Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women
Abstract
:1. Introduction
2. Results
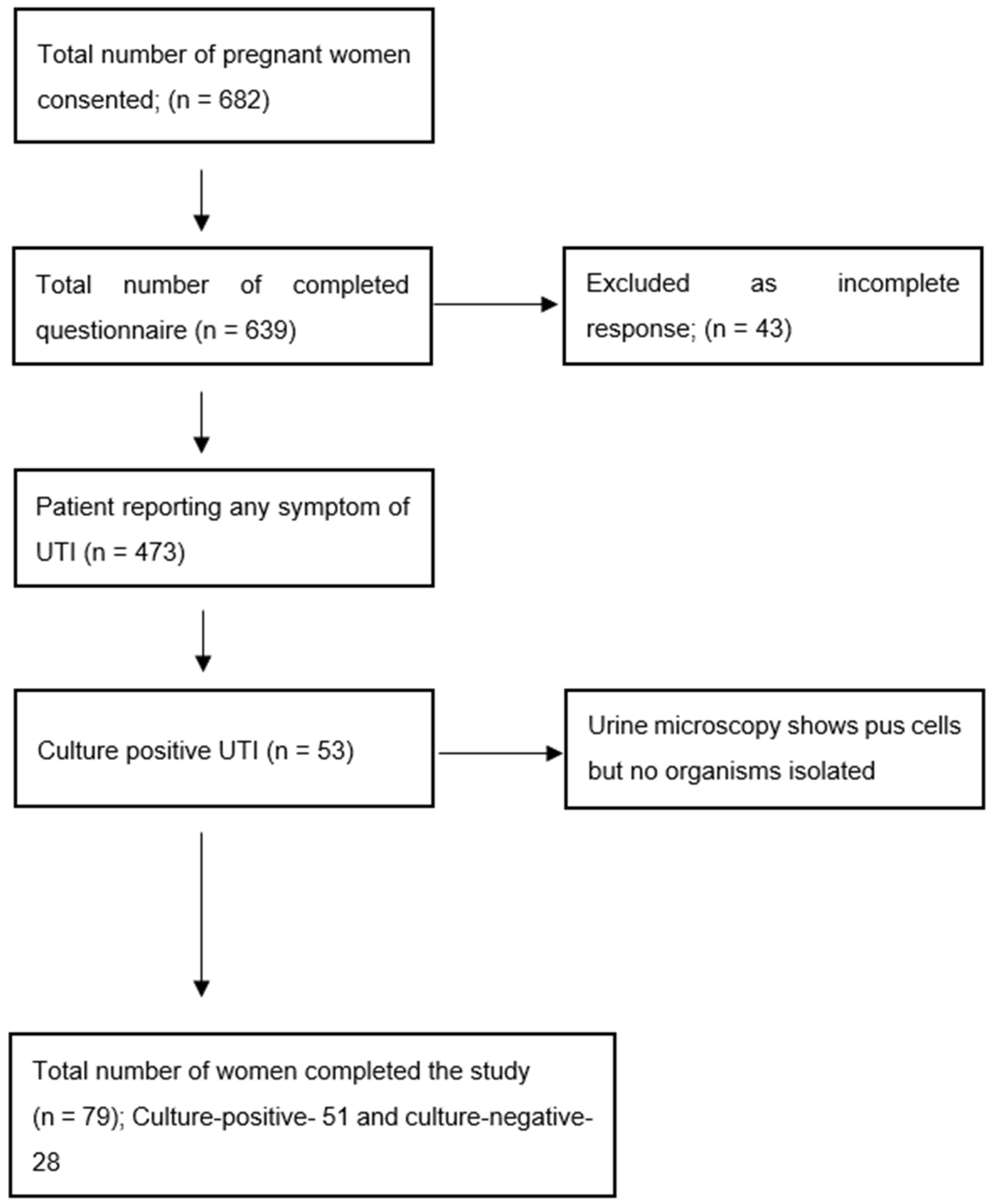
2.1. Prevalence
2.2. Demography and Risk Factors
2.3. Bacteriological Profile and Antibiotic Sensitivity
2.4. Clinical Profile and Pregnancy Outcome
3. Discussion
4. Materials and Methods
4.1. Sample Size and Sampling Technique
4.2. Questionnaire
4.3. Specimen Collection and Isolation
4.4. Susceptibility Testing
4.5. Follow Up
4.6. Outcomes
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, N.M.; O’Brien, V.P.; Hultgren, S.; Macones, G.; Lewis, W.G.; Lewis, A.L. Urinary tract infection as a preventable cause of pregnancy complications: Opportunities, challenges, and a global call to action. Glob. Adv. Health Med. 2013, 2, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, G.; Zehra, N.; Munir, A.A.; Haider, A. Risk factors of urinary tract infection in pregnancy. J. Pak. Med. Assoc. 2010, 60, 213–216. [Google Scholar] [PubMed]
- Abdel-Aziz Elzayat, M.; Barnett-Vanes, A.; Dabour, M.F.; Cheng, F. Prevalence of undiagnosed asymptomatic bacteriuria and associated risk factors during pregnancy: A cross-sectional study at two tertiary centres in Cairo, Egypt. BMJ Open 2017, 7, e013198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkar, N.; Banerjee, M.; Gupta, S.; Ahmad, A. Asymptomatic Bacteriuria among Pregnant Women Attending Tertiary Care Hospital in Lucknow, India. Dubai Med. J. 2021, 4, 18–25. [Google Scholar] [CrossRef]
- Lee, A.C.; Mullany, L.C.; Koffi, A.K.; Rafiqullah, I.; Khanam, R.; Folger, L.V.; Rahman, M.; Mitra, D.K.; Labrique, A.; Christian, P.; et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: Population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth 2020, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Alemu, A.; Moges, F.; Shiferaw, Y.; Tafess, K.; Kassu, A.; Anagaw, B.; Agegn, A. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res. Notes 2012, 5, 197. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, E.; Teshome, M.; Merid, Y.; Kibret, B.; Shimeliset, T. Asymptomatic urinary tract infection among pregnant women attending the antenatal clinic of Hawassa Referral Hospital, Southern Ethiopia. BMC Res. Notes 2014, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.A.; Al-Moslih, M.I. Prevalence of asymptomatic bacteriuria in pregnant women in Sharjah, United Arab Emirates. East. Mediterr. Health J. 2005, 11, 1045. [Google Scholar]
- Younis, M.; Ajroud, S.; Elgade, L.H.A.; Uahua, A.S.; Elzahaf, R.A. Prevalence of Urinary Tract Infection among Pregnant Women and Its Risk Factor in Derna City. Sch. Int. J. Obstet. Gynecol. 2019, 2, 219–223. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 91: Treatment of urinary tract infections in nonpregnant women. Obstet. Gynecol. 2008, 111, 785–794. [Google Scholar]
- Heytens, S.; De Sutter, A.; Coorevits, L.; Cools, P.; Boelens, J.; Van Simaey, L.; Christiaens, T.; Vaneechoutte, M.; Claeys, G. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin. Microbiol. Infect. 2017, 23, 647–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, M.; Lavasani, Z.; Norouzirad, R.; Najibpour, R.; Mohamadpour, M.; Nikpoor, A.R.; Raeisi, M.; Marzouni, H.Z. Prevalence of Urinary Tract Infection among Pregnant Women and Its Complications in Their Newborns during the Birth in the Hospitals of Dezful City, Iran, 2012–2013. Iran. Red Crescent Med. J. 2015, 17, e26946. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.S.; Callaghan, W.; Johnson, C.; Williams, L. Racial, ethnic, and economic disparities in the prevalence of pregnancy complications. Matern. Child Health J. 2009, 13, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.; Schito, G.; Botto, H.; Palou, J.; Mazzei, T. Surveillance study in Europe and Brazil on clinical aspects and microbial resistance epidemiology in females with cystitis (ARESC): Implications for empiric therapy. Eur. Urol. 2008, 54, 1164–1178. [Google Scholar] [CrossRef]
- De Backer, D.; Christiaens, T.; Heytens, S.; De Sutter, A.; Stobberingh, E.; Verschraegen, G. Evolution of bacterial susceptibility pattern of E. coli in uncomplicated urinary tract infections in a country with high antibiotic consumption: A comparison of two surveys with a 10 year interval. J. Antimicrob. Chemother. 2008, 62, 364–368. [Google Scholar] [CrossRef]
- Heytens, S.; Boelens, J.; Claeys, G.; De Sutter, A.; Christiaens, T. Uropathogen distribution and antimicrobial susceptibility in uncomplicated cystitis in Belgium, a high antibiotics prescribing country: 20-year surveillance. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 105–113. [Google Scholar] [CrossRef]
- Giesen, L.G.; Cousins, G.; Dimitrov, B.D.; van de Laar, F.A.; Fahey, T. Predicting acute uncomplicated urinary tract infection in women: A systematic review of the diagnostic accuracy of symptoms and signs. BMC Fam. Pract. 2010, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Knottnerus, B.J.; Geerlings, S.E.; Moll van Charante, E.P.; Ter Riet, G. Toward a simple diagnostic index for acute uncomplicated urinary tract infections. Ann. Fam. Med. 2013, 11, 442–451. [Google Scholar] [CrossRef]
- Little, P.; Moore, M.V.; Turner, S.; Rumsby, K.; Warner, G.; Lowes, J.A.; Smith, H.; Hawke, C.; Leydon, G.; Arscott, A.; et al. Effectiveness of five different approaches in management of urinary tract infection: Randomised controlled trial. BMJ 2010, 340, c199. [Google Scholar] [CrossRef] [Green Version]
- SIGN 160. Management of Suspected Bacterial Lower Urinary Tract Infection in Adult Women. September 2020. Available online: http://www.sign.ac.uk (accessed on 1 November 2022).
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Infectious Diseases Society of America, & European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.B.; Sheffield, J.S.; McIntire, D.D.; Wendel, G.D., Jr. Acute pyelonephritis in pregnancy. Obstet. Gynecol. 2005, 105, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The diagnosis of urinary tract infection: A systematic review. Dtsch. Arztebl. Int. 2010, 107, 361–367. [Google Scholar] [CrossRef]
- Delzell, J.E., Jr.; Lefevre, M.L. Urinary tract infections during pregnancy. Am. Fam. Physician 2000, 61, 713–721. [Google Scholar]
- McClure, E.M.; Goldenberg, R.L. Infection and stillbirth. Semin. Fetal Neonatal Med. 2009, 14, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, G.J.; Lee, A.C.; Baqui, A.H.; Tan, J.; Black, R.E. Risk of early-onset neonatal infection with maternal infection or colonization: A global systematic review and meta-analysis. PLoS Med. 2013, 10, e1001502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Guidelines on the Empiric Antibiotic Treatment of Urinary Tract Infections. Version 1: 1 September 2021. Available online: https://www.dha.gov.ae (accessed on 1 November 2022).
- Gupta, K. Urinary Tract Infections and Asymptomatic Bacteriuria in Pregnancy. Available online: https://www.uptodate.com (accessed on 1 November 2022).
- Werter, D.E.; Kazemier, B.M.; Schneeberger, C.; Mol, B.W.J.; de Groot, C.J.M.; Geerlings, S.E.; Pajkrt, E. Risk Indicators for Urinary Tract Infections in Low Risk Pregnancy and the Subsequent Risk of Preterm Birth. Antibiotics 2021, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Schnarr, J.; Smaill, F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. Eur. J. Clin. Investig. 2008, 38 (Suppl. 2), 50–57. [Google Scholar] [CrossRef] [PubMed]
- Golan, A.; Wexler, S.; Amit, A.; Gordon, D.; David, M.P. Asymptomatic bacteriuria in normal and high-risk pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 33, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.M.; Vazquez, J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst. Rev. 2019, 11, CD000490. [Google Scholar] [CrossRef]
- Moore, A.; Doull, M.; Grad, R.; Groulx, S.; Pottie, K.; Tonelli, M.; Courage, S.; Garcia, A.J.; Thombs, B.D. Canadian Task Force on Preventive Health Care. Recommendations on screening for asymptomatic bacteriuria in pregnancy. CMAJ Can. Med. Assoc. J. 2018, 190, E823–E830. [Google Scholar] [CrossRef] [Green Version]
- Kazemier, B.M.; Koningstein, F.N.; Schneeberger, C.; Ott, A.; Bossuyt, P.M.; de Miranda, E.; Vogelvang, T.E.; Verhoeven, C.J.M.; Langenveld, J.; Woiski, M.; et al. Maternal and neonatal consequences of treated and untreated asymptomatic bacteriuria in pregnancy: A prospective cohort study with an embedded randomised controlled trial. Lancet. Infect. Dis. 2015, 15, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, L.; Jacob, L.; Al Awadhi, R.; Yahya, L.O.; Catroon, K.M.; Soundararajan, L.P.; Wani, S.; Alabadla, S.; Hussein, Y.A. Urinary Tract Infection in Pregnancy and Its Effects on Maternal and Perinatal Outcome: A Retrospective Study. Cureus 2022, 14, e21500. [Google Scholar] [CrossRef]
- Bloukh, S.I.; Hassan, N.A.; AlAni, R.S.; Gacem, S.A. Urinary Tract Infection and Antibiotic Resistance among Pregnant and Non-pregnant females in UAE. Res. J. Pharm. Technol. 2021, 14, 461–465. [Google Scholar] [CrossRef]
- Faidah, H.S.; Ashshi, A.M.; Abou El-Ella, G.A.; Al-Ghamdi, A.K.; Mohamed, A.M. Urinary Tract Infections among Pregnant Women in Makkah, Saudi Arabia. Biomed. Pharmacol. J. 2013, 6, 1–7. Available online: http://biomedpharmajournal.org/?p=2581 (accessed on 30 September 2022). [CrossRef]
- AlZuheiri, S.T.; Dube, R.; Menezes, G.; Qasem, S. Clinical profile and outcome of Group B streptococcal colonization in mothers and neonates in Ras Al Khaimah, United Arab Emirates: A prospective observational study. Saudi Med. J. 2021, 9, 235–240. [Google Scholar] [CrossRef]
- Hamdan, H.Z.; Ziad, A.H.; Ali, S.K.; Adam, I. Epidemiology of urinary tract infections and antibiotics sensitivity among pregnant women at Khartoum North Hospital. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, M.C.; Fontes, M.C.; Lima, N.S.; Marques, K.M.G.; Santos, A.T.; Santos, L.C.B.; Gomes, M.C.T.; de Souza Júnior, V.R.; da Mota Sousa, C.F.; de Barros Correia Fontes, L. Subclinical hypothyroidism and recurrent infection of the urinary tract: A case report. J. Urol. Ren. Dis. 2019, 11, 1151–1152. [Google Scholar] [CrossRef]
- Oli, A.N.; Akabueze, V.B.; Ezeudu, C.E.; Eleje, G.U.; Ejiofor, O.S.; Ezebialu, I.U.; Oguejiofor, C.B.; Ekejindu, I.M.; Emechebe, G.O.; Okeke, K.N. Bacteriology and Antibiogram of Urinary Tract Infection Among Female Patients in a Tertiary Health Facility in South Eastern Nigeria. Open Microbiol. J. 2017, 11, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Glaser, A.P.; Schaeffer, A.J. Urinary tract infection and bacteriuria in pregnancy. Urol. Clin. North Am. 2015, 42, 547–560. [Google Scholar] [CrossRef]
- Taye, S.; Getachew, M.; Desalegn, Z.; Biratu, A.; Mubashir, K. Bacterial profile, antibiotic susceptibility pattern and associated factors among pregnant women with Urinary Tract Infection in Goba and Sinana Woredas, Bale Zone, Southeast Ethiopia. BMC Res. Notes 2018, 11, 799. [Google Scholar] [CrossRef] [Green Version]
- Rosana, Y.; Ocviyanti, D.; Halim, M.; Harlinda, F.Y.; Amran, R.; Akbar, W.; Billy, M.; Akhmad, S.R.P. Urinary tract infections among Indonesian pregnant women and its susceptibility pattern. Infect. Dis. Obstet. Gynecol. 2020, 2020, 9681632. [Google Scholar] [CrossRef] [PubMed]
- Committee on Obstetric Practice. Committee Opinion No. 717: Sulfonamides, Nitrofurantoin, and Risk of Birth Defects. Obstet. Gynecol. 2017, 130, e150–e152. [Google Scholar] [CrossRef]
- Siakwa, M.; Kpikpitse, D.; Azanu, W.; John, M.E.; Doe, P.F.; Ebu, N.I.; Hanson-Owoo, E. Maternal and perinatal outcomes among pregnant women with urinary tract infections. Int. J. Curr. Res. 2016, 8, 33366–33371. [Google Scholar]
- Romero, R.; Oyarzun, E.; Mazor, M.; Sirtori, M.; Hobbins, J.C.; Bracken, M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet. Gynecol. 1989, 73, 576–582. [Google Scholar] [PubMed]
- US Preventive Services Task Force; Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W., Jr.; Kubik, M.; et al. Screening for Asymptomatic Bacteriuria in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 322, 1188. [Google Scholar]
- Canadian Task Force on Preventive Health Care. Asymptomatic Bacteriuria in Pregnancy. 2018. Available online: https://canadiantaskforce.ca/guidelines/published-guidelines/asymptomatic-bacteriuria/ (accessed on 10 October 2020).
- World Health Organization (WHO). WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Sekikubo, M.; Hedman, K.; Mirembe, F.; Brauner, A. Antibiotic Overconsumption in Pregnant Women with Urinary Tract Symptoms in Uganda. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Artino, A.R., Jr.; La Rochelle, J.S.; Dezee, K.J.; Gehlbach, H. Developing questionnaires for educational research: AMEE Guide No. 87. Med. Teach. 2014, 36, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Clayson, D.; Wild, D.; Doll, H.; Keating, K.; Gondek, K. Validation of a patient-administered questionnaire to measure the severity and bothersomeness of lower urinary tract symptoms in uncomplicated urinary tract infection (UTI): The UTI Symptom Assessment questionnaire. BJU Int. 2005, 96, 350–359. [Google Scholar] [CrossRef]
- Di Vico, T.; Morganti, R.; Cai, T.; Naber, K.G.; Wagenlehner, F.M.E.; Pilatz, A.; Alidjanov, J.; Morelli, G.; Bartoletti, R. Acute Cystitis Symptom Score (ACSS): Clinical Validation of the Italian Version. Antibiotics 2020, 9, 104. [Google Scholar] [CrossRef]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Gupta, K. Recurrent Simple Cystitis in Women. Available online: https://www.uptodate.com (accessed on 30 September 2022).
| Parameter | Findings | Culture Positive (n = 53) (%) | Culture Negative (n = 32) (%) | Comparison |
|---|---|---|---|---|
| Age of the patient | ≤30 years | 29 (54.7) | 18 (56.2) | p = 0.890 Not significant |
| >30 years | 24 (45.2) | 14 (43.7) | ||
| Nationality | Emirati | 30 (56.6) | 20 (62.5) | p = 0.592 Not significant |
| Expatriate | 23 (43.3) | 12 (37.5) | ||
| BMI (kg/m2) | <25 | 7 (13.2) | 5 (15.6) | p > 0.05 Not significant |
| 25–29.9 | 12 (22.6) | 7 (21.8) | ||
| 30–34.9 | 19 (35.8) | 12 (37.5) | ||
| 35–39.9 | 7 (13.2) | 4 (12.5) | ||
| ≥40 | 8 (15%) | 4 (12.5) | ||
| Parity index | Primigravida | 17(32) | 9 (28.1) | p = 0.701 Not significant |
| Parity 1 or above | 36 (68) | 23 (71.8) | ||
| GA at presentation | First trimester (≤13 weeks) | 11 (20.7) | 7 (21.8) | |
| Second trimester (13+1–26+6 weeks) | 24 (45.2) | 15 (46.8) | ||
| Third trimester (≥27+0 weeks) | 18 (34) | 10 (31.2) | ||
| Risk factors | Diabetes 1 | 12 (22.6) | 5 (15.6) | Presence of any risk factors— p = 0.0001 significant |
| Previous history of UTI | 10 (19) | 1 (3.12) | ||
| Anemia 2 | 8 (15) | 2 (6.25) | ||
| Hypertension 3 | 4 (7.5) | 1(3.12) | ||
| Others 4 | 5 (9.4) | 1(3.12) |
| Symptoms (n) | Culture Positive (n = 53) (True Positive) | Culture-Negative UTI (Treated) (n = 32) | No UTI (n = 388) | OR * for Symptoms and Culture Positivity (95%CI) |
|---|---|---|---|---|
| Pain in the lower abdomen (n = 423) | 45 (84.9%) | 27 (84.3%) | 351 | OR = 1.041(0.309–3.511) p = 0.473 |
| Burning during urination (n = 61) | 26 (49%) | 15 (46.8%) | 20 | OR = 1.091 (0.453–2.628) p = 0.422 |
| Pain during urination (n = 23) | 11 (20.7%) | 7 (21.8%) | 5 | OR = 0.935 (0.321–2.725) p = 0.451 |
| Frequency of urination (n = 355) | 24 (45.2%) | 14 (43.7%) | 317 | OR = 1.064 (0.440–2.574) p = 0.445 |
| Urinary urgency (n = 49) | 24 (45.2%) | 14 (43.7%) | 11 | OR = 1.064 (0.440–2.574) p = 0.445 |
| Fever with/without chills (n = 47) | 26 (49%) | 15 (46.8%) | 6 | OR = 1.091 (0.453–2.628) p = 0.422 |
| Urinary incontinence (n = 19) | 7 (13.2%) | 3 (9.37%) | 9 | OR = 1.471 (0.352–6.148) p = 0.528 |
| Urinary retention (n = 3) | 2 (3.77%) | 0 | 1 |
| Organism Isolated (n) | Initial Antibiotic (n) | Second-Line Antibiotic (n) | Comment |
|---|---|---|---|
| E. coli (14) | Cefuroxime (14) | Meropenem + nitrofurantoin (1) Piperacillin + tazobactam (3) | -ESBL strain treated with meropenem and NFT (n = 1) -Pyelonephritis treated with piperacillin+ tazobactam (n = 3) |
| GBS (13) | Cefuroxime (7) BZP (4) Clindamycin (2) | BZP (10) Piperacillin + tazobactam (1) Vancomycin (2) | -Cases with known penicillin sensitivity were treated with vancomycin (n = 2) later as they showed clindamycin resistance -Pyelonephritis treated with piperacillin+ tazobactam (n = 1) |
| Klebsiella (11) | Cefuroxime (11) | Piperacillin + tazobactam (2) Gentamicin (1) NFT (1) | -ESBL strain treated with meropenem and NFT (n = 1) -Pyelonephritis treated with piperacillin+ tazobactam (n = 2) |
| Bacteroides (5) | Cefuroxime (5) | Metronidazole (4) Clindamycin (1) | |
| Gardnerella (2) Pseudomonas (3) Proteus (2) Citrobacter (2) Enterobacteria cloacae (1) | Cefuroxime (10) | Meropenem, cephazolin, Azithromycin (1) Nitrofurantoin (1) Piperacillin + tazobactam (3) | -Meropenem, cephazolin, azithromycin was used for enterobacteria cloacae -Pseudomonas treated with piperacillin+ tazobactam (n = 3) |
| No growth (32) | Cefuroxime (32) |
| Bacteria | Escherichia coli (n = 14) (Sensitivity %) | Group B Streptococcus (n = 13) (Sensitivity %) | Klebsiella pneumoniae (n = 11) (Sensitivity %) |
|---|---|---|---|
| Antibiotic sensitivity | Nitrofurantoin (n = 13, 92.8) Cefuroxime (n = 10, 71.4) Cefotaxime (n = 4, 28.5) Ceftriaxone (n = 9, 64.2) Ceftazidime (n = 10, 71.4) Cefepime (n = 11, 78.5) Gentamicin (n = 12, 85.7) Amikacin (n = 13, 92.8) Aztreonam (n = 7, 50) Meropenem (n = 13, 92.8) Ampicillin (n = 4, 28.5) Amoxicillin/Clavulanic acid (n = 10, 71.4) Piperacillin/Tazobactam (n = 13, 92.8) Ciprofloxacin (n = 3, 21.4) | Vancomycin (n = 13, 100) Linezolid (n = 12, 92.3) Ceftriaxone (n = 12, 92.3) Cefotaxime (n = 10, 76.9) Ampicillin (n = 10, 76.9) Benzylpenicillin (n = 12, 92.3) Levofloxacin (n = 11, 84.6) Erythromycin (n = 6, 46.1) Clindamycin (n = 6, 46.1) Trimethoprim/Sulfamethoxazole (n = 4, 30.7) | Nitrofurantoin (n = 10, 90.9) Cefuroxime (n = 7, 63.6) Cefotaxime (n = 7, 63.6) Ceftriaxone (n = 7, 63.6) Ceftazidime (n = 8, 72.7) Cefepime (n = 10, 90.9) Gentamicin (n = 10, 90.9) Amikacin (n = 10, 90.9) Aztreonam (n= 6, 54.5) Meropenem (n = 10, 90.9) Ampicillin (n = 4, 36.3) Amoxicillin/Clavulanic acid (n = 7, 63.6) Piperacillin/Tazobactam (n = 10, 90.9) Ciprofloxacin (n = 3, 27.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, R.; Al-Zuheiri, S.T.S.; Syed, M.; Harilal, L.; Zuhaira, D.A.L.; Kar, S.S. Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women. Antibiotics 2023, 12, 33. https://doi.org/10.3390/antibiotics12010033
Dube R, Al-Zuheiri STS, Syed M, Harilal L, Zuhaira DAL, Kar SS. Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women. Antibiotics. 2023; 12(1):33. https://doi.org/10.3390/antibiotics12010033
Chicago/Turabian StyleDube, Rajani, Shatha Taher Salman Al-Zuheiri, Mariyam Syed, Lekshmi Harilal, Dean Allah Layth Zuhaira, and Subhranshu Sekhar Kar. 2023. "Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women" Antibiotics 12, no. 1: 33. https://doi.org/10.3390/antibiotics12010033
APA StyleDube, R., Al-Zuheiri, S. T. S., Syed, M., Harilal, L., Zuhaira, D. A. L., & Kar, S. S. (2023). Prevalence, Clinico-Bacteriological Profile, and Antibiotic Resistance of Symptomatic Urinary Tract Infections in Pregnant Women. Antibiotics, 12(1), 33. https://doi.org/10.3390/antibiotics12010033






