Continuous Infusion of High Doses of Cefepime in Intensive Care Unit: Assessment of Steady-State Plasma Level and Incidence on Neurotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Collected Data
2.3. Cefepime Treatment and Assessment of Overdosing and Neurotoxicity
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Cefepime Treatment
3.2. TDM of Cefepime
3.3. Bacterial Data
3.4. Cefepime-Induced Neurotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: A systematic review. Crit. Care 2017, 21, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwyler, T.; Lenggenhager, L.; Abbas, M.; Lorenzini, K.; Hughes, S.; Huttner, B.; Karmime, A.; Uçkay, I.; von Dach, E.; Lescuyer, P.; et al. Cefepime plasma concentrations and clinical toxicity: A retrospective cohort study. Clin. Microbiol. Infect. 2017, 23, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The European Comittee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: http://eucast.org (accessed on 1 January 2022).
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care 2019, 23, 104. [Google Scholar]
- Lamoth, F.; Buclin, T.; Pascual, A.; Vora, S.; Bolay, S.; Decosterd, L. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob. Agents Chemother. 2010, 54, 4360–4367. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.; Marriott, D.; Gould, M.; Andresen, D.; Reuter, S.E.; Penm, J. A retrospective study to determine the cefepime-induced neurotoxicity threshold in hospitalized patients. J. Antimicrob. Chemother. 2020, 75, 718–725. [Google Scholar] [CrossRef]
- Boschung-Pasquier, L.; Atkinson, A.; Kastner, L.K.; Banholzer, S.; Haschke, M.; Buetti, N.; Furrer, D.I.; Hauser, C.; Jent, P.; Que, Y.A.; et al. Cefepime neurotoxicity: Thresholds and risk factors. A retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Vercheval, C.; Sadzot, B.; Maes, N.; Denooz, R.; Damas, P.; Frippiat, F. Continuous infusion of cefepime and neurotoxicity: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 731–735. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Loirat, P.; Alperovitch, A. Simplified acute physiological score for intensive care patients. Lancet 1983, 2, 741. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Nationale Institute of Cancer. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0; National Institute of Cancer: Bethesda, MD, USA, 2017.
- Organization (WHO), Uppsala Monitoring Centre. The Use of the WHO-UMC System for Standardised case Causality Assessment. WHO: Geneva, Switzerland, 2017. Available online: http://www.who-umc.org/graphics/4409.pdf (accessed on 6 April 2018).
- Maguigan, K.L.; Al-Shaer, M.H.; Peloquin, C.A. Antibiotics (Basel). Beta-Lactams Dosing in Critically Ill Patients with Gram-Negative Bacterial Infections: A PK/PD Approach. Antibiotics 2021, 10, 1154. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Shiu, J.; Wang, E.; Tejani, A.M.; Wasdell, M. Continuous versus intermittent infusions of antibiotics for the treatment of severe acute infections. Cochrane Data-Base Syst Rev. 2013, 2013, CD008481. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A Multicenter Randomized Trial of Continuous versus Inter-mittent β-Lactam Infusion in Severe Sepsis *. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Taccone, F.S.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [Green Version]
- Tam, V.H.; McKinnon, P.S.; Akins, R.L.; Rybak, M.J.; Drusano, G.L. Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 2002, 50, 425–428. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef] [Green Version]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, T.M.; Giannoni, E.; Majcherczyk, P.A.; Chioléro, R.; Schaller, M.D.; Berger, M.M.; Bolay, S.; Décosterd, L.A.; Bugnon, D.; Moreillon, P. Prospective monitoring of cefepime in intensive care unit adult patients. Crit. Care 2010, 14, R51. [Google Scholar] [CrossRef] [PubMed]
- Maan, G.; Keitoku, K.; Kimura, N.; Sawada, H.; Pham, A.; Yeo, J.; Hagiya, H.; Nishimura, Y. Cefepime-induced neurotoxicity: Systematic review. J. Antimicrob. Chemother. 2022, 77, 2908–2921. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; O’Horo, J.C.; Day, C.N.; Mandrekar, J.; Rabinstein, A.A. Cefepime is Associated with Acute Encephalopathy in Critically Ill Patients: A Retrospective Case-Control Study. Neurocrit. Care 2020, 33, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, P.; Santella, P.J. Pharmacokinetics of cefepime: A review. J. Antimicrob. Chemother. 1993, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Barradell, L.B.; Bryson, H.M. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1994, 47, 471–505. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb Company. Cefepime [Package Insert]; Bristol-Myers Squibb Company: Princeton, NJ, USA, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf (accessed on 25 December 2022).
- Lau, C.; Marriott, D.; Schultz, H.B.; Gould, M.; Andresen, D.; Wicha, S.G.; Alffenaar, J.W.; Penm, J.; Reuter, S.E. Assessment of cefepime toxicodynamics: Comprehensive examination of pharmacokinetic/pharmacodynamic targets for cefepime-induced neurotoxicity and evaluation of current dosing guidelines. Int. J. Antimicrob. Agents 2021, 58, 106443. [Google Scholar] [CrossRef]
- Fugate, J.E.; Kalimullah, E.A.; Hocker, S.E.; Clark, S.L.; Wijdicks, E.F.; Rabinstein, A.A. Cefepime neurotoxicity in the intensive care unit: A cause of severe, underappreciated encephalopathy. Crit Care 2013, 17, R264. [Google Scholar] [CrossRef] [Green Version]
- Capparelli, F.J.; Diaz, M.F.; Hlavnika, A.; Wainsztein, N.A.; Leiguarda, R.; Del Castillo, M.E. Cefepime- and cefixime-induced encephalopathy in a patient with normal renal function. Neurology 2005, 65, 1840. [Google Scholar] [CrossRef]
- Lindsay, H.; Gruner, S.; Brackett, J. Cefepime-Induced Neurotoxicity Despite Dose Adjustment for Renal Disease: A Brief Report and Review of the Literature. J. Pediatr. Infect. Dis. Soc. 2017, 6, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Li, H.T.; Lee, C.H.; Wu, T.; Cheng, M.Y.; Tseng, W.J.; Chang, C.W.; Hsieh, H.Y.; Chiang, H.I.; Lin, C.Y.; Chang, B.L.; et al. Clinical, Electroencephalographic Features and Prognostic Factors of Cefepime-Induced Neurotoxicity: A Retrospective Study. Neurocrit. Care 2019, 31, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Naeije, G.; Lorent, S.; Vincent, J.L.; Legros, B. Continuous epileptiform discharges in patients treated with cefepime or meropenem. Arch. Neurol. 2011, 68, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
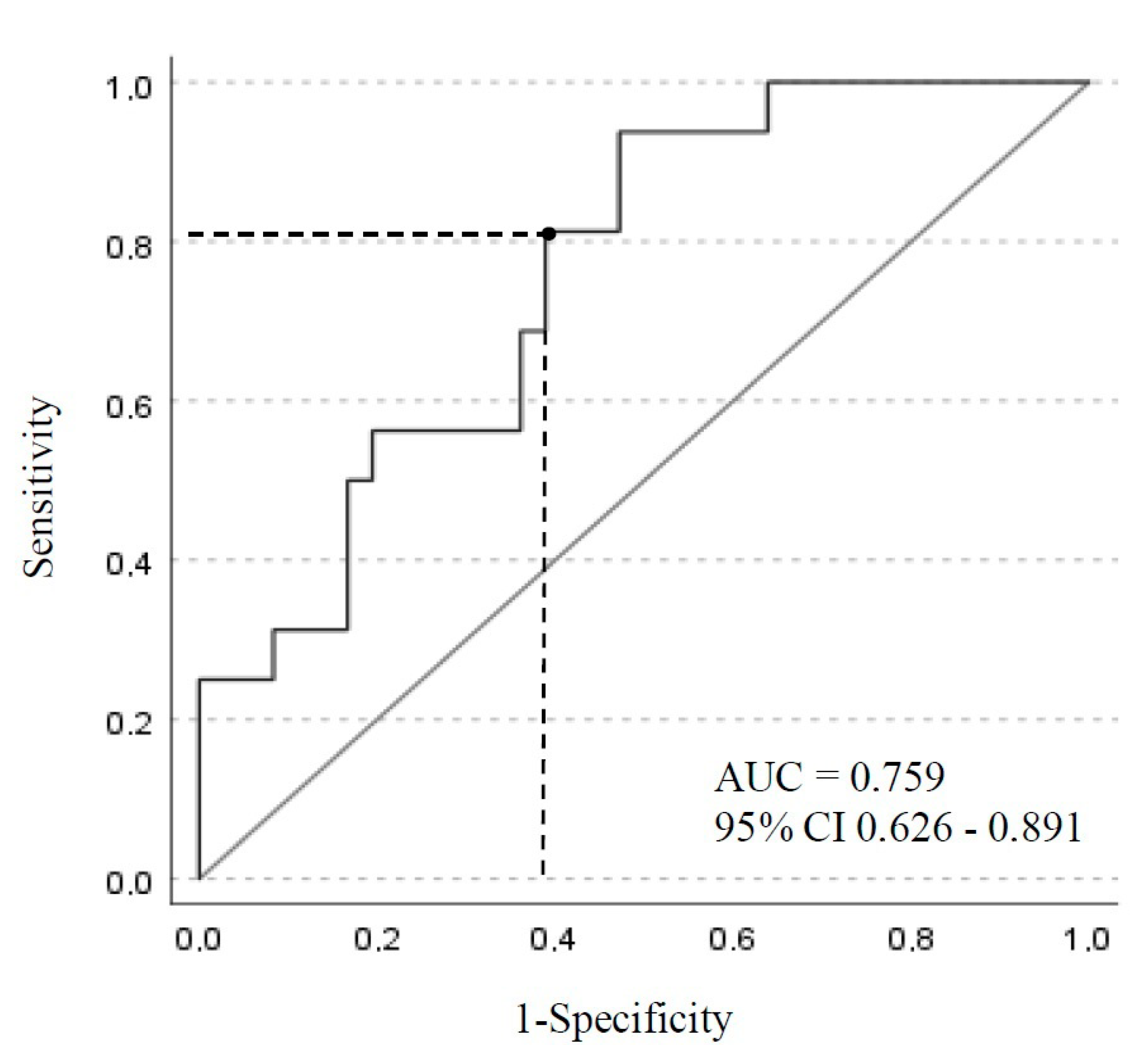
| Variable | Dosage < 35 mg/mL n = 15 | Dosage ≥ 35 mg/mL n = 63 | p Value |
|---|---|---|---|
| Age (years) | 63.3 ± 10.4 | 68.0 ± 11.2 | 0.06 |
| Age > 65 years | 5 (33.3) | 42 (66.6) | 0.01 |
| Male sex | 11 (73.3) | 47 (74.6) | 0.91 |
| Charlson score | 3.2 ± 1.6 | 4.0 ± 2.5 | 0.41 |
| SAPS II | 31.9 ± 7.8 | 42.4 ± 15.2 | 0.10 |
| BMI (kg/m2) | 28.3 ± 7.2 | 31.2 ± 8.9 | 0.25 |
| Chronic renal failure | 1 (6.6) | 8 (12.7) | 0.51 |
| Underlying neurological disease | 2 (13.3) | 11 (17.4) | 0.69 |
| Cirrhosis | 0 (0) | 4 (6.3) | 0.31 |
| Diabetes | 4 (26.6) | 19 (30.1) | 0.78 |
| Cardiac chronic failure | 4 (26.6) | 20 (31.7) | 0.70 |
| Indication for cefepime treatment | |||
| Nosocomial pneumonia | 8 (53.3) | 42 (66.6) | 0.33 |
| IAI | 2 (13.3) | 6 (9.5) | 0.66 |
| Bone and joint infection | 4 (26.6) | 10 (15.9) | 0.32 |
| Other | 1 (6.6) | 5 (7.9) | 0.86 |
| Start of cefepime treatment | |||
| Duration of ICU stay before treatment (days) | 11.0 ± 14.0 | 13.7 ± 13.8 | 0.27 |
| Serum albumin (mg/L) | 23.0 ± 6.2 | 21.6 ± 6.2 | 0.53 |
| SOFA score | 4.8 ± 2.5 | 4.9 ± 2.3 | 0.85 |
| Creatinine clearance (mL/min) | 90.9 ± 34.2 | 67.8 ± 39.7 | 0.02 |
| Creatinine clearance ≤ 60 mL/min | 3 (20) | 31 (49.2) | 0.04 |
| Daily dose (g) | 4.6 ± 1.2 | 5.4 ± 1.2 | 0.005 |
| Daily dose > 5 g | 6 (40) | 52 (82,5) | 0.0007 |
| Dose adjusted for creatinine clearance | 14 (93.3) | 48 (76.2) | 0.13 |
| Duration of ICU stay (days) | 25.4 ± 15.7 | 33.6 ± 30.8 | 0.56 |
| Number of deaths | 2 (13.3) | 12 (19) | 0.5 |
| Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Creatinine clearance < 60 mL/min | 8.0 | 1.52–42.6 | 0.01 |
| Daily dose of cefepime > 5 g | 11.2 | 2.44–51.28 | 0.001 |
| Microorganism | MIC ≤ 0.001 mg/L | MIC ≤ 1 mg/L | MIC = 4 mg/L | MIC = 8 mg/L | MIC > 8 mg/L |
|---|---|---|---|---|---|
| Proteus species | . | 5 | . | . | . |
| Enterobacter species | . | 13 | 1 | . | 2 |
| Klebsiella species | . | 3 | . | . | 2 |
| Escherichia coli | . | 6 | 1 | . | . |
| Serratia species | . | 3 | . | . | . |
| Morganella morganii | . | 2 | . | . | . |
| Citrobacter species | . | 3 | . | . | . |
| Hafnia alvei | . | 2 | . | . | . |
| Pseudomonas aeruginosa | 8 | . | . | 4 | 3 |
| Variable | Courses with Neurotoxicity (n = 16) | Courses without Neurotoxicity (n = 36) | p Value |
|---|---|---|---|
| Male sex | 10 (62.5) | 26 (72.2) | 0.48 |
| Mean age (years) | 72.3 ± 10.2 | 68.0 ± 10.6 | 0.21 |
| Charlson score | 6.0 ± 2.8 | 3.7 ± 1.9 | 0.01 |
| SAPS II | 49.3 ± 14.6 | 39.1 ± 14.3 | 0.04 |
| BMI, kg/m2 | 31.6 ± 5.6 | 30.9 ± 9.2 | 0.63 |
| Chronic renal failure | 6 (37) | 3 (8.3) | 0.01 |
| Cirrhosis | 3 (18.7) | 1 (2.7) | 0.04 |
| Diabetes mellitus | 8 (50) | 10 (27.7) | 0.12 |
| Chronic cardiac failure | 5 (31.2) | 15 (41.6) | 0.47 |
| Previous brain disease | 3 (18.7) | 5 (13.9) | 0.65 |
| ICU admission | |||
| Covid disease | 1 (6.2) | 7 (19.4) | 0.22 |
| Acute respiratory failure | 2 (12.5) | 9 (25) | 0.31 |
| Sepsis | 8 (50) | 17 (47.2) | 0.85 |
| Neurological disease | 2 (12.5) | 0 | 0.03 |
| other | 3 (18.7) | 3 (8.3) | 0.27 |
| Concomitant use of neurosedative drugs | 4 (25) | 9 (25) | 1 |
| Dexmedetomedine | 1 (6.2) | 4 (11.1) | 0.58 |
| Morphine | 2 (12.5) | 8 (22.2) | 0.41 |
| Benzodiazepine | 2 (12.5) | 10 (27.7) | 0.22 |
| Neuroleptics antipsychotics agents | 0 | 5 (13.9) | 0.11 |
| Daily dose of cefepime (g) | 5.1 ± 1.4 | 4.9 ± 1.4 | 0.75 |
| Creatinine clearance (mL/min) | 43.7 ± 33.1 | 72.1 ± 37.1 | 0.02 |
| Creatinine clearance < 60 mL/min | 13 (81.2) | 16 (44.4) | 0.01 |
| Dose adjusted for renal function | 10 (62.5) | 28 (77.7) | 0.25 |
| Presumed infection | |||
| Lower respiratory infection | 9 (56.2) | 18 (50) | 0.67 |
| IAI | 1 (6.2) | 4 (11.1) | 0.58 |
| Osteoarthritis | 4 (25) | 10 (27.7) | 0.83 |
| Other/undetermined | 2 (12.5) | 4 (11.1) | 0.88 |
| SOFA score on the day of dosage | 4.8 ± 2.2 | 4.5 ± 2.6 | 0.76 |
| Delivery of cefepime (days) | 3.3 ± 1.5 | 3.0 ± 2.4 | 0.11 |
| Cefepime plasma concentration (mg/L) | 85.7 ± 32.4 | 55.8 ± 24.9 | 0.005 |
| Duration of ICU stay (days) | 26.3 ± 33.1 | 25.6 ± 29.3 | 0.72 |
| Number of deaths | 1 (6.2) | 3 (8.3) | 0.79 |
| Variable | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Chronic renal failure | 7.0 | 1.27–38.6 | 0.02 |
| Plasma concentration ≥ 60 mg/mL | 5.6 | 1.24–26.1 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jean-Michel, V.; Homey, C.; Devos, P.; Delannoy, P.-Y.; Boussekey, N.; Caulier, T.; Leroy, O.; Georges, H. Continuous Infusion of High Doses of Cefepime in Intensive Care Unit: Assessment of Steady-State Plasma Level and Incidence on Neurotoxicity. Antibiotics 2023, 12, 69. https://doi.org/10.3390/antibiotics12010069
Jean-Michel V, Homey C, Devos P, Delannoy P-Y, Boussekey N, Caulier T, Leroy O, Georges H. Continuous Infusion of High Doses of Cefepime in Intensive Care Unit: Assessment of Steady-State Plasma Level and Incidence on Neurotoxicity. Antibiotics. 2023; 12(1):69. https://doi.org/10.3390/antibiotics12010069
Chicago/Turabian StyleJean-Michel, Vanessa, Corentin Homey, Patrick Devos, Pierre-Yves Delannoy, Nicolas Boussekey, Thomas Caulier, Olivier Leroy, and Hugues Georges. 2023. "Continuous Infusion of High Doses of Cefepime in Intensive Care Unit: Assessment of Steady-State Plasma Level and Incidence on Neurotoxicity" Antibiotics 12, no. 1: 69. https://doi.org/10.3390/antibiotics12010069
APA StyleJean-Michel, V., Homey, C., Devos, P., Delannoy, P.-Y., Boussekey, N., Caulier, T., Leroy, O., & Georges, H. (2023). Continuous Infusion of High Doses of Cefepime in Intensive Care Unit: Assessment of Steady-State Plasma Level and Incidence on Neurotoxicity. Antibiotics, 12(1), 69. https://doi.org/10.3390/antibiotics12010069






