A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oils (EOs) Extraction and Chemical Characterisation
2.2. Antioxidant Activity of Essential Oils: DPPH and ABTS Assays
2.3. Antimicrobial Activity: MIC of the EOs and Growth Inhibition Percentage of Their Headspace Fractions
2.4. Cytotoxicity and Mutagen Protection Properties of the Amazonian EOs
3. Materials and Methods
3.1. Plant Material and Isolation of Essential Oil
3.2. Chemicals
3.3. Gas Chromatography—Flame Ionization Detector (FID)
3.4. Gas Chromatography—Mass Spectrometry
3.5. Headspace Gas Chromatography—Mass Spectrometry
3.6. Biological Activities of Essential Oils (EOs)
3.6.1. Antioxidant Properties
3.6.2. Antimicrobial Activity
3.6.3. HP-TLC-Bioautographic Assay of the Antioxidant and Antimicrobial Most Interesting Results
3.6.4. Amazonian Essential Oils Cytotoxicity and Mutagen Protection Properties: Highest Uneffective Dose (HUD) and Ames Test Properly Modified
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical profiling, antioxidant, antimicrobial and cholinesterase inhibitory effects of essential oils isolated from the leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Meenua, M.; Padhanb, B.; Patel, M.; Patel, R.; Xu, B. Antibacterial activity of essential oils from different parts of plants against Salmonella and Listeria spp. Food Chem. 2023, 404, 34723. [Google Scholar] [CrossRef]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of essential oil in aromatic plants under changing environment. J. App. Res. Med. Ar. Plants 2023, 32, 100441. [Google Scholar] [CrossRef]
- Singh, I.R.; Pulikkal, A.K. Preparation, stability and biological activity of essential oil-based nano emulsions: A comprehensive review. Open Nano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Sarmah, P.; Das, B.; Saikia, J.; Konwar, P.; Mudoi, K.D.; Saikia, S.P.; Banik, D. An insight on the immunomodulatory potential of wood oil of Aquilaria malaccensis Lam. with an emphasis on related phytomedicine, biomarkers, pharmacology, and toxicity. S. Afr. J. Bot. 2022, 151, 695–712. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, F.; Yu, C. Therapeutic potential of Curcuma oil and its terpenoids in gynecological cancers. Biomed. Pharmacother. 2023, 157, 114016. [Google Scholar] [CrossRef]
- Lee, M.-K.; Lim, S.; Song, J.-A.; Kim, M.-E.; Hur, M.-H. The effects of aromatherapy essential oil inhalation on stress, sleep quality and immunity in healthy adults: Randomized controlled trial. Eur. J. Integr. Med. 2017, 12, 79–86. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Poitevin, C.G.; Bischoff, A.M.; Beger, M.; da Luz, T.S.; Mazarotto, E.J.; Benatto, A.; Martins, C.E.N.; Sales Maia, B.H.L.N.; Sari, R.; et al. Insecticidal and antifungal activities of Melaleuca rhaphiophylla essential oil against insects and seed-borne pathogens in stored products. Ind. Crop. Prod. 2022, 182, 114871. [Google Scholar] [CrossRef]
- Elumalai, K.; Krishnappa, K.; Pandiyan, J.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Barnard, D.R.; Vijayakumar, N.; Govindarajan, M. Characterization of secondary metabolites from Lamiaceae plant leaf essential oil: A novel perspective to combat medical and agricultural pests. Physiol. Mol. Plant Pathol. 2022, 117, 101752. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Zhao, L.-L.; Shao, Y.-X.; Liao, X.-D.; Zhang, L.-Y.; Luo, X.-G. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J. Integr. Agr. 2019, 18, 2123–2132. [Google Scholar] [CrossRef]
- Coimbra, A.; Ferreira, S.; Duarte, A.P. Biological properties of Thymus zygis essential oil with emphasis on antimicrobial activity and food application. Food Chem. 2022, 393, 133370. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Guerrini, A.; Maietti, S.; Bruni, R.; Paganetto, G.; Poli, F.; Scalvenzi, L.; Radice, M.; Saro, K.; Sacchetti, G. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: A new functional food ingredient? Food Chem. 2011, 126, 837–848. [Google Scholar] [CrossRef]
- Rossi, D.; Guerrini, A.; Paganetto, G.; Bernacchia, G.; Conforti, F.; Statti, G.; Maietti, S.; Poppi, I.; Tacchini, M.; Sacchetti, G. Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil as possible mutagen-protective food ingredient against heterocyclic amines from cooked food. Food Chem. 2013, 139, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Noshad, M.; Behbahani, B.A.; Nikfarjam, Z. Chemical composition, antibacterial activity and antioxidant activity of Citrus bergamia essential oil: Molecular docking simulations. Food Biosci. 2022, 50, 102123. [Google Scholar] [CrossRef]
- Arab, H.A.; Bahadori, F.; Mirza, M.; Badi, H.N.; Kalate-Jari, S. Variability in essential oil composition and phenolic acid profile of Thymus daenensis Celak. populations from Iran. Ind. Crop. Prod. 2022, 178, 114345. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Curcuma longa L. Rhizome Essential Oil from Extraction to Its Agri-Food Applications. A Review. Plants 2021, 10, 44. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytosci. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, G.; Medici, A.; Maietti, S.; Radice, M.; Muzzoli, M.; Manfredini, S.; Braccioli, E.; Bruni, R. Composition and Functional Properties of the Essential Oil of Amazonian Basil, Ocimum micranthum Willd., Labiatae in Comparison with Commercial Essential Oils. J. Agric. Food Chem. 2004, 52, 3486–3491. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Radice, M.; Toma, L.; Severini, F.; Boccolini, D.; Bella, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; et al. Larvicidal activity of Ocimum campechianum, Ocotea quixos and Piper aduncum essential oils against Aedes aegypti. Parasite 2019, 26, 23. [Google Scholar] [CrossRef] [Green Version]
- Tacchini, M.; Guevara, M.P.E.; Grandini, A.; Maresca, I.; Radice, M.; Angiolella, L.; Guerrini, A. Ocimum campechianum Mill. from Amazonian Ecuador: Chemical Composition and Biological Activities of Extracts and Their Main Constituents (Eugenol and Rosmarinic Acid). Molecules 2021, 26, 84. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Falcao, M.A.; Fianco, A.L.B.; Lucas, A.M.; Pereira, M.A.A.; Torres, F.C.; Vargas, R.M.F.; Cassel, E. Determination of antibacterial activity of vacuum distillation fractions of lemongrass essential oil. Phytochem. Rev. 2012, 11, 405–412. [Google Scholar] [CrossRef]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Cuong, P.V.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Dat, N.Y. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020, 5924856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Komatsu, K.; Toume, K.; Zhu, S.; Tanaka, K.; Hayashi, S.; Anjiki, N.; Kawahara, N.; Takano, A.; Miyake, K.; et al. Essential oil composition of Curcuma species and drugs from Asia analyzed by headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry. J. Nat. Med. 2022, 77, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [CrossRef] [Green Version]
- Liju, V.B.; Jeena, K.; Kuttan, R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa L. Indian J. Pharmacol. 2011, 43, 526–531. [Google Scholar] [CrossRef]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; de Souza Rodrigues dos Santos, P.A.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Vázquez-Briones, M.C.; Hernández, L.R.; Guerrero-Beltrán, J.A. Physicochemical and Antioxidant Properties of Cymbopogon citratus Essential Oil. J. Food Res. 2015, 4, 36–45. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action (a review). Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Guerrini, A.; Rossi, D.; Paganetto, G.; Tognolini, M.; Muzzoli, M.; Romagnoli, C.; Antonioni, F.; Vertuani, S.; Medici, A.; Bruni, A.; et al. Chemical characterization (GC-MS and NMR fingerprinting) and bioactivities of South-African Pelargonium capitatum (L.) L’Herit. (Geraniaceae) essential oil. Chem. Biodivers. 2011, 8, 624–642. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.-H. Management of Highly Resistant Gram-Negative Infections in the Intensive Care Unit in the Era of Novel Antibiotics. Infect. Dis. Clin. N. Am. 2022, 36, 791–823. [Google Scholar] [CrossRef] [PubMed]
- Eva, L.; Gernot, Z.; Josefa, L.; Kathrin, H.; Shiva, P.-A.; Martin, H.; Thomas, V.; Gebhard, F.; Andrea, J.G. Contaminated Handwashing Sinks as the Source of a Clonal Outbreak of KPC-2-Producing Klebsiella oxytoca on a Hematology Ward. Antimicrob. Agents Chemother. 2015, 59, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Gerver, S.M.; Nsonwu, O.; Thelwall, S.; Brown, C.S.; Hope, R. Trends in rates of incidence, fatality and antimicrobial resistance among isolates of Pseudomonas spp. causing bloodstream infections in England between 2009 and 2018: Results from a national voluntary surveillance scheme. J. Hosp. Infect. 2022, 120, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.; Barclay, M.; Danial, J.; Philip, C.; Perry, M.; Etherson, M.; Henderson, N. Risk factors for antimicrobial resistance in patients with Escherichia coli bacteraemia related to urinary tract infection. Infect. Prev. Prac. 2022, 4, 100248. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Cr. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Yew, X.Y.; Sivasamugham, L.A. Antibacterial activity of Cymbopogon citratus against clinically important bacteria. S. Afr. J. Chem. Eng. 2020, 34, 26–30. [Google Scholar] [CrossRef]
- Albino, S.; Robazza, A.W.S.; Schittler, L.; Gomes, G.A. Synergistic and antimicrobial properties of commercial turmeric (Curcuma longa) essential oil against pathogenic bacteria. Food Sci. Technol. 2012, 32, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber Officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res.-Envir. Muta. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Thaís, F.L.; De Souza, C.T.; Pinho, R.A.; de Oliveira Marques, S.; Luiz, G.P.; dos Santos Tramontin, N.; da Silveira, P.C.L.; de Andrade, V.M.; Muller, A.P. Effects of Zingiber officinale extract supplementation on metabolic and genotoxic parameters in diet-induced obesity in mice. Brit. J. Nutr. 2021, 126, 970–981. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L.). Food Chem. Toxicol. 2013, 53, 52–61. [Google Scholar] [CrossRef]
- Ahmad, I.; Aqil, F.; Owais, M. Modern Phytomedicine: Turning Medicinal Plants into Drugs; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Costa, C.A.R.A.; Bidinotto, B.T.; Takahira, R.K.; Salvadori, D.M.F.; Barbisan, L.F.; Costa, M. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem. Toxicol. 2011, 49, 2268–2272. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, W. Official Methods of Analysis of AOAC International, Revision 3; AOAC International: Gaithersburg, MA, USA, 2010; ISBN 0935584676. [Google Scholar]
- Maietti, S.; Rossi, D.; Guerrini, A.; Useli, C.; Romagnoli, C.; Poli, F.; Bruni, R.; Sacchetti, G. Multivariate analysis approach to the study of chemical and functional properties of chemo-diverse plant derivatives: Lavender essential oils. Flav. Fragr. J. 2013, 28, 144–145. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Mukku, V.J.; El Mousadik, A.; Msanda, F.; El Aouad, N. A review on Moroccan Thymus species: Traditional uses, essential oils chemical composition and biological effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E.; Tognolini, M.; Maldonado, M.E.; Bruni, R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Phar. 2009, 27, 39–48. [Google Scholar] [CrossRef]
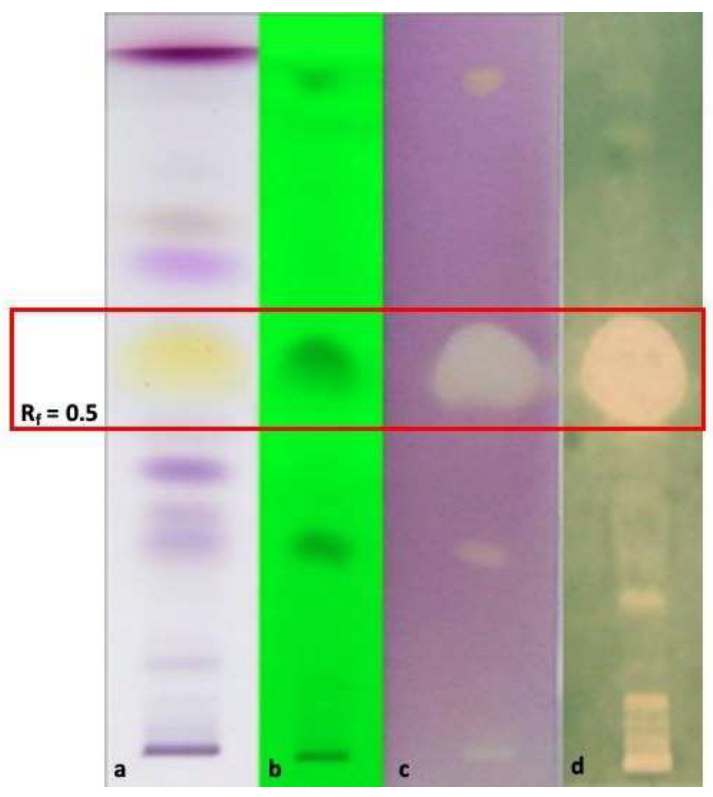

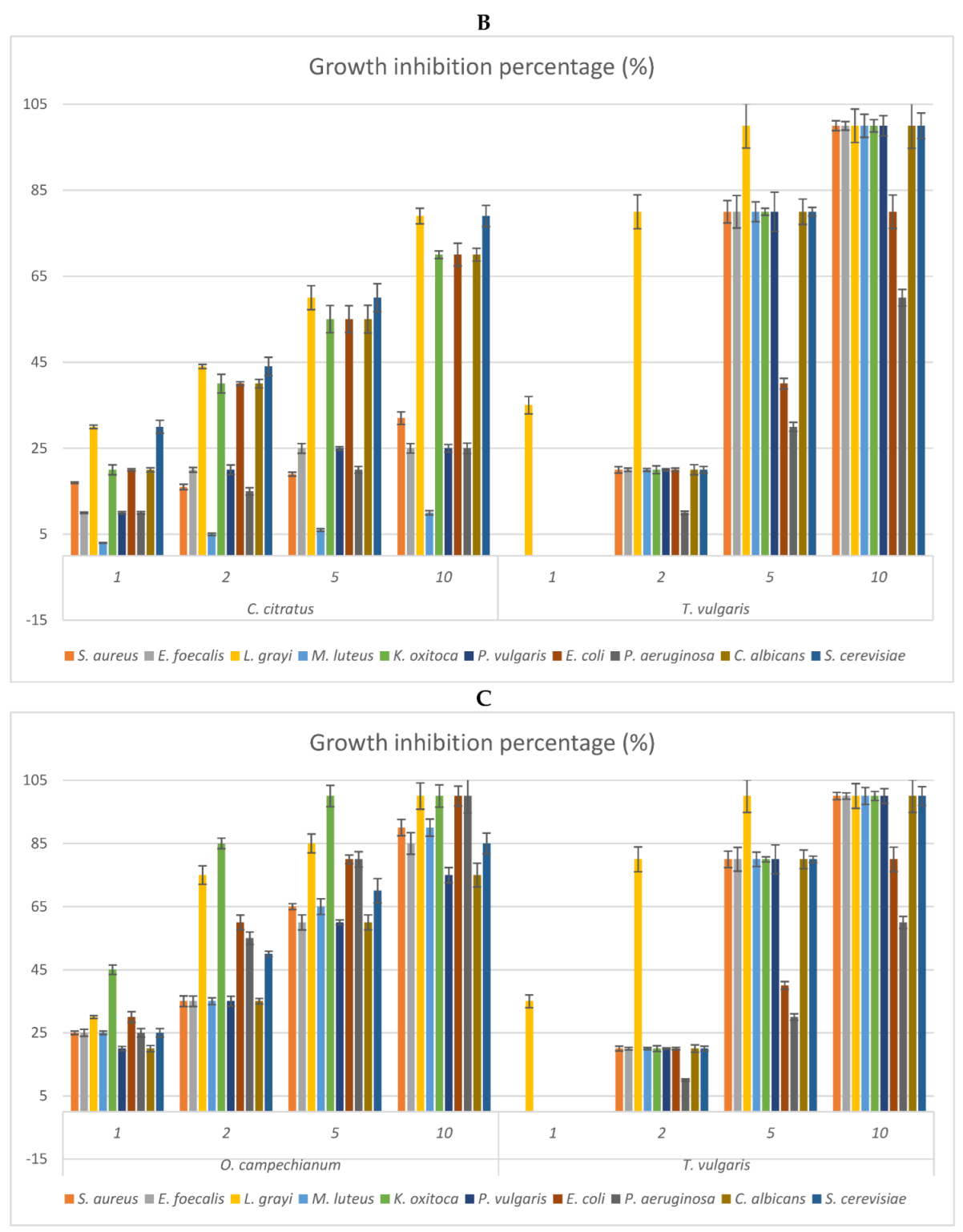
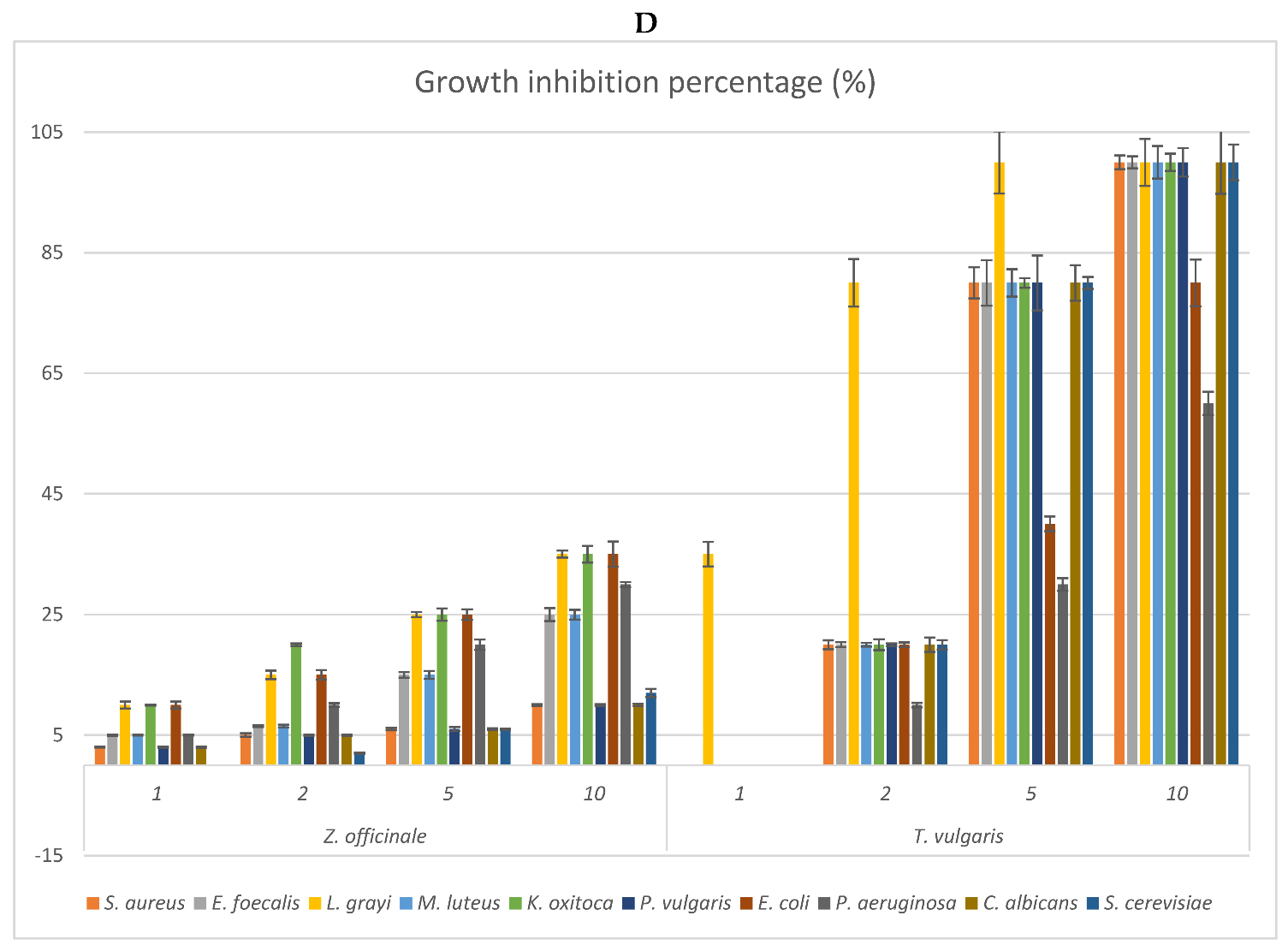
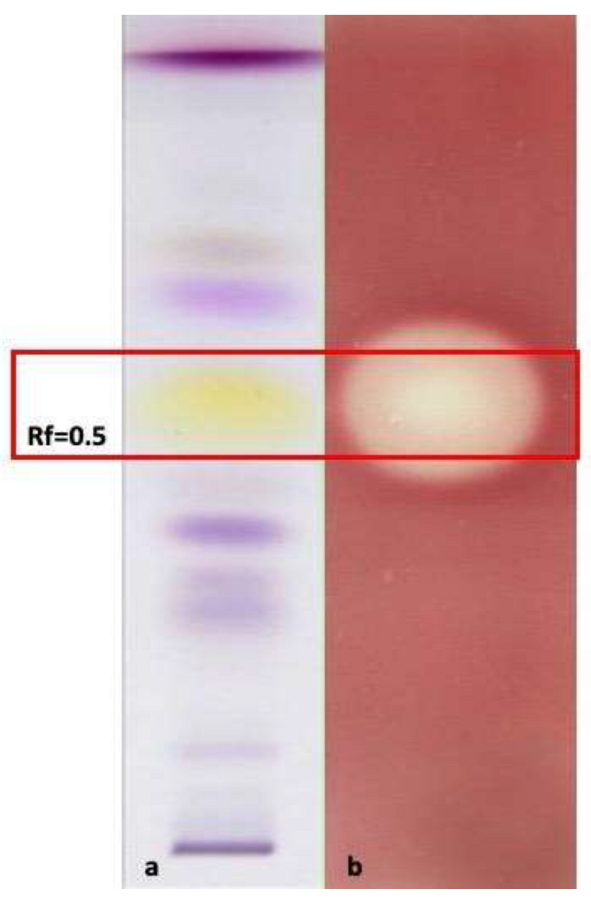
| Plant Species | Part Used | Hydro-Distillation Yield (mL/Kg Fresh Plant Material) | Density (g/mL) |
|---|---|---|---|
| Curcuma longa | Rhizome | 2.6 ± 0.4 | 0.8975 |
| Cymbopogon citratus | Aerial parts | 3.0 ± 0.5 | 0.8760 |
| Ocimum campechianum | Aerial parts | 7.0 ± 0.8 | 0.9310 |
| Zingiber officinale | Rhizome | 4.0 ± 0.6 | 0.8870 |
| N | Compound a | AI b | Curcuma longa | Cymbopogon citratus | Ocimum campechianum | Zingiber officinale |
|---|---|---|---|---|---|---|
| Area % | ||||||
| 1 | α-Pinene | 932 | 0.47 | - c | Tr d | 4.16 |
| 2 | Camphene | 946 | - | - | tr | 14.72 |
| 3 | Sabinene | 969 | 1.31 | - | tr | - |
| 4 | β-Pinene | 974 | - | - | 0.49 | 0.47 |
| 5 | Methyl-5-hepten-2-one | 981 | - | 0.41 | - | 0.69 |
| 6 | Myrcene | 988 | 0.36 | - | 0.19 | 1.80 |
| 7 | α-Phellandrene | 1002 | 9.81 | - | - | 0.71 |
| 8 | α-Terpinene | 1014 | 0.25 | - | - | - |
| 9 | p-Cymene | 1020 | 2.17 | - | - | - |
| 10 | Limonene | 1024 | 1.61 | tr | 0.17 | 5.75 |
| 11 | 1,8-Cineole | 1026 | 7.85 | - | 7.36 | 8.61 |
| 12 | cis-Ocimene | 1032 | - | - | 2.87 | - |
| 13 | trans-Ocimene | 1044 | - | - | tr | - |
| 14 | γ-Terpinene | 1054 | 0.46 | - | - | - |
| 15 | Terpinolene | 1086 | 2.30 | - | - | 0.49 |
| 16 | Linalool | 1095 | 0.26 | 0.46 | 1.87 | 0.95 |
| 17 | allo-Ocimene | 1128 | - | - | 0.16 | - |
| 18 | 1-Terpineol | 1130 | - | - | - | - |
| 19 | Citronellal | 1148 | 0.22 | 4.54 | - | - |
| 20 | Borneol | 1165 | - | - | 0.19 | 3.59 |
| 21 | cis-Isocitral | 1160 | - | 0.54 | - | - |
| 22 | 4-Terpineol | 1174 | 0.31 | - | - | - |
| 23 | trans-Isocitral | 1177 | - | 0.89 | - | - |
| 24 | α-Terpineol | 1186 | 0.47 | - | 0.59 | 1.53 |
| 25 | γ-Terpineol | 1199 | - | - | - | - |
| 26 | Nerol | 1227 | - | 2.64 | - | - |
| 27 | Neral | 1235 | - | 14.37 | - | 3.98 |
| 28 | Geraniol | 1249 | - | 39.43 | - | 0.44 |
| 29 | Geranial | 1264 | tr | 17.29 | - | 5.16 |
| 30 | Bornyl acetate | 1287 | - | - | - | 0.38 |
| 31 | Thymol | 1289 | tr | - | - | - |
| 32 | Carvacrol | 1298 | tr | - | - | - |
| 33 | δ-Elemene | 1335 | - | - | tr | - |
| 34 | Eugenol | 1356 | - | - | 50.97 | - |
| 35 | Neryl acetate | 1359 | - | 0.52 | - | - |
| 36 | β-Elemene | 1389 | - | - | 4.85 | 0.35 |
| 37 | Geranyl acetate | 1379 | - | 7.96 | - | - |
| 38 | E-Caryophyllene | 1417 | 0.38 | 2.46 | 10.21 | - |
| 39 | α-Humulene | 1452 | - | 0.41 | 2.05 | - |
| 40 | allo-Aromadendrene | 1458 | - | - | 0.53 | - |
| 41 | Germacrene D | 1484 | - | 0.47 | - | 1.45 |
| 42 | ar-Curcumene | 1479 | 1.22 | - | - | 3.80 |
| 43 | β-Selinene | 1489 | - | - | 1.66 | - |
| 44 | Bicyclogermacrene | 1500 | - | - | 4.11 | - |
| 45 | trans-Muurola-4(14)5-diene | 1493 | - | - | - | 2.24 |
| 46 | Germacrene A | 1508 | - | - | 2.76 | - |
| 47 | α-Zingiberene | 1493 | 1.25 | - | - | 15.45 |
| 48 | α-Bisabolene | 1506 | 0.27 | - | - | - |
| 49 | α-(E,E)-Farnesene | 1505 | - | - | - | 8.52 |
| 50 | δ-Cadinene | 1522 | - | 0.56 | - | tr |
| 51 | β-Sesquiphellandrene | 1521 | 2.20 | - | 6.87 | |
| 52 | Germacrene B | 1559 | - | - | 0.79 | 0.95 |
| 53 | trans-Nerolidol | 1561 | - | - | 0.51 | |
| 54 | α-Cadinene | 1537 | - | 1.13 | - | - |
| 55 | ar-Turmerol | 1582 | 1.38 | - | - | - |
| 56 | Spathulenol | 1577 | - | - | 4.42 | - |
| 57 | Helifolen-12-ale A | 1592 | 1.45 | - | - | - |
| 58 | Apiole | 1620 | - | 5.02 | - | - |
| 59 | β-Biotol | 1612 | 2.03 | - | - | - |
| 60 | β-Eudesmol | 1649 | - | - | - | 0.98 |
| 61 | α-Cadinol | 1654 | - | 0.49 | - | - |
| 62 | ar-Turmerone | 1668 | 23.35 | - | - | 2.06 |
| 63 | α-Turmerone | 1671 | 22.81 | - | - | 0.87 |
| 64 | β-Turmerone | 1707 | 15.27 | - | - | 0.56 |
| Total identified | 99.46 | 99.59 | 96.24 | 98.04 | ||
| Monoterpene hydrocarbons | 18.74 | 0.00 | 3.88 | 28.10 | ||
| Monoterpene oxygenated: Alcohols: | 9.11 | 89.05 | 60.98 | 25.33 | ||
| 1.04 | 42.53 | 53.62 | 6.51 | |||
| Aliphatics | 1.04 | 42.53 | 2.65 | 6.51 | ||
| Phenolics | - | - | 50.97 | - | ||
| Esters | - | 8.48 | - | 0.38 | ||
| Aldehydes and ketones | 0.22 | 38.04 | - | 9.83 | ||
| Ethers | 7.85 | - | 7.36 | 8.61 | ||
| Sesquiterpene hydrocarbons | 5.32 | 5.03 | 26.96 | 39.63 | ||
| Sesquiterpene oxygenated | 66.29 | 5.51 | 4.42 | 4.98 | ||
| N | Compound a | AI b | Curcuma longa | Cymbopogon citratus | Ocimum campechianum | Zingiber officinale |
|---|---|---|---|---|---|---|
| Area % | ||||||
| 1 | Tricyclene | 921 | -d | - | - | 0.78 |
| 2 | α-Pinene | 932 | 8.28 | 2.24 | 4.66 | 16.47 |
| 3 | Camphene | 946 | 3.49 | 2.51 | 50.39 | |
| 4 | Sabinene | 969 | 9.76 | 0.97 | 0.26 | - |
| 5 | β-Pinene | 974 | - | - | 10.61 | 4.40 |
| 6 | Myrcene | 981 | 1.35 | - | 3.24 | 4.34 |
| 7 | Methyl-5-hepten-2-one | 988 | - | 7.11 | - | - |
| 8 | α-Phellandrene | 1002 | 32.33 | 0.91 | - | 2.25 |
| 9 | α-Terpinene | 1014 | - | 0.70 | - | - |
| 10 | Limonene | 1024 | 8.15 | 5.30 | 1.73 | 8.1 |
| 11 | 1,8-Cineole | 1026 | 29.2 | - | 29.1 | 9.1 |
| 12 | cis-Ocimene | 1032 | - | 4.76 | 19.49 | - |
| 13 | trans-Ocimene | 1044 | 0.18 | 6.64 | 0.29 | - |
| 14 | γ-Terpinene | 1054 | 1.65 | 2.03 | - | - |
| 15 | Terpinolene | 1086 | 7.13 | 0.54 | 0.17 | 0.48 |
| 16 | Linalool | 1095 | 0.16 | 0.78 | 2.11 | 0.25 |
| 17 | allo-Ocimene | 1128 | - | 2.33 | 8.43 | - |
| 18 | Citronellal | 1148 | - | 11.07 | - | - |
| 19 | Borneol | 1165 | - | 0.97 | 0.11 | 0.57 |
| 20 | 4-Terpineol | 1160 | 0.16 | 1.87 | - | - |
| 21 | α-Terpineol | 1174 | 0.12 | 1.16 | 0.22 | 0.19 |
| 22 | cis-Isocitral | 1177 | - | 15.27 | - | - |
| 23 | trans-Isocitral | 1186 | - | 10.89 | - | - |
| 24 | γ-Terpineol | 1199 | - | 12.92 | - | - |
| 25 | Nerol | 1227 | - | 3.15 | - | - |
| 26 | Neral | 1235 | - | 3.45 | - | 0.57 |
| 27 | Geraniol | 1249 | 0.17 | tr c | - | - |
| 28 | Geranial | 1264 | 0.14 | tr c | - | 0.70 |
| 29 | Thymol | 1289 | - | tr c | - | - |
| 30 | δ-Elemene | 1335 | - | - | 0.18 | - |
| 31 | Eugenol | 1356 | 0.19 | - | 7.01 | 0.15 |
| 32 | β-Elemene | 1389 | - | - | 2.88 | 0.07 |
| 33 | E-Caryophyllene | 1417 | 0.09 | - | 4.95 | 0.06 |
| 34 | α-Humulene | 1452 | - | - | 0.71 | - |
| 35 | allo-Aromadendrene | 1458 | - | - | 0.21 | - |
| 36 | ar-Curcumene | 1479 | 0.11 | - | - | 0.14 |
| 37 | β-Selinene | 1489 | - | - | 0.46 | - |
| 38 | Bicyclogermacrene | 1500 | - | - | 0.67 | - |
| 39 | trans-Muurola-4(14)5-diene | 1493 | - | - | - | 0.13 |
| 40 | α-Zingiberene | 1493 | 0.09 | - | - | 0.48 |
| 41 | α-(E,E)-Farnesene | 1505 | - | - | - | 0.23 |
| 42 | β-Sesquiphellandrene | 1521 | 0.08 | - | - | 0.15 |
| 43 | ar-Turmerone | 1668 | 0.26 | - | - | - |
| 44 | α-Turmerone | 1671 | 0.23 | - | - | - |
| 45 | β-Turmerone | 1707 | 0.18 | - | - | - |
| Total identified | 100.00 | 98.55 | 100.00 | 100.00 | ||
| Monoterpene hydrocarbons | 68.83 | 29.91 | 51.39 | 87.21 | ||
| Monoterpene oxygenated: | 30.14 | 68.64 | 38.55 | 11.53 | ||
| Alcohols: | 0.80 | 20.85 | 9.45 | 1.16 | ||
| Aliphatics | 0.61 | 20.85 | 2.44 | 1.01 | ||
| Phenolics | 0.19 | - | 7.01 | 0.15 | ||
| Esters | - | - | - | - | ||
| Aldehydes and Ketones | 0.14 | 47.79 | - | 1.27 | ||
| Ethers | 29.2 | - | 29.10 | 9.10 | ||
| Sesquiterpene hydrocarbons | 0.37 | - | 10.06 | 1.26 | ||
| Sesquiterpenes oxygenated | 0.66 | - | - | - | ||
| DPPH | ABTS | |
|---|---|---|
| Essential Oils | IC50 (mg/mL) | |
| Curcuma longa | 16.512 ± 2.452 | 0.871 ± 0.132 |
| Cymbopogon citratus | 2.270 ± 0.340 | 4.322 ± 0.651 |
| Ocimum campechianum | 0.012 ± 0.003 | 0.0013 ± 0.0004 |
| Zingiber officinale | 5.478 ± 0.082 | 0.563 ± 0.080 |
| Thymus vulgaris | 0.325 ± 0.038 | 0.288 ± 0.041 |
| Trolox® | 0.0060 ± 0.0010 | 0.0024 ± 0.0003 |
| MIC (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| C. longa | C. citratus | O. campechianum | Z. officinale | T. vulgaris | CPh (mg/mL) | ||
| Gram + | S. aureus | 48.75 | 9.31 | 8.67 | 17.74 | 1.93 | 3.50 × 10−3 |
| E. foecalis | 89.50 | 9.31 | 8.67 | 44.35 | 1.93 | 3.50 × 10−3 | |
| L. grayi | 8.98 | 3.50 | 1.70 | 8.87 | 0.97 | 1.80 × 10−3 | |
| M. luteus | 89.50 | 17.34 | 9.31 | 44.35 | 1.93 | 3.50 × 10−3 | |
| Gram − | K. oxytoca | 89.75 | 4.34 | 0.75 | 8.87 | 1.93 | 1.80 × 10−3 |
| P. vulgaris | 89.75 | 8.67 | 4.66 | 17.74 | 1.93 | 3.50 × 10−3 | |
| E. coli | 44.88 | 4.66 | 1.70 | 8.87 | 4.84 | 3.00 × 10−3 | |
| P. aeruginosa | 89.50 | 9.31 | 1.70 | 10.80 | 9.67 | 3.00 × 10−3 | |
| Flu (μg/mL) | |||||||
| Yeasts | C. albicans | 89.75 | 4.70 | 4.10 | 17.74 | 1.93 | 0.125 |
| S. cerevisiae | 8.98 | 3.45 | 2.25 | 88.70 | 1.93 | 0.500 | |
| A | ||||
|---|---|---|---|---|
| Z. officinale (mg/plate) | TA98-S9 | TA98+S9 | TA100-S9 | TA100+S9 |
| 0.00 | 38.0 ± 6.0 | 45.0 ± 4.1 | 101.0 ± 11.7 | 147.0 ± 13.7 |
| 0.01 | 39.0 ± 5.1 | 43.0 ± 3.1 | 105.0 ± 7.0 | 141.0 ± 11.3 |
| 0.05 | 37.0 ± 7.4 | 48.0 ± 3.0 | 98.0 ± 5.4 | 133.0 ± 8.9 |
| 0.10 | 33.0 ± 4.9 | 44.0 ± 3.3 | 100.0 ± 5.9 | 144.0 ± 7.7 |
| 0.50 | 34.0 ± 4.7 | 39.0 ± 3.2 | 96.0 ± 5.3 | 138.0 ± 6.6 |
| 1.00 | 32.0 ± 2.4 | 37.0 ± 3.3 | 99.0 ± 6.6 | 140.0 ± 6.7 |
| 2.50 | 22 ± 2.0 * | 20.0 ± 1.5 * | 88.0 ± 2.7 | 136.0 ± 5.5 |
| 5.00 | 10.0 ± 0.3 * | 9.0 ± 0.4 * | 29.0 ± 4.1 * | 119.0 ± 6.3 |
| 10.00 | - * | - * | 5.0 ± 0.0 * | 58.0 ± 1.0 * |
| B | ||||
| C. longa(mg/plate) | TA98-S9 | TA98+S9 | TA100-S9 | TA100+S9 |
| 0.00 | 38.0 ± 4.1 | 45.0 ± 3.9 | 101.0 ± 6.3 | 147.0 ± 9.6 |
| 0.01 | 38.0 ± 3.9 | 46.0 ± 4.1 | 99.0 ± 9.9 | 145.0 ± 8.4 |
| 0.05 | 35.0 ± 3.1 | 39.0 ± 3.0 | 98.0 ± 8.5 | 143.0 ± 7.1 |
| 0.10 | 41.0 ± 4.3 | 42.0 ± 2.8 | 100.0 ± 4.7 | 144.0 ± 6.5 |
| 0.50 | 39.0 ± 4.7 | 43.0 ± 2.0 | 91.0 ± 6.8 | 141.0 ± 11.2 |
| 1.00 | 33.0 ± 2.0 | 42.0 ± 3.1 | 101.0 ± 5.0 | 133.0 ± 6.7 |
| 2.50 | 12.0 ± 1.0 * | 40.0 ± 4.7 | 98.0 ± 2.7 | 61.0 ± 3.5 * |
| 5.00 | - * | 27.0 ± 1.0 * | 71.0 ± 3.1 * | 33.0 ± 2.2 * |
| 10.00 | - * | 7.0 ± 0.3 * | 11.0 ± 0.5 * | - * |
| C | ||||
| O. campechianum(mg/plate) | TA98-S9 | TA98+S9 | TA100-S9 | TA100+S9 |
| 0.00 | 38.0 ± 4.1 | 45.0 ± 5.6 | 101.0 ± 9.9 | 147.0 ± 12.3 |
| 0.01 | 33.0 ± 3.7 | 44.0 ± 6.1 | 104.0 ± 12.3 | 138.0 ± 9.8 |
| 0.05 | 35.0 ± 3.5 | 46.0 ± 4.8 | 96.0 ± 10.0 | 140.0 ± 11.1 |
| 0.10 | 40.0 ± 4.0 | 39.0 ± 4.7 | 100.0 ± 8.7 | 143.0 ± 7.8 |
| 0.50 | 37.0 ± 2.8 | 41.0 ± 3.9 | 99.0 ± 9.3 | 152.0 ± 10.7 |
| 1.00 | 37.0 ± 3.4 | 39.0 ± 2.5 | 95.0 ± 6.8 | 140.0 ± 9.5 |
| 2.50 | 20.0 ± 1.1 * | 35.0 ± 2.8 | 66.0 ± 5.1 * | 93.0 ± 6.2 |
| 5.00 | 6.0 ± 0.3 * | 11.0 ± 0.6 * | 42.0 ± 3.3 * | 18.0 ± 0.9 * |
| 10.00 | - * | 2.0 ± 0.1 * | 10.0 ± 0.7 * | - * |
| D | ||||
| C. citratus(mg/plate) | TA98-S9 | TA98+S9 | TA100-S9 | TA100+S9 |
| 0.00 | 38.0 ± 4.1 | 45.0 ± 2.7 | 101.0 ± 7.8 | 147.0 ± 13.4 |
| 0.01 | 39.0 ± 3.7 | 44.0 ± 3.8 | 111.0 ± 9.3 | 144.0 ± 12.5 |
| 0.05 | 37.0 ± 3.3 | 40.0 ± 2.9 | 106.0 ± 10.1 | 138.0 ± 10.7 |
| 0.10 | 36.0 ± 4.0 | 42.0 ± 3.2 | 100.0 ± 8.8 | 140.0 ± 11.5 |
| 0.50 | 39.0 ± 3.8 | 46.0 ± 3.8 | 96.0 ± 9.0 | 127.0 ± 13.2 |
| 1.00 | 35.0 ± 2.7 | 43.0 ± 2.8 | 92.0 ± 7.5 | 133.0 ± 8.4 |
| 2.50 | 33.0 ± 3.0 | 38.0 ± 3.0 | 89.0 ± 6.6 | 115.0 ± 9.1 |
| 5.00 | 30.0 ± 3.2 | 11.0 ± 0.6 * | 53.0 ± 3.6 * | 66.0 ± 4.4 * |
| 10.00 | 15.0 ± 0.8 * | - * | 20.0 ± 1.3 * | 31.0 ± 2.6 * |
| A | ||||
|---|---|---|---|---|
| Z. officinale (mg/plate) | TA98-S9 | TA98+S9 AA | TA100-S9 SA | TA100+S9 AA |
| 0.00 | 506.0 ± 65.9 | 1218.0 ± 101.6 | 1188.0 ± 128.4 | 581.0 ± 68.7 |
| 0.01 | 498.0 ± 56.4 | 1231.0 ± 76.8 | 1212.0 ± 76.5 | 592.0 ± 56.3 |
| 0.02 | 512.0 ± 81.2 | 1287.0 ± 74.3 | 1203.0 ± 59.7 | 567.0 ± 44.7 |
| 0.05 | 538.0 ± 53.4 | 1183.0 ± 81.4 | 1225.0 ± 65.0 | 543.0 ± 38.7 |
| 0.10 | 410.0 ± 26.4 | 1114.0 ± 52.3 | 1116.0 ± 73.1 | 534.0 ± 33.3 |
| 0.20 | 185.0 ± 22.2 * | 1036.0 ± 38.5 | 1197.0 ± 29.9 | 519.0 ± 27.4 |
| 0.50 | 28.0 ± 3.6 * | 228.0 ± 11.0 * | 821.0 ± 44.7 * | 233.0 ± 6.5 * |
| 1.00 | - * | - * | - * | 161.0 ± 4.8 * |
| B | ||||
| C. longa(mag/plate) | TA98-S9 NF | TA98+S9 AA | TA100-S9 SA | TA100+S9 AA |
| 0.00 | 363.0 ± 28.4 | 1233.0 ± 74.4 | 1179.0 ± 99.4 | 508.0 ± 33.6 |
| 0.01 | 380.0 ± 22.6 | 1208.0 ± 68.7 | 1103.0 ± 81.5 | 489.0 ± 48.7 |
| 0.02 | 361.0 ± 30.7 | 1177.0 ± 59.5 | 1121.0 ± 60.0 | 515.0 ± 36.4 |
| 0.05 | 352.0 ± 17.5 | 1165.0 ± 66.3 | 1136.0 ± 55.7 | 553.0 ± 30.4 |
| 0.10 | 227.0 ± 11.8 * | 974.0 ± 52.1 | 1148.0 ± 68.4 | 421.0 ± 12.5 * |
| 0.20 | 191.0 ± 9.9 * | 370.0 ± 27.8 * | 1154.0 ± 61.7 | 192.0 ± 13.7 * |
| 0.50 | 35.0 ± 2.3 * | 57.0 ± 3.8 * | 825.0 ± 31.4 * | 37.0 ± 2.4 * |
| 1.00 | - * | - * | 266.0 ± 21.0 * | - * |
| C | ||||
| O. campechianum(mg/plate) | TA98-S9 NF | TA98+S9 AA | TA100-S9 SA | TA100+S9 AA |
| 0.00 | 431.0 ± 38.1 | 643.0 ± 42.8 | 1034.0 ± 77.6 | 889.0 ± 71.5 |
| 0.01 | 397.0 ± 27.5 | 592.0 ± 38.6 | 1006.0 ± 69.4 | 881.0 ± 68.2 |
| 0.02 | 202.0 ± 31.4 * | 388.0 ± 27.9 * | 1015.0 ± 55.8 | 827.0 ± 59.7 |
| 0.05 | 189.0 ± 22.9 * | 247.0 ± 17.5 * | 1069.0 ± 48.1 | 712.0 ± 63.4 |
| 0.10 | 168.0 ± 12.8 * | 210.0 ± 12.4 * | 1063.0 ± 53.7 | 406.0 ± 28.6 * |
| 0.20 | 64.0 ± 3.4 * | 78.0 ± 4.4 * | 560.0 ± 32.6 * | 287.0 ± 7.8 * |
| 0.50 | - * | - * | 592.0 ± 40.1 * | 85.0 ± 5.0 * |
| 1.00 | - * | - * | 101.0 ± 22.0 * | - * |
| D | ||||
| C. citratus(mg/plate) | TA98-S9 NF | TA98+S9 AA | TA100-S9 SA | TA100+S9 AA |
| 0.00 | 201.0 ± 17.6 | 377.0 ± 26.7 | 787.0 ± 68.5 | 683.0 ± 50.7 |
| 0.01 | 198.0 ± 15.4 | 371.0 ± 22.3 | 788.0 ± 71.4 | 655.0 ± 39.4 |
| 0.02 | 199.0 ± 16.8 | 389.0 ± 18.0 | 795.0 ± 58.3 | 679.0v42.6 |
| 0.05 | 202.0 ± 13.6 | 385.0 ± 19.2 | 808.0 ± 62.7 | 677.0 ± 28.6 |
| 0.10 | 194.0 ± 7.9 | 276.0 ± 11.4 * | 814.0 ± 55.5 | 560.0 ± 31.5 * |
| 0.20 | 81.0 ± 6.1 * | 83.0 ± 7.6 * | 698.0 ± 42.0 | 375.0 ± 11.3 * |
| 0.50 | - * | - * | 372.0 ± 12.8 * | 173.0 ± 10.7 * |
| 1.00 | - * | - * | 80.0 ± 6.6 * | 62.0 ± 3.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, A.; Tacchini, M.; Chiocchio, I.; Grandini, A.; Radice, M.; Maresca, I.; Paganetto, G.; Sacchetti, G. A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae). Antibiotics 2023, 12, 177. https://doi.org/10.3390/antibiotics12010177
Guerrini A, Tacchini M, Chiocchio I, Grandini A, Radice M, Maresca I, Paganetto G, Sacchetti G. A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae). Antibiotics. 2023; 12(1):177. https://doi.org/10.3390/antibiotics12010177
Chicago/Turabian StyleGuerrini, Alessandra, Massimo Tacchini, Ilaria Chiocchio, Alessandro Grandini, Matteo Radice, Immacolata Maresca, Guglielmo Paganetto, and Gianni Sacchetti. 2023. "A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae)" Antibiotics 12, no. 1: 177. https://doi.org/10.3390/antibiotics12010177
APA StyleGuerrini, A., Tacchini, M., Chiocchio, I., Grandini, A., Radice, M., Maresca, I., Paganetto, G., & Sacchetti, G. (2023). A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae). Antibiotics, 12(1), 177. https://doi.org/10.3390/antibiotics12010177










