/vaccines/acip/recs/grade/influenza-older-adults.html (accessed on 29 October 2022).
- ECDC. European Centre for Disease Prevention and Control. Systematic Review of the Efficacy, Effectiveness and Safety of Newer and Enhanced Seasonal Influenza Vaccines for the Prevention of Laboratory Confirmed Influenza in Individuals Aged 18 Years and Over. Stockholm: ECDC. 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-vaccines-systematic-review-efficacy.pdf (accessed on 29 October 2022).
- Public Health Agency of Canada. Literature Review Update on the Efficacy and Effectiveness of high-Dose (Fluzone®® High-Dose) and MF59-Adjuvanted (Fluad®®) Trivalent Inactivated Influenza Vaccines in Adults 65 Years of Age and Older. 2018. Available online: https://publications.gc.ca/site/eng/9.852907/publication.html (accessed on 29 October 2022).
- (NACI), National Advisory Committee on Immunization. Supplemental Statement—Mammalian Cell Culture-Based Influenza Vaccines. 2020. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/mammalian-cell-culture-based-influenza-vaccines/naci-sppl-stmt-ammalian-cell-based-influenza-vaccines-en.pdf (accessed on 29 October 2022).
- Institute, R.K. Anlage zum Epidemiologischen Bulletin Nr. 1|7. Januar 2021 (Online Vorab). 2021. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/01_21_Anhang.pdf?__blob=publicationFile (accessed on 29 October 2022).
- Aldeán, J.Á.; Hevia, A.C.; Cenoz, M.G.; Sanz, I.J.; Margüello, E.R.; Herrero, F.S.; Astiz, T.V.; Rojas, A.G. Revisión y Análisis de las Evaluaciones Públicas de Organismos Internacionales Sobre los Niveles de Evidencia de las Nuevas Vacunas Antigripales. 2022. Available online: https://www.vacunas.sanofipasteur.es/dam/jcr:c7b41ade-00cd-458f-8d61-98828c46b8dd/Resumen_analisis_organismos_internacionales.pdf (accessed on 29 October 2022).
- EMA. Guideline on Influenza Vaccines. Non-Clinical and Clinical Module. 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf (accessed on 29 October 2022).
- (NACI), National Advisory Committee on Immunization. Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022–2023. 2022. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2022-2023/naci-2022-2023-statement.pdf (accessed on 29 October 2022).
- Grohskopf, L. Influenza Vaccines for Persons Aged >65 Years: Evidence to Recommendations (EtR) Framework. 2022. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf (accessed on 29 October 2022).
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K.; Morgan, R.L.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022–2023 Influenza Season. MMWR Recomm. Rep. 2022, 71, 1–28. [Google Scholar] [CrossRef]
- T, B.-S. DANFLU-1: High-Dose vs. Standard-Dose Influenza Vaccine in Elderly Adults. 2022. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2022/08/25/19/13/sat-339am-DANFLU-1-esc-2022 (accessed on 29 October 2022).
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Samson, S.I.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine 2021, 39 (Suppl. 1), A24–A35. [Google Scholar] [CrossRef]
- Kuodi, P.; Gorelik, Y.; Zayyad, H.; Wertheim, O.; Wiegler, K.B.; Abu Jabal, K.; Dror, A.A.; Nazzal, S.; Glikman, D.; Edelstein, M. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: Cross-sectional study 2020-21, Israel. NPJ Vaccines 2022, 7, 101. [Google Scholar] [CrossRef]
- Hansen, C.L.; Chaves, S.S.; Demont, C.; Viboud, C. Mortality Associated With Influenza and Respiratory Syncytial Virus in the US, 1999–2018. JAMA Netw. Open 2022, 5, e220527. [Google Scholar] [CrossRef]
- Gil-Prieto, R.; Gonzalez-Escalada, A.; Marin-Garcia, P.; Gallardo-Pino, C.; Gil-de-Miguel, A. Respiratory Syncytial Virus Bronchiolitis in Children up to 5 Years of Age in Spain: Epidemiology and Comorbidities: An Observational Study. Medicine 2015, 94, e831. [Google Scholar] [CrossRef]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef] [Green Version]
- Pastula, S.T.; Hackett, J.; Coalson, J.; Jiang, X.; Villafana, T.; Ambrose, C.; Fryzek, J. Hospitalizations for Respiratory Syncytial Virus Among Adults in the United States, 1997–2012. Open Forum. Infect. Dis. 2017, 4, ofw270. [Google Scholar] [CrossRef] [Green Version]
- Coultas, J.A.; Smyth, R.; Openshaw, P.J. Respiratory syncytial virus (RSV): A scourge from infancy to old age. Thorax 2019, 74, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Atamna, A.; Babich, T.; Froimovici, D.; Yahav, D.; Sorek, N.; Ben-Zvi, H.; Leibovici, L.; Bishara, J.; Avni, T. Morbidity and mortality of respiratory syncytial virus infection in hospitalized adults: Comparison with seasonal influenza. Int. J. Infect. Dis. 2021, 103, 489–493. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current State of Respiratory Syncytial Virus Disease and Management. Infect Dis. Ther. 2021, 10, 5–16. [Google Scholar] [CrossRef]
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory syncytial virus: Diagnosis, prevention and management. Ther. Adv. Infect Dis. 2019, 6, 2049936119865798. [Google Scholar] [CrossRef]
- Volling, C.; Hassan, K.; Mazzulli, T.; Green, K.; Al-Den, A.; Hunter, P.; Mangat, R.; Ng, J.; McGeer, A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorazka, M.; Flury, D.; Herzog, K.; Albrich, W.C.; Vuichard-Gysin, D. Clinical outcomes of adults hospitalized for laboratory confirmed respiratory syncytial virus or influenza virus infection. PLoS ONE 2021, 16, e0253161. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Rodriguez-Fernandez, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, J.; Moorthy, V.; Giersing, B.; Friede, M.; Hombach, J.; Arora, N.; Modjarrad, K.; Smith, P.G.; Karron, R.; Graham, B.; et al. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7394–7395. [Google Scholar] [CrossRef]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardaji, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2022, in press. [CrossRef]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef]
- Ison, M.G.; Papi, A.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; Van Zyl-Smit, R.N.; Dezutter, N.; De Schrevel, N.; et al. A Respiratory Syncytial Virus (RSV) Prefusion F Protein Candidate Vaccine (RSVPreF3 OA) is Efficacious in Adults ≥ 60 Years of Age (YOA); Abstract IDWeek; Oxford University Press: Washington, DC, USA, 2022. [Google Scholar]
- Shen, D.P.; Vermeulen, F.; Debeer, A.; Lagrou, K.; Smits, A. Impact of COVID-19 on viral respiratory infection epidemiology in young children: A single-center analysis. Front. Public Health 2022, 10, 931242. [Google Scholar] [CrossRef]
- Perez, A.; Lively, J.Y.; Curns, A.; Weinberg, G.A.; Halasa, N.B.; Staat, M.A.; Szilagyi, P.G.; Stewart, L.S.; McNeal, M.M.; Clopper, B.; et al. Respiratory Virus Surveillance Among Children with Acute Respiratory Illnesses—New Vaccine Surveillance Network, United States, 2016–2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1253–1259. [Google Scholar] [CrossRef]
- Casanova, C.; Kuffer, M.; Leib, S.L.; Hilty, M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg. Microbes Infect. 2021, 10, 2202–2204. [Google Scholar] [CrossRef]
- Rios-Silva, M.; Trujillo, X.; Huerta, M.; Benites-Godinez, V.; Guzman-Esquivel, J.; Bricio-Barrios, J.A.; Mendoza-Cano, O.; Lugo-Radillo, A.; Murillo-Zamora, E. Reemerging Influenza Virus Infections during the Dominance of the Omicron SARS-CoV-2 Variant in Mexico. Pathogens 2022, 11, 1181. [Google Scholar] [CrossRef]
- Fink, A.L.; Klein, S.L. Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology 2015, 30, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernan, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth Dose of BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef]
- Link-Gelles, R. Association between COVID-19 mRNA Vaccination and COVID-19 Illness and Severity during Omicron BA.4 and BA.5 Sublineage Periods. 2022. Available online: https://www.medrxiv.org/content/10.1101/2022.10.04.22280459v1 (accessed on 29 October 2022).
- Nadesalingam, A.; Cantoni, D.; Wells, D.A.; Aguinam, E.T.; Ferrari, M.; Smith, P.; Chan, A.; Carnell, G.; Ohlendorf, L.; Einhauser, S.; et al. Paucity and discordance of neutralising antibody responses to SARS-CoV-2 VOCs in vaccinated immunodeficient patients and health-care workers in the UK. Lancet Microbe 2021, 2, e416–e418. [Google Scholar] [CrossRef]
- Davis-Gardner, M. mRNA Bivalent Booster Enhances Neutralization against BA.2.75.2 and BQ.1.1. 2022. Available online: https://europepmc.org/article/ppr/ppr565823 (accessed on 29 October 2022).
- Guerrini, G.; Magri, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of nanoparticles-based vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef]
- Kandeil, W.; Atanasov, P.; Avramioti, D.; Fu, J.; Demarteau, N.; Li, X. The burden of pertussis in older adults: What is the role of vaccination? A systematic literature review. Expert. Rev. Vaccines 2019, 18, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Kondratiuk, T.P.; Bychenok, S.F.; Prishchepa, L.A.; Babich, L.G.; Kurskii, M.D. Isolation and characteristics of the plasma membrane fraction from the swine myometrium. Ukr. Biokhim. Zh. 1986, 58, 50–56. [Google Scholar]
- Jenkins, V.A.; Savic, M.; Kandeil, W. Pertussis in high-risk groups: An overview of the past quarter-century. Hum. Vaccines Immunother. 2020, 16, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Aris, E.; Harrington, L.; Bhavsar, A.; Simeone, J.C.; Ramond, A.; Papi, A.; Vogelmeier, C.F.; Meszaros, K.; Lambrelli, D.; Mukherjee, P. Burden of Pertussis in COPD: A Retrospective Database Study in England. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 157–169. [Google Scholar] [CrossRef]
- Bhavsar, A.; Aris, E.; Harrington, L.; Simeone, J.C.; Ramond, A.; Lambrelli, D.; Papi, A.; Boulet, L.P.; Meszaros, K.; Jamet, N.; et al. Burden of Pertussis in Individuals with a Diagnosis of Asthma: A Retrospective Database Study in England. J. Asthma Allergy 2022, 15, 35–51. [Google Scholar] [CrossRef]

| Entity | NACI [66,67,71] | ECDC [65] | STIKO [68] | ATAGI [61,62,63] | ACIP [64] |
|---|---|---|---|---|---|
| Publication date | May 2018 (HD and adj) August 2020 (TC) September 2022 (REC) | October 2020 | January 2021 | November 2020 (adj) March 2021 (TC) March 2022 (HD) | August 2022 |
| HD | A (Maximum) | +++ (Moderate) | ++++ (High) | ++ (Low) +++ (moderate) | High Support HD |
| Recombinant | B (Limited) | +++ (Moderate) | +++ (Moderate) | Not evaluated | Moderate Do not support one against the other |
| Tissue culture | I (Insufficient) | No evidence | ++ (Low) | + (very low) | Not evaluated |
| Adjuvanted | I (Insufficient) * | No evidence | ++ (Low) | + (very low) | Moderate * Do not support one against the other |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redondo, E.; Rivero-Calle, I.; Mascarós, E.; Ocaña, D.; Jimeno, I.; Gil, Á.; Díaz-Maroto, J.L.; Linares, M.; Onieva-García, M.Á.; González-Romo, F.; et al. Vaccination against Community-Acquired Pneumonia in Spanish Adults: Practical Recommendations by the NeumoExperts Prevention Group. Antibiotics 2023, 12, 138. https://doi.org/10.3390/antibiotics12010138
Redondo E, Rivero-Calle I, Mascarós E, Ocaña D, Jimeno I, Gil Á, Díaz-Maroto JL, Linares M, Onieva-García MÁ, González-Romo F, et al. Vaccination against Community-Acquired Pneumonia in Spanish Adults: Practical Recommendations by the NeumoExperts Prevention Group. Antibiotics. 2023; 12(1):138. https://doi.org/10.3390/antibiotics12010138
Chicago/Turabian StyleRedondo, Esther, Irene Rivero-Calle, Enrique Mascarós, Daniel Ocaña, Isabel Jimeno, Ángel Gil, José Luis Díaz-Maroto, Manuel Linares, María Ángeles Onieva-García, Fernando González-Romo, and et al. 2023. "Vaccination against Community-Acquired Pneumonia in Spanish Adults: Practical Recommendations by the NeumoExperts Prevention Group" Antibiotics 12, no. 1: 138. https://doi.org/10.3390/antibiotics12010138
APA StyleRedondo, E., Rivero-Calle, I., Mascarós, E., Ocaña, D., Jimeno, I., Gil, Á., Díaz-Maroto, J. L., Linares, M., Onieva-García, M. Á., González-Romo, F., Yuste, J., & Martinón-Torres, F. (2023). Vaccination against Community-Acquired Pneumonia in Spanish Adults: Practical Recommendations by the NeumoExperts Prevention Group. Antibiotics, 12(1), 138. https://doi.org/10.3390/antibiotics12010138







