Synergistic Activity of Pep16, a Promising New Antibacterial Pseudopeptide against Multidrug-Resistant Organisms, in Combination with Colistin against Multidrug-Resistant Escherichia coli, In Vitro and in a Murine Peritonitis Model
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Strains
2.2. Antibiotics
2.3. In Vitro Experiments
2.3.1. MICs
2.3.2. Bacteriostatic and Bactericidal Synergy Tests
2.4. In Vivo Experiments
2.4.1. Ethics
2.4.2. Survival Study
2.4.3. Pep16 Pharmacokinetic Analysis
2.4.4. Murine Peritoneal Infection Model
2.5. Statistical Analysis
3. Results
3.1. In Vitro Studies
3.1.1. Minimum Inhibitory Concentrations
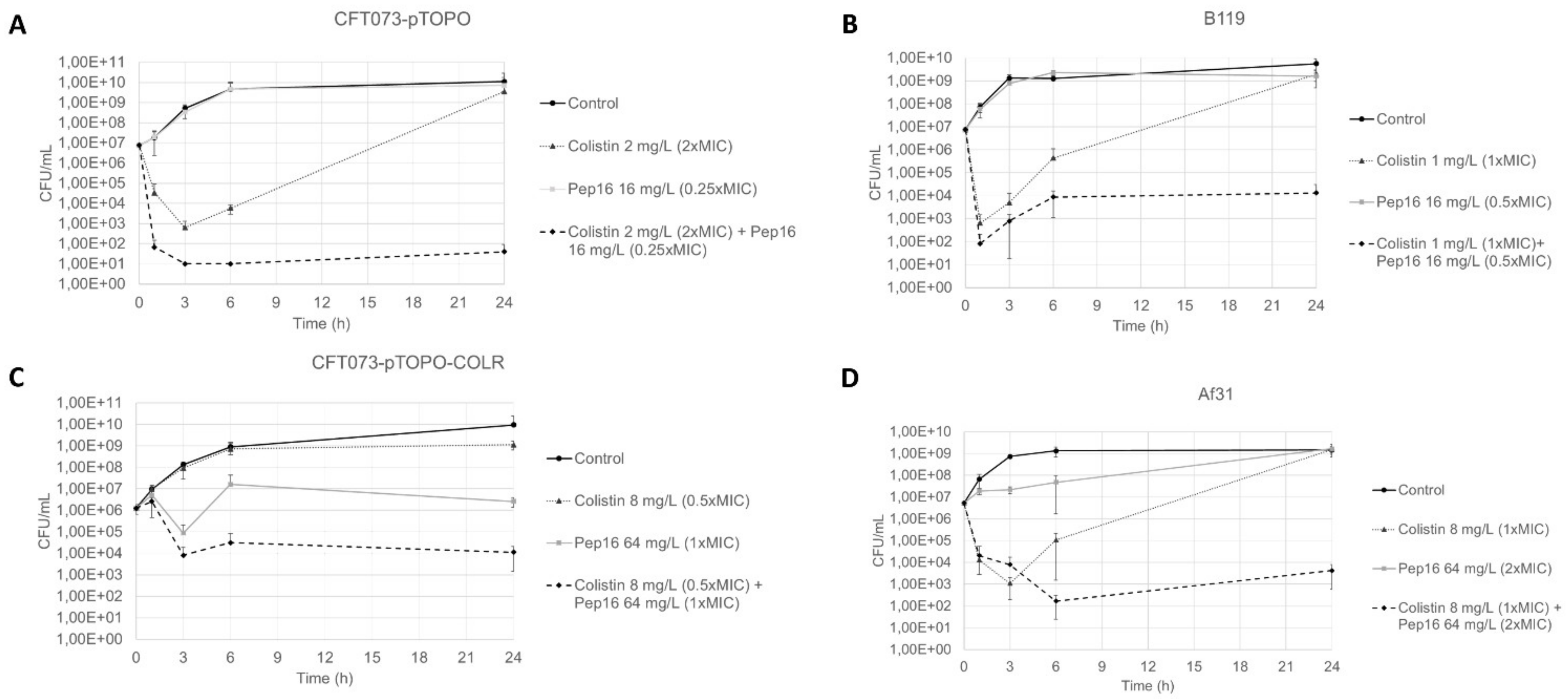
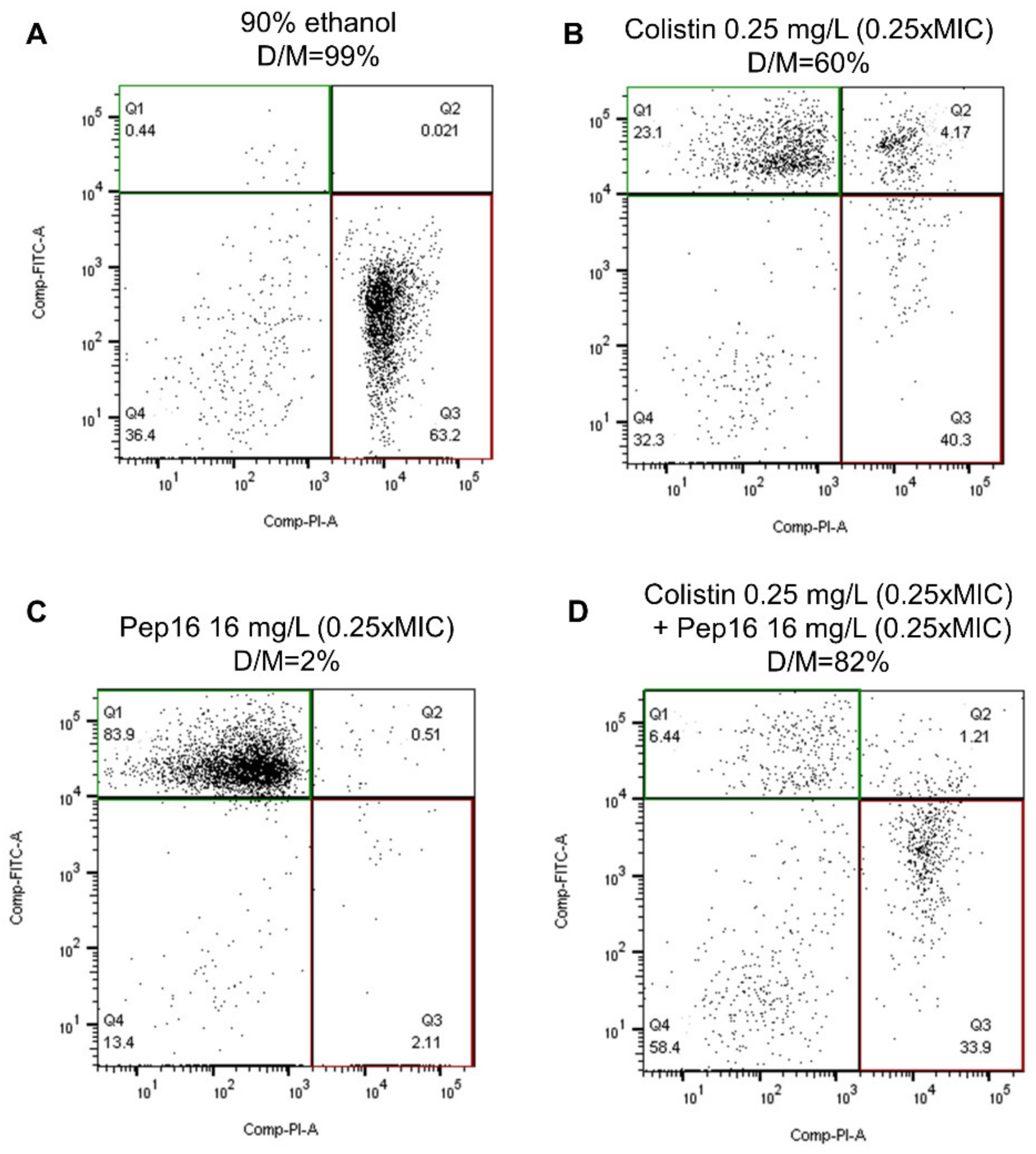
3.1.2. Synergy Studies
3.2. In Vivo Studies
3.2.1. Toxicity Study
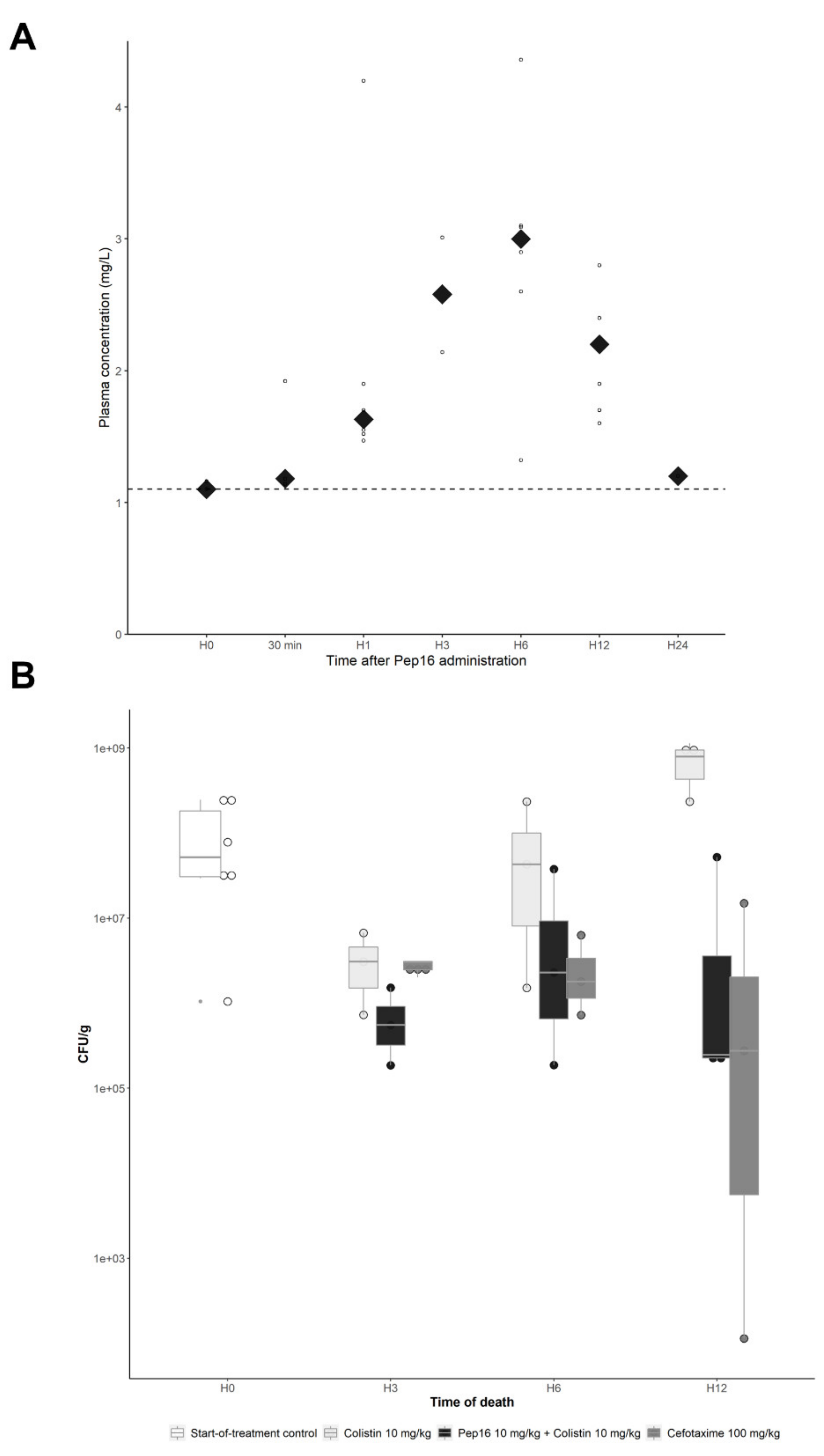
3.2.2. Pep16 Pharmacokinetic Analysis
3.2.3. Murine Peritoneal Infection Model—In Vivo Time–Kill Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laxminarayan, R.; Van Boeckel, T.; Frost, I.; Kariuki, S.; Khan, E.A.; Limmathurotsakul, D.; Larsson, D.G.J.; Levy-Hara, G.; Mendelson, M.; Outterson, K.; et al. The Lancet Infectious Diseases Commission on Antimicrobial Resistance: 6 Years Later. Lancet Infect. Dis. 2020, 20, e51–e60. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-β-Lactamases: A Last Frontier for β-Lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Fantin, B.; Poujade, J.; Grégoire, N.; Chau, F.; Roujansky, A.; Kieffer, N.; Berleur, M.; Couet, W.; Nordmann, P. The Inoculum Effect of Escherichia coli Expressing Mcr-1 or Not on Colistin Activity in a Murine Model of Peritonitis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 1563.e5–1563.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef]
- Abdelaziz, S.M.; Aboshanab, K.M.; Yahia, I.S.; Yassien, M.A.; Hassouna, N.A. Correlation between the Antibiotic Resistance Genes and Susceptibility to Antibiotics among the Carbapenem-Resistant Gram-Negative Pathogens. Antibiotics 2021, 10, 255. [Google Scholar] [CrossRef]
- Dénervaud Tendon, V.; Poirel, L.; Nordmann, P. Transferability of the Mcr-1 Colistin Resistance Gene. Microb. Drug Resist. 2017, 23, 813–814. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.-L.; Shen, Y.; Zhang, Y.; Yang, J.-W.; Shu, L.-B.; Zhou, H.-W.; Wang, Y.; Wang, B.; Zhang, R.; et al. Rapid Increase in Prevalence of Carbapenem-Resistant Enterobacteriaceae (CRE) and Emergence of Colistin Resistance Gene Mcr-1 in CRE in a Hospital in Henan, China. J. Clin. Microbiol. 2018, 56, e01932-17. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.-R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Cantón, R.; et al. Effect of Appropriate Combination Therapy on Mortality of Patients with Bloodstream Infections Due to Carbapenemase-Producing Enterobacteriaceae (INCREMENT): A Retrospective Cohort Study. Lancet Infect. Dis. 2017, 17, 726–734. [Google Scholar] [CrossRef]
- Le Menestrel, A.; Guerin, F.; Chau, F.; Massias, L.; Benchetrit, L.; Cattoir, V.; Fantin, B.; de Lastours, V. Activity of the Combination of Colistin and Fosfomycin against NDM-1-Producing Escherichia coli with Variable Levels of Susceptibility to Colistin and Fosfomycin in a Murine Model of Peritonitis. J. Antimicrob. Chemother. 2021, 77, 155–163. [Google Scholar] [CrossRef]
- Nicolas, I.; Bordeau, V.; Bondon, A.; Baudy-Floc’h, M.; Felden, B. Novel Antibiotics Effective against Gram-Positive and -Negative Multi-Resistant Bacteria with Limited Resistance. PLoS Biol. 2019, 17, e3000337. [Google Scholar] [CrossRef]
- Berleur, M.; Guérin, F.; Massias, L.; Chau, F.; Poujade, J.; Cattoir, V.; Fantin, B.; de Lastours, V. Activity of Fosfomycin Alone or Combined with Temocillin in Vitro and in a Murine Model of Peritonitis Due to KPC-3- or OXA-48-Producing Escherichia coli. J. Antimicrob. Chemother. 2018, 73, 3074–3080. [Google Scholar] [CrossRef]
- Cheminet, G.; de Lastours, V.; Poirel, L.; Chau, F.; Peoc’h, K.; Massias, L.; Fantin, B.; Nordmann, P. Dimercaptosuccinic Acid in Combination with Carbapenems against Isogenic Strains of Escherichia coli Producing or Not Producing a Metallo-β-Lactamase in Vitro and in Murine Peritonitis. J. Antimicrob. Chemother. 2020, 75, 3593–3600. [Google Scholar] [CrossRef]
- Alexandre, K.; Chau, F.; Guérin, F.; Massias, L.; Lefort, A.; Cattoir, V.; Fantin, B. Activity of Temocillin in a Lethal Murine Model of Infection of Intra-Abdominal Origin Due to KPC-Producing Escherichia coli. J. Antimicrob. Chemother. 2016, 71, 1899–1904. [Google Scholar] [CrossRef] [Green Version]
- Berenbaum, M.C. A Method for Testing for Synergy with Any Number of Agents. J. Infect. Dis. 1978, 137, 122–130. [Google Scholar] [CrossRef]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of Three Different in Vitro Methods of Detecting Synergy: Time-Kill, Checkerboard, and E Test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [Green Version]
- Chau, F.; Lefort, A.; Benadda, S.; Dubée, V.; Fantin, B. Flow Cytometry as a Tool to Determine the Effects of Cell Wall-Active Antibiotics on Vancomycin-Susceptible and -Resistant Enterococcus Faecalis Strains. Antimicrob. Agents Chemother. 2011, 55, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Rossi, B.; Soubirou, J.F.; Chau, F.; Massias, L.; Dion, S.; Lepeule, R.; Fantin, B.; Lefort, A. Cefotaxime and Amoxicillin-Clavulanate Synergism against Extended-Spectrum-β-Lactamase-Producing Escherichia coli in a Murine Model of Urinary Tract Infection. Antimicrob. Agents Chemother. 2016, 60, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangchinda, W.; Pati, N.; Maknakhon, N.; Seenama, C.; Tiengrim, S.; Thamlikitkul, V. Collateral Damage of Using Colistin in Hospitalized Patients on Emergence of Colistin-Resistant Escherichia coli and Klebsiella Pneumoniae Colonization and Infection. Antimicrob. Resist. Infect. Control 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-Inhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
| E. coli Isolates | Origin | Inoculum (CFU/mL) | MICs | ||
|---|---|---|---|---|---|
| Pep16 (mg/L) | Colistin (mg/L) (S ≤ 2; R > 2) | Cefotaxime (mg/L) (S ≤ 1; R > 2) | |||
| CFT073-pTOPO | Derived from CFT073 clinical isolate (UTI) | 105 | 64 | 1 | 0.06 |
| 106 | 128 | 2 | 1 | ||
| 107 | 1024 | 32 | 8 | ||
| CFT073-pTOPO-COLR | In vivo mutant from CFT073 | 105 | 64 | 16 | 0.06 |
| Af31 | Clinical isolate (UTI) | 105 | 32 | 8 | 0.06 |
| B119 | Clinical isolate (UTI) | 105 | 32 | 1 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chosidow, S.; Fantin, B.; Nicolas, I.; Mascary, J.-B.; Chau, F.; Bordeau, V.; Verdier, M.-C.; Rocheteau, P.; Guérin, F.; Cattoir, V.; et al. Synergistic Activity of Pep16, a Promising New Antibacterial Pseudopeptide against Multidrug-Resistant Organisms, in Combination with Colistin against Multidrug-Resistant Escherichia coli, In Vitro and in a Murine Peritonitis Model. Antibiotics 2023, 12, 81. https://doi.org/10.3390/antibiotics12010081
Chosidow S, Fantin B, Nicolas I, Mascary J-B, Chau F, Bordeau V, Verdier M-C, Rocheteau P, Guérin F, Cattoir V, et al. Synergistic Activity of Pep16, a Promising New Antibacterial Pseudopeptide against Multidrug-Resistant Organisms, in Combination with Colistin against Multidrug-Resistant Escherichia coli, In Vitro and in a Murine Peritonitis Model. Antibiotics. 2023; 12(1):81. https://doi.org/10.3390/antibiotics12010081
Chicago/Turabian StyleChosidow, Samuel, Bruno Fantin, Irène Nicolas, Jean-Baptiste Mascary, Françoise Chau, Valérie Bordeau, Marie-Clemence Verdier, Pierre Rocheteau, Francois Guérin, Vincent Cattoir, and et al. 2023. "Synergistic Activity of Pep16, a Promising New Antibacterial Pseudopeptide against Multidrug-Resistant Organisms, in Combination with Colistin against Multidrug-Resistant Escherichia coli, In Vitro and in a Murine Peritonitis Model" Antibiotics 12, no. 1: 81. https://doi.org/10.3390/antibiotics12010081






