Clostridioides difficile in South American Camelids in Germany: First Insights into Molecular and Genetic Characteristics and Antimicrobial Resistance
Abstract
:1. Introduction
2. Results
2.1. Prevalence and Molecular Characteristics
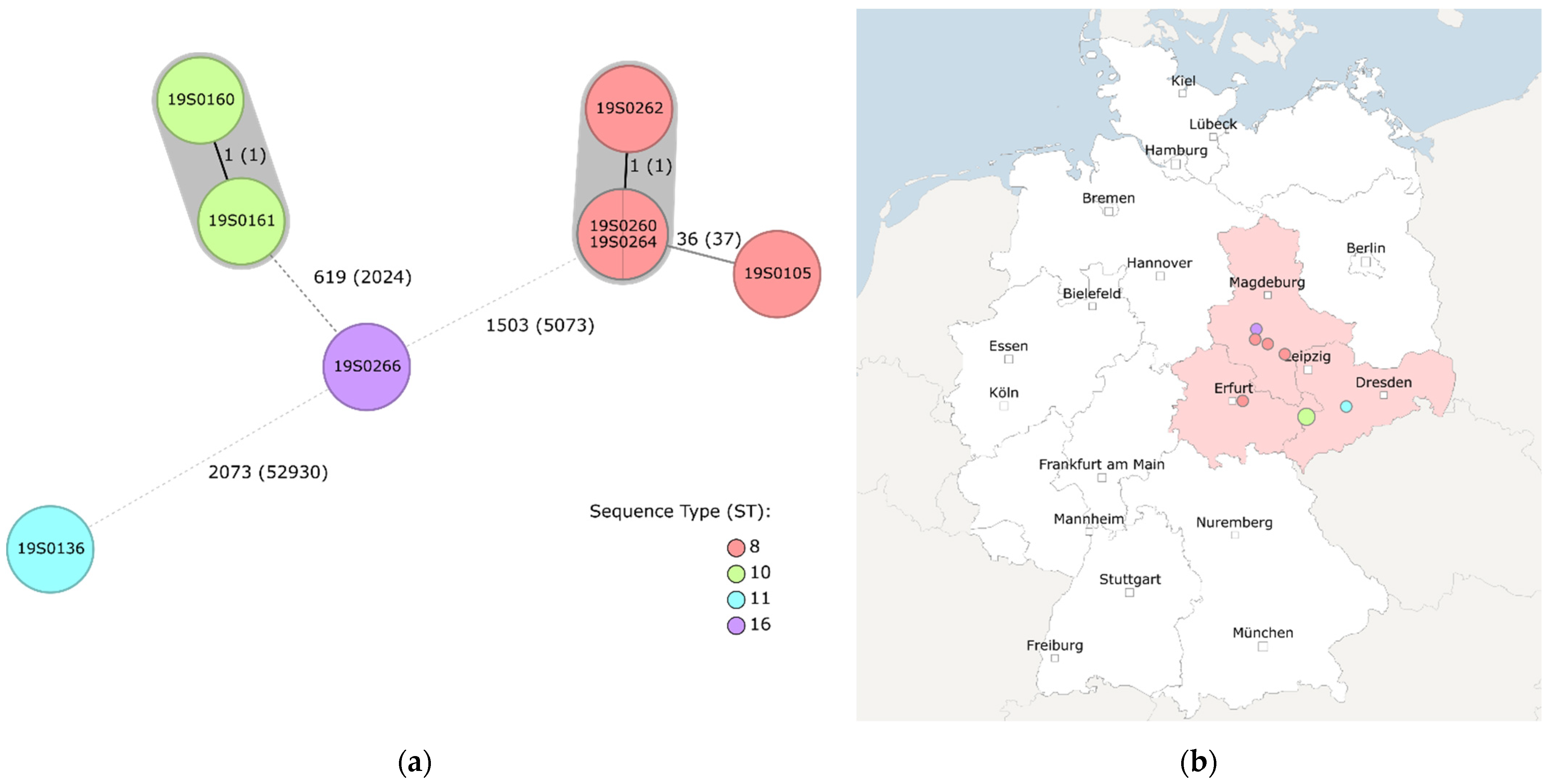
2.2. Whole Genome Sequencing (WGS)
2.3. Antimicrobial Susceptibility Testing (AST)
3. Discussion
3.1. Clostridioides difficile and (South American) Camelids in Literature—A Short Review
3.2. Prevalence of Clostridioides difficile in German South American Camelids
3.3. Isolates of Different Toxinogenic Ribotypes Resistant to Antimicrobial Agents Were Detected
3.4. South American Camelids and Antimicrobial Agents
3.5. Further Investigation Is Needed
4. Materials and Methods
4.1. Sampling
4.2. Isolation of C. difficile
4.3. Molecular Characterisation
4.4. Whole Genome Sequencing
4.5. Antimicrobial Susceptibility Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weese, J.S. Clostridium (Clostridioides) difficile in animals. J. Vet. Diagn. Investig. 2020, 32, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Meléndez, A.; Morfin-Otero, R.; Villarreal-Treviño, L.; Baines, S.D.; Camacho-Ortíz, A.; Garza-González, E. Molecular epidemiology of predominant and emerging Clostridioides difficile ribotypes. J. Microbiol. Methods 2020, 175, 105974. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Healthcare-associated infections: Clostridium difficile infections. In ECDC. Annual Epidemiological Report for 2016; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- Ofori, E.; Ramai, D.; Dhawan, M.; Mustafa, F.; Gasperino, J.; Reddy, M. Community-acquired Clostridium difficile: Epidemiology, ribotype, risk factors, hospital and intensive care unit outcomes, and current and emerging therapies. J. Hosp. Infect. 2018, 99, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.R.; Seyboldt, C.; Rupnik, M. Non-human C. difficile Reservoirs and Sources: Animals, Food, Environment. Adv. Exp. Med. Biol. 2018, 1050, 227–243. [Google Scholar] [CrossRef]
- Moloney, G.; Eyre, D.W.; Mac Aogáin, M.; McElroy, M.C.; Vaughan, A.; Peto, T.E.A.; Crook, D.W.; Rogers, T.R. Human and Porcine Transmission of Clostridioides difficile Ribotype 078, Europe. Emerg. Infect. Dis. 2021, 27, 2294–2300. [Google Scholar] [CrossRef]
- Knetsch, C.W.; Connor, T.R.; Mutreja, A.; van Dorp, S.M.; Sanders, I.M.; Browne, H.P.; Harris, D.; Lipman, L.; Keessen, E.C.; Corver, J.; et al. Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Eurosurveillance 2014, 19, 20954. [Google Scholar] [CrossRef] [Green Version]
- Sebaihia, M.; Wren, B.W.; Mullany, P.; Fairweather, N.F.; Minton, N.; Stabler, R.; Thomson, N.R.; Roberts, A.P.; Cerdeño-Tárraga, A.M.; Wang, H.; et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 2006, 38, 779–786. [Google Scholar] [CrossRef]
- O’Grady, K.; Knight, D.R.; Riley, T.V. Antimicrobial resistance in Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2459–2478. [Google Scholar] [CrossRef]
- Neubert, S.; von Altrock, A.; Wendt, M.; Wagener, M.G. Llama and Alpaca Management in Germany-Results of an Online Survey among Owners on Farm Structure, Health Problems and Self-Reflection. Animals 2021, 11, 102. [Google Scholar] [CrossRef]
- González-Santamarina, B.; Schnee, C.; Köhler, H.; Weber, M.; Methner, U.; Seyboldt, C.; Berens, C.; Menge, C. Survey on shedding of selected pathogenic, zoonotic or antimicrobial resistant bacteria by South American camelids in Central Germany. Berl. Munch. Tierarztl. Wochenschr. 2022, 135, 1–16. [Google Scholar] [CrossRef]
- García-Soto, S.; Abdel-Glil, M.Y.; Tomaso, H.; Linde, J.; Methner, U. Emergence of Multidrug-Resistant Salmonella enterica Subspecies enterica Serovar Infantis of Multilocus Sequence Type 2283 in German Broiler Farms. Front. Microbiol. 2020, 11, 1741. [Google Scholar] [CrossRef] [PubMed]
- Clostridioides Difficile cgMLST. Available online: https://www.cgmlst.org/ncs/schema/12556067/ (accessed on 22 August 2022).
- Baktash, A.; Corver, J.; Harmanus, C.; Smits, W.K.; Fawley, W.; Wilcox, M.H.; Kumar, N.; Eyre, D.W.; Indra, A.; Mellmann, A.; et al. Comparison of Whole-Genome Sequence-Based Methods and PCR Ribotyping for Subtyping of Clostridioides difficile. J. Clin. Microbiol. 2022, 60, e0173721. [Google Scholar] [CrossRef] [PubMed]
- Jünemann, S.; Sedlazeck, F.J.; Prior, K.; Albersmeier, A.; John, U.; Kalinowski, J.; Mellmann, A.; Goesmann, A.; von Haeseler, A.; Stoye, J.; et al. Updating benchtop sequencing performance comparison. Nat. Biotechnol. 2013, 31, 294–296. [Google Scholar] [CrossRef] [Green Version]
- BIOMÉRIEUX Resource Center. Available online: https://resourcecenter.biomerieux.com/ (accessed on 2 January 2023).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 2 January 2023).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-135-2. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC Distribution Website. Available online: https://mic.eucast.org/ (accessed on 18 February 2022).
- Fayez, M.; El-Ghareeb, W.R.; Elmoslemany, A.; Alsunaini, S.J.; Alkafafy, M.; Alzahrani, O.M.; Mahmoud, S.F.; Elsohaby, I. Genotyping and Antimicrobial Susceptibility of Clostridium perfringens and Clostridioides difficile in Camel Minced Meat. Pathogens 2021, 10, 1640. [Google Scholar] [CrossRef]
- Esfandiari, Z.; Weese, J.S.; Ezzatpanah, H.; Chamani, M.; Shoaei, P.; Yaran, M.; Ataei, B.; Maracy, M.R.; Ansariyan, A.; Ebrahimi, F.; et al. Isolation and characterization of Clostridium difficile in farm animals from slaughterhouse to retail stage in Isfahan, Iran. Foodborne Pathog. Dis. 2015, 12, 864–866. [Google Scholar] [CrossRef]
- Rahimi, E.; Jalali, M.; Weese, J.S. Prevalence of Clostridium difficile in raw beef, cow, sheep, goat, camel and buffalo meat in Iran. BMC Public Health 2014, 14, 119. [Google Scholar] [CrossRef] [Green Version]
- McNamara, S.E.; Abdujamilova, N.; Somsel, P.; Gordoncillo, M.J.; DeDecker, J.M.; Bartlett, P.C. Carriage of Clostridium difficile and other enteric pathogens among a 4-H avocational cohort. Zoonoses Public Health 2011, 58, 192–199. [Google Scholar] [CrossRef]
- Blasi, F.; Lovito, C.; Albini, E.; Bano, L.; Dalmonte, G.; Drigo, I.; Maresca, C.; Massacci, F.R.; Orsini, S.; Primavilla, S.; et al. Clostridioides difficile in Calves in Central Italy: Prevalence, Molecular Typing, Antimicrobial Susceptibility and Association with Antibiotic Administration. Animals 2021, 11, 515. [Google Scholar] [CrossRef]
- Bandelj, P.; Blagus, R.; Briski, F.; Frlic, O.; Rataj, A.V.; Rupnik, M.; Ocepek, M.; Vengust, M. Identification of risk factors influencing Clostridium difficile prevalence in middle-size dairy farms. Vet. Res. 2016, 47, 41. [Google Scholar] [CrossRef]
- Knight, D.R.; Riley, T.V. Prevalence of gastrointestinal Clostridium difficile carriage in Australian sheep and lambs. Appl. Environ. Microbiol. 2013, 79, 5689–5692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbanti, F.; Spigaglia, P. Microbiological characteristics of human and animal isolates of Clostridioides difficile in Italy: Results of the Istituto Superiore di Sanità in the years 2006–2016. Anaerobe 2020, 61, 102136. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, E.; Stelzer, Y.; Berger, F.; Jazmati, N. Changes in Clostridium (Clostridioides) difficile PCR-Ribotype Distribution and Antimicrobial Resistance in a German Tertiary Care Hospital Over the Last 10 Years. Curr. Microbiol. 2019, 76, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.W.; Wilson, R.B. Clostridium difficile colitis and zoonotic origins-a narrative review. Gastroenterol. Rep. 2018, 6, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, D.R.; Riley, T.V. Genomic Delineation of Zoonotic Origins of Clostridium difficile. Front. Public Health 2019, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Carriço, J.A.; Rossi, M.; Moran-Gilad, J.; van Domselaar, G.; Ramirez, M. A primer on microbial bioinformatics for nonbioinformaticians. Clin. Microbiol. Infect. 2018, 24, 342–349. [Google Scholar] [CrossRef] [Green Version]
- Quainoo, S.; Coolen, J.P.M.; van Hijum, S.A.F.T.; Huynen, M.A.; Melchers, W.J.G.; van Schaik, W.; Wertheim, H.F.L. Whole-Genome Sequencing of Bacterial Pathogens: The Future of Nosocomial Outbreak Analysis. Clin. Microbiol. Rev. 2017, 30, 1015–1063. [Google Scholar] [CrossRef] [Green Version]
- Bletz, S.; Janezic, S.; Harmsen, D.; Rupnik, M.; Mellmann, A. Defining and Evaluating a Core Genome Multilocus Sequence Typing Scheme for Genome-Wide Typing of Clostridium difficile. J. Clin. Microbiol. 2018, 56, e01987-17. [Google Scholar] [CrossRef] [Green Version]
- Mac Aogáin, M.; Kilkenny, S.; Walsh, C.; Lindsay, S.; Moloney, G.; Morris, T.; Jones, S.; Rogers, T.R. Identification of a novel mutation at the primary dimer interface of GyrA conferring fluoroquinolone resistance in Clostridium difficile. J. Glob. Antimicrob. Resist. 2015, 3, 295–299. [Google Scholar] [CrossRef]
- Abdrabou, A.M.M.; Ul Habib Bajwa, Z.; Halfmann, A.; Mellmann, A.; Nimmesgern, A.; Margardt, L.; Bischoff, M.; von Müller, L.; Gärtner, B.; Berger, F.K. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany, 2014–2019. Int. J. Med. Microbiol. 2021, 311, 151507. [Google Scholar] [CrossRef]
- Freeman, J.; Vernon, J.; Pilling, S.; Morris, K.; Nicholson, S.; Shearman, S.; Longshaw, C.; Wilcox, M.H. The ClosER study: Results from a three-year pan-European longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011–2014. Clin. Microbiol. Infect. 2018, 24, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krijger, I.M.; Meerburg, B.G.; Harmanus, C.; Burt, S.A. Clostridium difficile in wild rodents and insectivores in the Netherlands. Lett. Appl. Microbiol. 2019, 69, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, M.; Stewart, N.K.; Smith, C.; Vakulenko, S.B. Intrinsic Class D β-Lactamases of Clostridium difficile. mBio 2018, 9, e01803-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avberšek, J.; Pirš, T.; Pate, M.; Rupnik, M.; Ocepek, M. Clostridium difficile in goats and sheep in Slovenia: Characterisation of strains and evidence of age-related shedding. Anaerobe 2014, 28, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Dupont, P.-Y.; Gilpin, B.J.; Cornelius, A.J. Isolation and characterization of Clostridium difficile from a small survey of wastewater, food and animals in New Zealand. Lett. Appl. Microbiol. 2020, 70, 29–35. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Yang, L.; Gu, X.-X.; Chen, P.-X.; Fu, J.-L.; Jiang, H.-X. The first isolation of Clostridium difficile RT078/ST11 from pigs in China. PLoS ONE 2019, 14, e0212965. [Google Scholar] [CrossRef] [Green Version]
- Sholeh, M.; Krutova, M.; Forouzesh, M.; Mironov, S.; Sadeghifard, N.; Molaeipour, L.; Maleki, A.; Kouhsari, E. Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 158. [Google Scholar] [CrossRef]
- Ammam, F.; Marvaud, J.-C.; Lambert, T. Distribution of the vanG-like gene cluster in Clostridium difficile clinical isolates. Can. J. Microbiol. 2012, 58, 547–551. [Google Scholar] [CrossRef]
- Peltier, J.; Courtin, P.; El Meouche, I.; Catel-Ferreira, M.; Chapot-Chartier, M.-P.; Lemée, L.; Pons, J.-L. Genomic and expression analysis of the vanG-like gene cluster of Clostridium difficile. Microbiology 2013, 159, 1510–1520. [Google Scholar] [CrossRef] [Green Version]
- Ammam, F.; Meziane-Cherif, D.; Mengin-Lecreulx, D.; Blanot, D.; Patin, D.; Boneca, I.G.; Courvalin, P.; Lambert, T.; Candela, T. The functional vanGCd cluster of Clostridium difficile does not confer vancomycin resistance. Mol. Microbiol. 2013, 89, 612–625. [Google Scholar] [CrossRef]
- Gesetz zur Vorbeugung vor und Bekämpfung von Tierseuchen (Tiergesundheitsgesetz—TierGesG), § 2, 4. Available online: https://www.gesetze-im-internet.de/tiergesg/__2.html (accessed on 2 January 2023).
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0037 (accessed on 2 January 2023).
- Emmerich, I.U.; Ganter, M.; Wittek, T. Dosierungsvorschläge für Arzneimittel bei Kleinen Wiederkäuern und Neuweltkameliden: MemoVet, 2., Vollst. Aktualisierte und Überarb. Aufl.; Schattauer: Stuttgart, Germany, 2016; ISBN 978-3-7945-3168-4. [Google Scholar]
- González-Santamarina, B.; Weber, M.; Menge, C.; Berens, C. Comparative Genomic analysis of antimicrobial-resistant Escherichia coli from South American Camelids in Central Germany. Microorganisms 2022, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Zidaric, V.; Zemljic, M.; Janezic, S.; Kocuvan, A.; Rupnik, M. High diversity of Clostridium difficile genotypes isolated from a single poultry farm producing replacement laying hens. Anaerobe 2008, 14, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kato, N.; Watanabe, K.; Iwai, N.; Nakamura, H.; Yamamoto, T.; Suzuki, K.; Kim, S.M.; Chong, Y.; Wasito, E.B. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 1998, 36, 2178–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, N.; Ou, C.Y.; Kato, H.; Bartley, S.L.; Brown, V.K.; Dowell, V.R.; Ueno, K. Identification of toxigenic Clostridium difficile by the polymerase chain reaction. J. Clin. Microbiol. 1991, 29, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, S.; Rupnik, M.; Gibert, M.; Brazier, J.; Duerden, B.; Popoff, M. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 2000, 186, 307–312. [Google Scholar] [CrossRef]
- Indra, A.; Huhulescu, S.; Schneeweis, M.; Hasenberger, P.; Kernbichler, S.; Fiedler, A.; Wewalka, G.; Allerberger, F.; Kuijper, E.J. Characterization of Clostridium difficile isolates using capillary gel electrophoresis-based PCR ribotyping. J. Med. Microbiol. 2008, 57, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- WEBRIBO. Available online: https://webribo.ages.at/ (accessed on 2 January 2023).
- FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 25 August 2022).
- Shovill. Available online: https://github.com/tseemann/shovill (accessed on 25 August 2022).
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Prokka. Available online: https://github.com/tseemann/prokka (accessed on 25 August 2022).
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Snippy. Available online: https://github.com/tseemann/snippy (accessed on 25 August 2022).
- Seemann, T. Abricate, Github. Available online: https://github.com/tseemann/abricate (accessed on 17 October 2022).
| Isolate | Farm-ID | Federal State | Ribotype (RT) | WEBRIBO-ID | Sequence Type (ST) | Toxin A | Toxin B | Binary Toxin |
|---|---|---|---|---|---|---|---|---|
| tcdA | tcdB | cdtA/cdtB | ||||||
| 19S0105 | 008 | Thuringia | AI-75 | PR28224 | 8 | + | + | −/− |
| 19S0136 | 016 | Saxony | 078 | PR28225 | 11 | + | + | +/+ |
| 19S0160 | 018 | Saxony | 015 | PR26350 | 10 | + | + | −/− |
| 19S0161 | 018 | Saxony | 015 | PR28235 | 10 | + | + | −/− |
| 19S0260 | 039 | Saxony-Anhalt | 002/2 | PR28226 | 8 | + | + | −/− |
| 19S0262 | 040 | Saxony-Anhalt | 002/2 | PR28226 | 8 | + | + | −/− |
| 19S0264 | 041 | Saxony-Anhalt | 002/2 | PR26352 | 8 | + | + | −/− |
| 19S0266 | 042 | Saxony-Anhalt | 029 | PR28237 | 16 | + | + | −/− |
| Isolate | 19S0105 | 19S0136 | 19S0160 | 19S0161 | 19S0260 | 19S0262 | 19S0264 | 19S0266 | CD630 |
|---|---|---|---|---|---|---|---|---|---|
| 19S0105 | 0 | 91994 | 9286 | 9286 | 116 | 116 | 121 | 9306 | 9147 |
| 19S0136 | 91994 | 0 | 91623 | 91623 | 91999 | 91999 | 91996 | 91714 | 92383 |
| 19S0160 | 9286 | 91623 | 0 | 6 | 9310 | 9310 | 9307 | 3955 | 10527 |
| 19S0161 | 9286 | 91623 | 6 | 0 | 9310 | 9310 | 9307 | 3953 | 10531 |
| 19S0260 | 116 | 91999 | 9310 | 9310 | 0 | 0 | 5 | 9330 | 9173 |
| 19S0262 | 116 | 91999 | 9310 | 9310 | 0 | 0 | 5 | 9330 | 9173 |
| 19S0264 | 121 | 91996 | 9307 | 9307 | 5 | 5 | 0 | 9327 | 9168 |
| 19S0266 | 9306 | 91714 | 3955 | 3953 | 9330 | 9330 | 9327 | 0 | 10484 |
| CD630 | 9147 | 92383 | 10527 | 10531 | 9173 | 9173 | 9168 | 10484 | 0 |
| Isolate | Resistance Genes |
|---|---|
| 19S0105 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0136 | aadE; blaCDD-1; tet(40); tet(M); vanZ1 |
| 19S0160 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0161 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0260 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0262 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0264 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| 19S0266 | blaCDD-1; vanG; vanR; vanS; vanT; vanZ1 |
| Isolate | Protein | Amino Acid Substitutions |
|---|---|---|
| 19S0136 | GyrA | Thr-82-Val * Lys-413-Asn |
| GyrB | Gln-160-His Ser-366 #-Val Ser-416-Ala * | |
| 19S0160 | GyrB | Val-130-Ile |
| 19S0161 | GyrB | Val-130-Ile |
| Isolate (RT) | Antimicrobials | MEZ | VAN | MOX | CIP | AMP | PEN | AMC | MEP | ERY | CLI | CHL | TET | LZD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP/ECOFF (mg/L) | >2 a | >2 a | ≤2/≥8 b | 32 c | ≤0.5/≥2 b | ≤0.5/≥2 b | ≤4/2/≥16/8 b | ≤4/≥16 b | 4 c | ≤2/≥8 b | ≤8/≥32 b | ≤4/≥16 b | - | |
| 19S0105 (RT AI-75) | 0.19 | 0.75 | 1.5 | >32 | 1.5 | 1.5 | 0.75 | 1.5 | 2 | 8 | 12 | 0.38 | 3 | |
| 19S0136 (RT 078) | 0.125 | 0.75 | >32 | >32 | 0.75 | 1.5 | 0.38 | 0.75 | >256 | 2 | 6 | 16 | 3 | |
| 19S0160 (RT 015) | 0.38 | 0.75 | 1.5 | >32 | 1 | 1 | 1 | 1.5 | 2 | 12 | 6 | 0.38 | 6 | |
| 19S0161 (RT 015) | 0.125 | 0.75 | 1 | >32 | 1.5 | 1 | 0.75 | 1.5 | 2 | 12 | 6 | 0.38 | 4 | |
| 19S0260 (RT 002/2) | 0.38 | 1 | 1.5 | >32 | 1.5 | 1.5 | 0.5 | 1.5 | 2 | 8 | 8 | 0.19 | 3 | |
| 19S0262 (RT 002/2) | 0.38 | 0.75 | 1.5 | >32 | 1.5 | 1.5 | 0.75 | 1.5 | 2 | 8 | 8 | 0.25 | 3 | |
| 19S0264 (RT 002/2) | 0.38 | 0.75 | 1.5 | >32 | 1 | 1 | 0.5 | 1 | 2 | 12 | 8 | 0.094 | 3 | |
| 19S0266 (RT 029) | 0.38 | 1 | 1 | >32 | 3 | 4 | 0.75 | 1.5 | 2 | 6 | 8 | 0.5 | 8 | |
| PCR-MIX and -Programme | Test Components | Working Concentration | cdd3 | tcdA—Non-Repetitive Region | tcdA—Repetitive Region | tcdB | cdtA | cdtB |
|---|---|---|---|---|---|---|---|---|
| PCR-Mix | DNA | As obtained by DNA isolation | 2 µL | 2 µL | 2 µL | 2 µL | 2 µL | 2 µL |
| DreamTaq Buffer (Thermo Fisher Scientific, Waltham, MA USA) | 10 x | 2.5 µL | 2.5 µL | 2.5 µL | 2.5 µL | 2.5 µL | 2.5 µL | |
| dNTP-Mix (Carl Roth, Karlsruhe, Germany) | 10 mM | 0.5 µL | 1 µL | 0.5 µL | 1 µL | 0.5 µL | 0.5 µL | |
| Primer 1 a | 10 pmol/µL | 0.5 µL | 1 µL | 1 µL | 1 µL | 1 µL | 1 µL | |
| Primer 2 a | 10 pmol/µL | 0.5 µL | 1 µL | 1 µL | 1 µL | 1 µL | 1 µL | |
| DreamTaq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA USA) | 5 U/µL | 0.1 µL | 0.2 µL | 0.1 µL | 0.2 µL | 0.1 µL | 0.1 µL | |
| MgCl2 (Qiagen, Hilden, Germany) | 25 mM | 1 µL | 1 µL | 1 µL | 1 µL | 1 µL | 1 µL | |
| H2O | 17.9 µL | 16.3 µL | 16.9 µL | 16.3 µL | 16.9 µL | 16.9 µL | ||
| PCR-Programme | Cycles | 35 | 35 | 35 | 35 | 35 | 35 | |
| Initial Denaturation | 2 min, 95 °C | 2 min, 95 °C | 2 min, 95 °C | 2 min, 95 °C | 2 min, 95 °C | 2 min, 95 °C | ||
| Denaturation | 30 s, 95 °C | 30 s, 95 °C | 30 s, 95 °C | 30 s, 95 °C | 30 s, 95 °C | 30 s, 95 °C | ||
| Annealing | 45 s, 50 °C | 30 s, 55 °C | 30 s, 62 °C | 30 s, 55 °C | 45 s, 52 °C | 45 s, 52 °C | ||
| Elongation | 1 min, 72 °C | 30 s, 72 °C | 1,5 min 72 °C | 30 s, 72 °C | 40 s, 72 °C | 40 s, 72 °C | ||
| Finale Extension | 8 min, 72 °C | 5 min, 72 °C | 8 min, 72 °C | 5 min, 72 °C | 8 min, 72 °C | 8 min, 72 °C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dost, I.; Abdel-Glil, M.; Schmoock, G.; Menge, C.; Berens, C.; González-Santamarina, B.; Wiegand, E.; Neubauer, H.; Schwarz, S.; Seyboldt, C. Clostridioides difficile in South American Camelids in Germany: First Insights into Molecular and Genetic Characteristics and Antimicrobial Resistance. Antibiotics 2023, 12, 86. https://doi.org/10.3390/antibiotics12010086
Dost I, Abdel-Glil M, Schmoock G, Menge C, Berens C, González-Santamarina B, Wiegand E, Neubauer H, Schwarz S, Seyboldt C. Clostridioides difficile in South American Camelids in Germany: First Insights into Molecular and Genetic Characteristics and Antimicrobial Resistance. Antibiotics. 2023; 12(1):86. https://doi.org/10.3390/antibiotics12010086
Chicago/Turabian StyleDost, Ines, Mostafa Abdel-Glil, Gernot Schmoock, Christian Menge, Christian Berens, Belén González-Santamarina, Elisabeth Wiegand, Heinrich Neubauer, Stefan Schwarz, and Christian Seyboldt. 2023. "Clostridioides difficile in South American Camelids in Germany: First Insights into Molecular and Genetic Characteristics and Antimicrobial Resistance" Antibiotics 12, no. 1: 86. https://doi.org/10.3390/antibiotics12010086







