Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans
Abstract
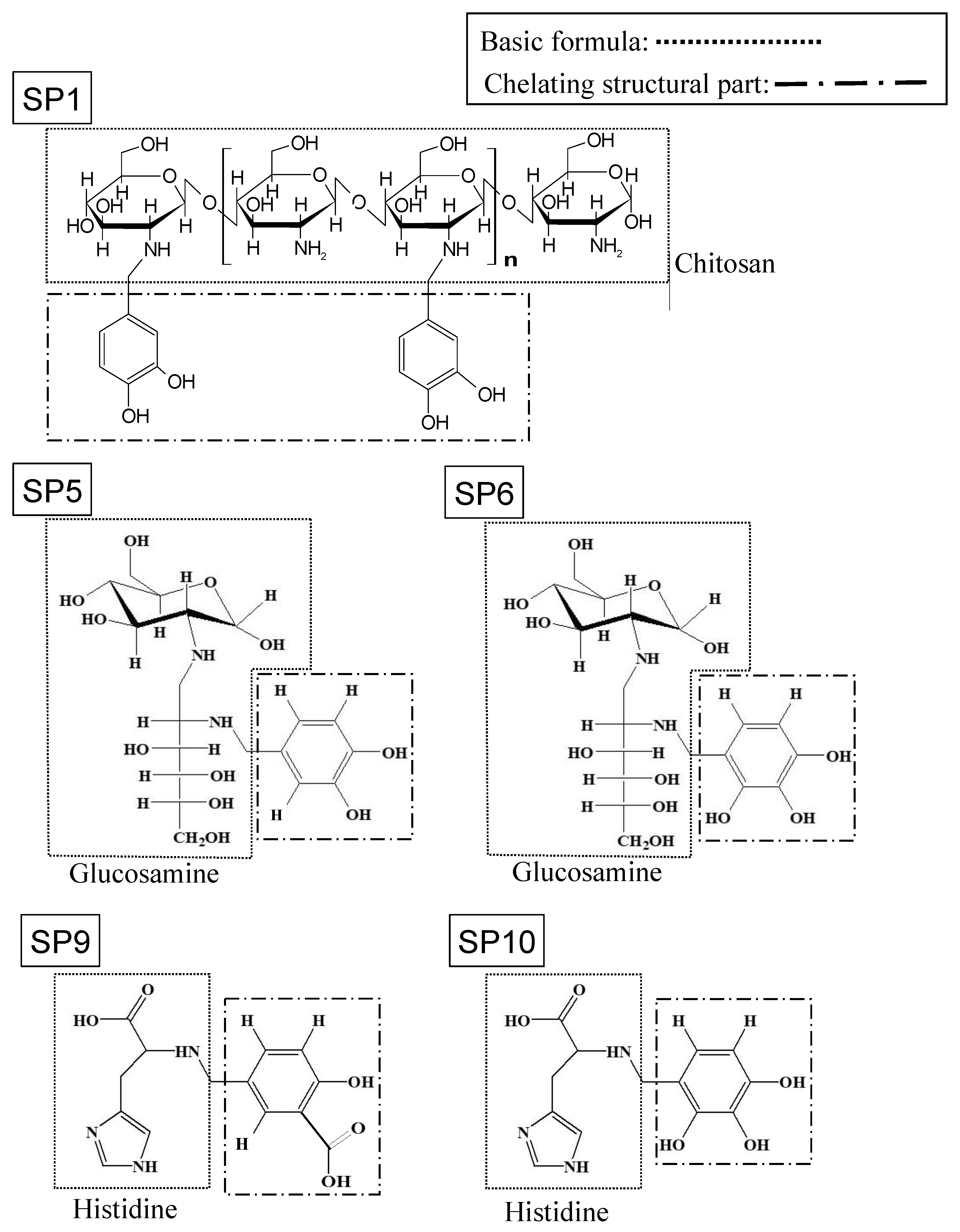
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacteria
2.3. Human Cells
2.4. Bacterial Growth Assay
2.5. Bacterial Morphological Observation
2.6. Bacterial Viability Analysis
2.7. Biofilm Forming Assay
2.8. Bacterial Iron Uptake Assay
2.9. Cell Toxicity Test on Human Cells
2.10. Statistical Analysis
3. Results
3.1. SPs Suppresses Bacterial Growth
3.2. SP6 and SP10 Did Not Affect Bacterial Morphology
3.3. SP6 and SP10 Did Not affect Bacterial Viability
3.4. SP6 and SP10 Suppresses Biofilm Formation
3.5. SP6 and SP10 Suppresses Bacterial Iron Uptake
3.6. SP6 and SP10 Was as Cytotoxic as Povidone-Iodine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| DFO | deferoxamine |
| DFX | deferasirox |
| SPs | super-polyphenols |
| P. gingivalis | Porphyromonas gingivalis |
| S. mutans | Streptococcus mutans |
| TSBY | tryptic soy broth with yeast extract |
| TSBY-s | tryptic soy broth without dextrose with yeast extractand 1% sucrose |
| CFU | colony forming unit |
| rpm | revolutions per minute |
| HGKs | human gingival keratinocytes |
| PBS | phosphate buffered saline |
| SEM | scanning electron microscope |
| ANOVA | analysis of variance |
| P | p-value |
| MIC | minimum inhibitory concentration |
References
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol. 2000 2006, 42, 80–87. [Google Scholar] [CrossRef]
- Cisar, J.O.; Kolenbrander, P.E.; McIntire, F.C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 1979, 24, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The pathogenic factors from oral streptococci for systemic diseases. Int. J. Mol. Sci. 2019, 20, 4571. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Naganuma, T.; Kurita, N.; Omae, K.; Ohnishi, T.; Yoshioka, T.; Ito, F.; Takeshima, T.; Fukuma, S.; Hamaguchi, S.; et al. Social Isolation/Lonliness and Tooth Loss in Community-Dwelling Older Adults: The Sukagawa Study. Innov. Aging. 2023, 7, igad065. [Google Scholar] [CrossRef]
- Almagor, J.; Temkin, E.; Benenson, I.; Fallach, N.; Carmeli, Y.; DRIVE-AB Consortium. The impact of antibiotic use on transmission of resistant bacteria in hospitals: Insights from an agent-based model. PLoS ONE 2018, 13, e0197111. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014.
- Griffiths, E. The iron-uptake systems of pathogenic bacteria. In Iron and Infection; Bullen, J.J., Griffiths, E., Eds.; John Wiley & Sons: Chichester, UK, 1987; pp. 69–137. [Google Scholar]
- Warning, W.S.; Werkman, C.H. Iron deficiency in bacterial metabolism. Arch. Biochem. 1944, 4, 75–87. [Google Scholar]
- Bullen, J.J. The significance of iron in infection. Rev. Infect. Dis. 1981, 3, 1127–1138. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron and infection. Microbiol. Rev. 1978, 42, 45–66. [Google Scholar] [CrossRef]
- Wooldridge, K.G.; Williams, P.H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 1993, 12, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. Int. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Aranha, H.; Strachan, R.C.; Arceneaux, J.E.; Byers, B.R. Effect of trace metals on growth of Streptococcus mutans in a Teflon chemostat. Infect. Immun. 1982, 35, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Bramanti, T.E.; Ebersole, J.L.; Holt, S.C. Hemolytic activity in the periodontopathogen Porphyromonas gingivalis: Kinetics of enzyme release and localization. Infect. Immun. 1991, 59, 1932–1940. [Google Scholar] [CrossRef]
- Moon, J.H.; Herr, Y.; Kim, S.W.; Lee, J.Y. In vitro activity of deferoxamine against Porphyromonas gingivalis. FEMS Microbiol. Lett. 2011, 323, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, C.; Lee, H.S.; Kim, S.W.; Lee, J.Y. Antibacterial and antibiofilm effects of iron chelators against Prevotella intermedia. J. Med. Microbiol. 2013, 62, 1307–1316. [Google Scholar] [CrossRef]
- Lee, J.W.; Yoon, S.S.; Shen, Z.X.; Ganser, A.; Hsu, H.-C.; Habr, D.; Domokos, G.; Roubert, B.; Porter, J.B.; EPIC Study Investigators. Iron chelation therapy with deferasirox in patients with aplastic anemia: A subgroup analysis of 116 patients from the EPIC trial. Blood 2010, 116, 2448–2454. [Google Scholar] [CrossRef]
- Vichinsky, E.; Onyekwere, O.; Porter, J.; Swerdlow, P.; Eckman, J.; Lane, P.; Files, B.; Hassell, K.; Kelly, P.; Wilson, F. A randomized comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br. J. Haematol. 2007, 136, 501–508. [Google Scholar] [CrossRef]
- Ohara, T.; Tomono, Y.; Boyi, X.; Yingfu, S.; Omori, K.; Matsukawa, A. A novel, nontoxic iron chelator, super-polyphenol, effectively induces apoptosis in human cancer cell lines. Oncotarget 2018, 9, 32751–32760. [Google Scholar] [CrossRef]
- Hirai, K. History and prospect of development of antibacterial agents of Japanese origin. Jpn. J. Chemotherapy. 2020, 68, 499–509. [Google Scholar]
- Ferrer, M.; Méndez-García, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef]
- Scoglio, M.; Cappellini, M.D.; D’Angelo, E.; Bianchetti, M.G.; Lava, S.A.; Agostoni, C.; Milani, G.P. Kidney Tubular Damage Secondary to Deferasirox: Systematic Literature Review. Children 2021, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Bereswill, S.; Mousavi, S.; Weschka, D.; Buczkowski, A.; Schmidt, S.; Heimesaat, M.M. Iron Deprivation by Oral Deferoxamine Application Alleviates Acute Campylobacteriosis in a Clinical Murine Campylobacter jejuni Infection Model. Biomolecules 2022, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, T.; Ohara, T.; Noma, K.; Nishiwaki, N.; Katsura, Y.; Kato, T.; Sato, H.; Tomono, Y.; Kikuchi, S.; Tazawa, H.; et al. Nanog is a promising chemoresistant stemness marker and therapeutic target by iron chelators for esophageal cancer. Int. J. Cancer 2021, 149, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ohara, T.; Chen, Y.; Hamada, Y.; Li, C.; Fujisawa, M.; Yoshimura, T.; Matsukawa, A. Highly Metastatic Subpopulation of TNBC Cells Has Limited Iron Metabolism and Is a Target of Iron Chelators. Cancers 2023, 15, 468. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinoda-Ito, Y.; Omori, K.; Ito, T.; Nakayama, M.; Ikeda, A.; Ito, M.; Ohara, T.; Takashiba, S. Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans. Antibiotics 2023, 12, 1562. https://doi.org/10.3390/antibiotics12111562
Shinoda-Ito Y, Omori K, Ito T, Nakayama M, Ikeda A, Ito M, Ohara T, Takashiba S. Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans. Antibiotics. 2023; 12(11):1562. https://doi.org/10.3390/antibiotics12111562
Chicago/Turabian StyleShinoda-Ito, Yuki, Kazuhiro Omori, Takashi Ito, Masaaki Nakayama, Atsushi Ikeda, Masahiro Ito, Toshiaki Ohara, and Shogo Takashiba. 2023. "Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans" Antibiotics 12, no. 11: 1562. https://doi.org/10.3390/antibiotics12111562
APA StyleShinoda-Ito, Y., Omori, K., Ito, T., Nakayama, M., Ikeda, A., Ito, M., Ohara, T., & Takashiba, S. (2023). Novel Iron Chelators, Super-Polyphenols, Show Antimicrobial Effects against Cariogenic Streptococcus mutans. Antibiotics, 12(11), 1562. https://doi.org/10.3390/antibiotics12111562







