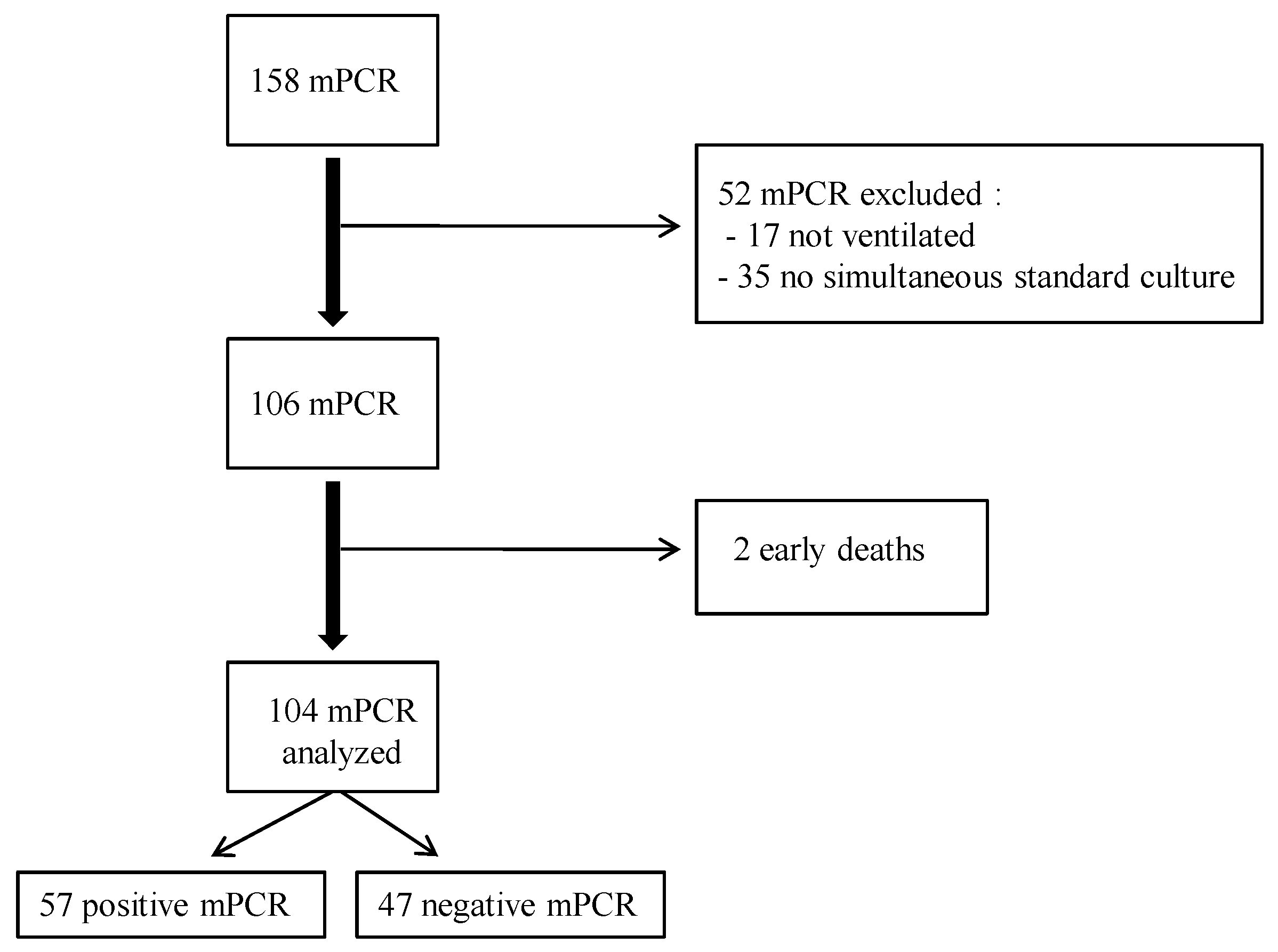
Performance and Impact on Antibiotic Prescriptions of a Multiplex PCR in a Real-Life Cohort of Critically Ill Patients with Suspected Ventilated Pneumonia: A Retrospective Monocentric Observational Study
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Data
2.2. Microbiological Data
2.3. Performance of the m-PCR
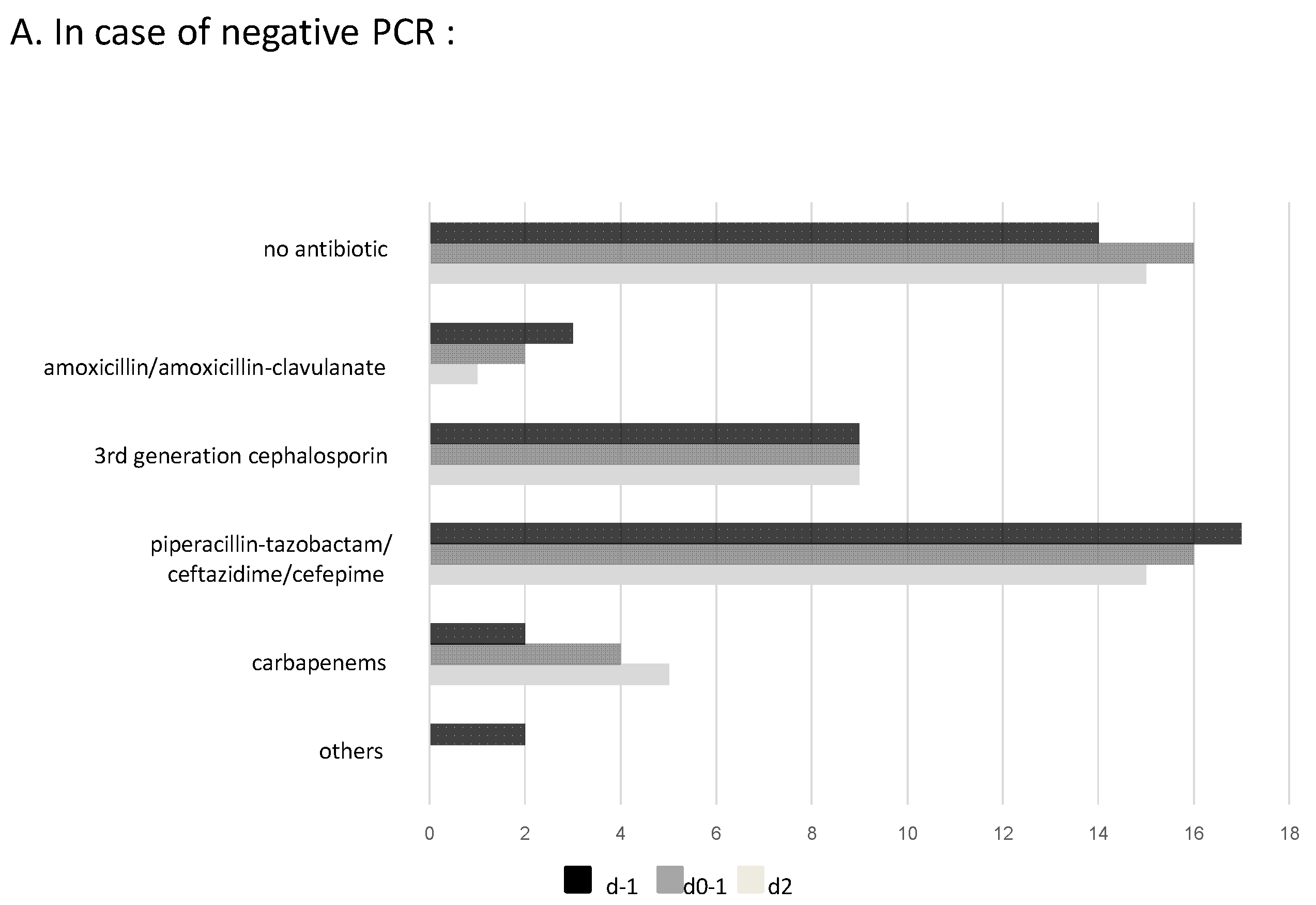
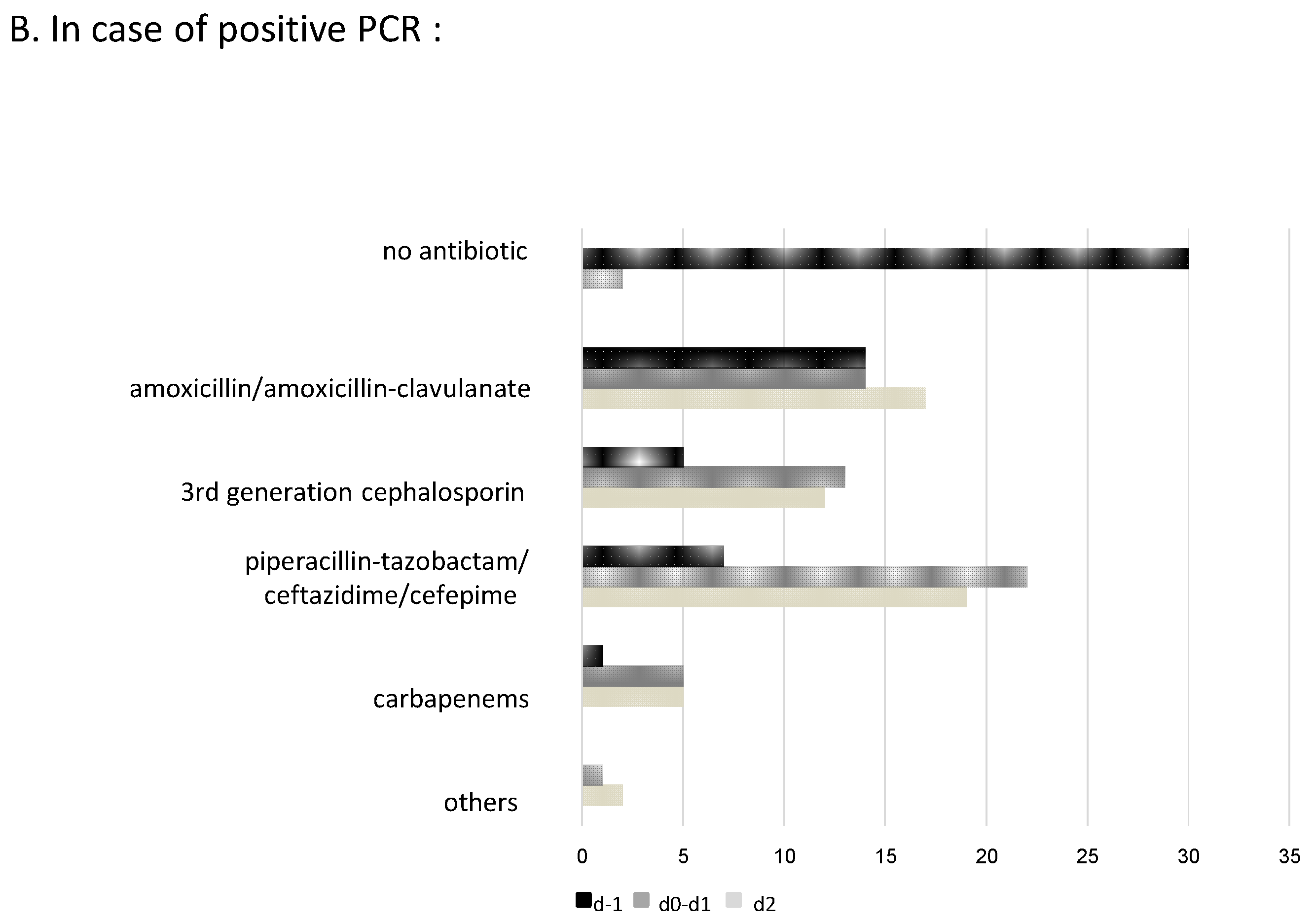
2.4. Impact of m-PCR Results on Antibiotic Prescription and Additional Modifications after Culture Results
2.5. Factors Associated with Appropriate Antibiotic Strategy after m-PCR Results
3. Discussion
4. Patients and Methods
4.1. Setting and Study Population
4.2. Microbiological Analysis
4.3. Data Collection
4.4. Endpoints
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F.; et al. International nosocomial infection control consortium report, data summary of 50 countries for 2010-2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Ohannessian, R.; Gustin, M.-P.; Bénet, T.; Gerbier-Colomban, S.; Girard, R.; Argaud, L.; Rimmelé, T.; Guerin, C.; Bohé, J.; Piriou, V.; et al. Estimation of extra length of stay attribuable to hospital-acquired infections in adult ICUs using a time-dependent multistate model. Crit. Care Med. 2018, 46, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attribuable mortality of ventilator-associated pneumonia: Ameta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Jorda, A.; Gabler, C.; Blaschke, A.; Wölfl-Duchek, M.; Gelbenegger, G.; Nussbaumer-Pröll, A.; Radtke, C.; Zeitlinger, M.; Bergmann, F. Community-acquired and hospital-acquired bacterial co-infections in patients hospitalized with COVID-19 or influenza: A retrospective cohort study. Infection, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Nseir, S.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Makris, D.; Geronimi, C.B.; et al. Relationship between ventilator-associated pneumonia and mortality in COVID-19 patients: A planned ancillary analysis of the coVAPid cohort. Crit. Care 2021, 25, 177. [Google Scholar] [CrossRef]
- Kalil, A.C.; Cawcutt, K.A. Is ventilator-associated pneumonia more frequent in patients with coronavirus disease 2019? Crit. Care Med. 2021, 50, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, M.; Plata-Menchaca, E.P.; Nuvials, F.X.; Roca, O.; Ferrer, R. Risk factors and outcomes of ventilator-associated pneumonia in COVID-19 patients: A propensity score matched analysis. Crit. Care 2021, 25, 235. [Google Scholar] [CrossRef]
- Cour, M.; Simon, M.; Argaud, L.; Monneret, G.; Venet, F. Effects of dexamethasone on immune dysfunction and ventilator-associated pneumonia in COVID-19 acute respiratory distress syndrome: An observational study. J. Intensive Care 2021, 9, 64. [Google Scholar] [CrossRef]
- Leroy, O.; Meybeck, A.; d’Escrivan, T.; Devos, P.; Kipnis, E.; Georges, H. Impact of adequacy of initial antimicrobial therapy on prognosis of patients with ventilator-associated pneumonia. Intensive Care Med. 2003, 29, 2170–2173. [Google Scholar] [CrossRef]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumoniain adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, J.; Panigada, M.; Klompas, M.; Berra, L. Ventilator-associated pneumonia among SARS-CoV-2 acute respiratory distress syndrome patients. Curr. Opin. Crit. Care 2022, 28, 74–82. [Google Scholar] [CrossRef]
- François, B.; Laterre, P.F.; Luyt, C.E.; Chastre, J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit. Care 2020, 24, 289. [Google Scholar] [CrossRef] [PubMed]
- Peiffer-Smadja, N.; Bouadma, L.; Mathy, V.; Allouche, K.; Patrier, J.; Reboul, M.; Montravers, P.; Timsit, J.-F.; Armand-Lefevre, L. Performance and impact of a multiplex PCR in ICU patients with ventilator-associated pneumonia or ventilated hospital-acquired pneumonia. Crit. Care 2020, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Maataoui, N.; Chemali, L.; Patrier, J.; Dinh, A.T.; Le Fèvre, L.; Lortat-Jacob, B.; Marzouk, M.; D’Humières, C.; Rondinaud, E.; Ruppé, E.; et al. Impact of rapid multiplex PCR on management of antibiotic therapy in COVID-19-positive patients hospitalized in intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Crémet, L.; Gaborit, B.; Bouras, M.; Drumel, T.; Guillotin, F.; Poulain, C.; Persyn, E.; Lakhal, K.; Rozec, B.; Vibet, M.-A.; et al. Evaluation of the FilmArray® Pneumonia Plus Panel for rapid diagnosis of hospital-acquired pneumonia in intensive care unit patients. Front. Microbiol. 2020, 11, 2080. [Google Scholar] [CrossRef]
- Murphy, C.N.; Fowler, R.; Balada-Llasat, J.M.; Carroll, A.; Stone, H.; Akerele, O.; Buchan, B.; Windham, S.; Hopp, A.; Ronen, S.; et al. Multicenter evaluation of the BioFire FilmArray Pneumonia/Pneumonia Plus Panel for detection and quantification of agents of lower respiratory tract infection. J. Clin. Microbiol. 2020, 58, e00128-20. [Google Scholar] [CrossRef]
- Buchan, B.W.; Windham, S.; Balada-Llasat, J.-M.; Leber, A.; Harrington, A.; Relich, R.; Murphy, C.; Bard, J.D.; Naccache, S.; Ronen, S.; et al. Practical comparison of the BioFire FilmArray Pneumonia Panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adulthospitalized patients with lower respiratory tract infections. J. Clin. Microbiol. 2020, 58, e00135-20. [Google Scholar] [CrossRef]
- Monard, C.; Pehlivan, J.; Auger, G.; Alviset, S.; Tran Dinh, A.; Duquaire, P.; Gastli, N.; d’Humières, C.; Maamar, A.; Boibieux, A.; et al. Multicenter evaluation of a syndromicrapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit. Care 2020, 24, 434. [Google Scholar] [CrossRef]
- Paz, V.; D’Agostino, M.L.; Garibaldi, F.; Orellana, R.; Paniagua, M.; Santillan, A. Multiplex PCR in the empirical antibiotic treatment of patients with SARS-CoV-2 and bacterial respiratory superinfection. Infect. Prev. Pr. 2022, 4, 100227. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ruan, S.Y.; Pan, S.C.; Lee, T.F.; Chien, J.Y.; Hsueh, P.R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Sansot, M.; Fradin, E.; Chenouard, R.; Kempf, M.; Kouatchet, A.; Lasocki, S.; Lemarié, C.; Eveillard, M.; Pailhoriès, H. Performance of the extended use of the FilmArray® BCID panel kit for bronchoalveolar lavage analysis. Mol. Biol. Rep. 2019, 46, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Clark, T.W. Rapid syndromic molecular testing in pneumonia: The current landscape and future potential. J. Infect. 2019, 80, 1–7. [Google Scholar] [CrossRef]
- Tellapragada, C.; Ydsten, K.A.; Ternhag, A.; Giske, C.G. Evaluation of a pneumonia multiplex PCR panel for detection of bacterial respiratory tract pathogens from serial specimens collected from hospitalized COVID-19 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1093–1098. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Guillotin, F.; Poulain, C.; Gaborit, B.; Bouras, M.; Cinotti, R.; Lakhal, K.; Vourc, M.; Rozec, B.; Asehnoune, K.; Vibet, M.-A.; et al. Potential impact of rapid multiplex PCR on antimicrobial therapy guidance for ventilated hospital-acquired pneumonia in critically ill patients, a prospective observationnal clinical and economic Study. Front. Cell Infect. Microbiol. 2022, 12, 804611. [Google Scholar] [CrossRef] [PubMed]
- Erich, B.J.; Kilic, A.; Palavecino, E.; Williamson, J.; Johnson, J.; Ohl, C.; Luther, V.; Beardsley, J. Evaluation of the potential impact of a multiplex rapid diagnostic panel in critically ill patients with hospital-acquired pneumonia. Cureus 2022, 14, e21716. [Google Scholar] [CrossRef]
- Tabah, A.; Cotta, M.O.; Garnacho-Montero, J.; Schouten, J.; Roberts, J.A.; Lipman, J.; Tacey, M.; Timsit, J.-F.; Leone, M.; Zahar, J.R.; et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin. Infect. Dis. 2016, 62, 1009–1017. [Google Scholar] [CrossRef]
- Darie, A.M.; Khanna, N.; Jahn, K.; Osthoff, M.; Bassetti, S.; Osthoff, M.; Schumann, D.M.; Albrich, W.C.; Hirsch, H.; Brutsche, M.; et al. Fast multiplex bacterial PCR of bronchoalveolar lavage for antibiotic stewardship in hospitalised patients with pneumonia at risk of Gram-negative bacterial infection (Flagship II): A multicentre, randomised, controlled trial. Lancet Respir. Med. 2022, 10, 877–887. [Google Scholar] [CrossRef]
- Posteraro, B.; Cortazzo, V.; Liotti, F.M.; Menchinelli, G.; Ippoliti, C.; De Angelis, G.; La Sorda, M.; Capalbo, G.; Vargas, J.; Antonelli, M.; et al. Diagnosis and treatment of bacterial pneumonia in critically ill patients with COVID-19 using a multiplex PCR assay: A large italian hospital’s five-month experience. Microbiol. Spectr. 2021, 9, e0069521. [Google Scholar] [CrossRef] [PubMed]
- Fartoukh, M.; Nseir, S.; Mégarbane, B.; Cohen, Y.; Lafarge, A.; Contou, D.; Thille, A.W.; Galerneau, L.-M.; Reizine, F.; Cour, M.; et al. Respiratory multiplex PCR and procalcitonin to reduce antibiotic exposure in severe SARS-CoV-2 pneumonia: A multicentre randomized controlled trial. Clin. Microbiol. Infect. 2023, 29, 734–743. [Google Scholar] [CrossRef] [PubMed]
- De Bus, L.; Depuydt, P.; Steen, J.; Dhaese, S.; De Smet, K.; Tabah, A.; Akova, M.; Cotta, M.O.; De Pascale, G.; Dimopoulos, G.; et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: The DIANA study. Intensive Care Med. 2020, 46, 1404–14177. [Google Scholar] [CrossRef] [PubMed]
- Novy, E.; Goury, A.; Thivilier, C.; Guillard, T. Algorithm for rational use of Film Array Pneumonia Panel in bacterial coinfections of critically ill ventilated COVID-19 patients. Diagn. Microbiol. Infect. Dis. 2021, 101, 115507. [Google Scholar] [CrossRef] [PubMed]
- Recommendations 2019 by the European Committee on Antimicrobial Susceptibility Reading EUCAST. Available online: https://www.sfm-microbiologieorg/wp-content/uploads/2019/02/CASFM2019_v1.0.pdf (accessed on 16 December 2022).
- Weiss, E.; Zahar, J.-R.; Lesprit, P.; Ruppe, E.; Leone, M.; Chastre, J.; Lucet, J.-C.; Paugam-Burtz, C.; Brun-Buisson, C.; Timsit, J.-F.; et al. Elaboration of a consensual definition of de-escalation allowing a ranking of ß-lactams. Clin. Microbiol. Infect. 2015, 21, 649.e1–649.e10. [Google Scholar] [CrossRef]
| Patients Characteristics | Data |
|---|---|
| Demographic characteristics | |
| Sexe (male) | 71 (68%) |
| Age (years) | 62 ± 11 |
| Comorbidities | |
| diabetes | 43 (41%) |
| arterial hypertension | 46 (43%) |
| respiratory chronic insufficiency | 24 (23%) |
| renal chronic insufficiency | 10 (9%) |
| cancer | 5 (5%) |
| hemopathy | 16 (15%) |
| immunodepression | 22 (21%) |
| Clinical characteristics on the day of the PCR test | |
| SARS-CoV-2 infection | 76 (73%) |
| SAPS II | 49 ± 21 |
| receiving antibiotics | 90 (87%) |
| septic shock | 71 (67%) |
| PaO2/FiO2 | 150 ± 69 |
| duration of mechanical ventilation (days) | 5.5 ± 7.2 |
| Microbiological diagnostic | |
| ETA | 85 (82%) |
| BAL | 19 (18%) |
| delay of PCR results (hours) | 17.8 ± 14.6 |
| positive PCR | 57 (55%) |
| positive culture | 39 (38%) |
| Outcome | |
| total duration of stay in ICU (days) | 35 ± 39 |
| Deaths in ICU | 56 (54%) |
| Microorganisms | Identified by PCR n = 104 | Isolated by Culture n = 104 |
|---|---|---|
| Gram-positive | ||
| S. aureus | 22 (21%) | 8 (8%) |
| S. pneumoniae | 5 (5%) | 2 (2%) |
| S. agalactiae | 1 (1%) | 0 |
| E. faecalis | 0 | 1 (1%) |
| Gram-negative | ||
| H. influenzae | 14 (13%) | 1 (1%) |
| E. coli | 12 (12%) | 6 (6%) |
| E. aerogenes | 10 (10%) | 7 (7%) |
| P. Aeruginosa | 9 (9%) | 6 (6%) |
| K. Pneumoniae | 6 (6%) | 5 (5%) |
| M. catarrhalis | 4 (4%) | 0 |
| Proteus spp. | 4 (4%) | 2 (2%) |
| E. cloacae | 3 (3%) | 2 (2%) |
| S. marcescens | 1 (1%) | 0 |
| K. oxytoca | 1 (1%) | 1 (1%) |
| H. alvei | 0 | 4 (4%) |
| S. maltophilia | 0 | 2 (2%) |
| M. morganii | 0 | 1 (1%) |
| Fungi | ||
| Aspergillus spp. | 0 | 4 (4%) |
| All Cultures (ETA and BAL) | ||||
|---|---|---|---|---|
| All m-PCR (ETA and BAL) | positive | negative | ||
| positive | 14 | 34 | 48 | |
| negative | 14 | 42 | 56 | |
| 28 | 76 | 104 | ||
| Cultures in ETA | ||||
| m-PCR in ETA | positive | negative | ||
| positive | 12 | 30 | 42 | |
| negative | 9 | 34 | 43 | |
| 21 | 64 | 85 | ||
| Results of PCR n = 104 | Appropriateness of Antibiotic Strategy | Number (%) |
|---|---|---|
| Negative PCR n = 47 | Appropriate strategy | 16/47 (34%) |
| Antibiotics discontinuation | 8/47 (17%) | |
| Lack of antibiotic initiation | 8/47 (17%) | |
| Positive PCR n = 57 | Appropriate strategy | 51/57 (89%) |
| Appropriate initiation | 33/57 (58%) | |
| Appropriate escalation | 9/57 (16%) | |
| Appropriate de-escalation | 7/57 (12%) | |
| Optimization | 2/57 (4%) |
| Variables | Factors Associated with an Appropriate Antibiotic Strategy | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| OR [CI95%] | p | OR [CI95%] | p | |
| Sexe (Men) | 2.43 [1.05–5.70] | 0.1 | 1.29 [0.31–6.27] | 0.72 |
| Age | 1.00 [0.96–1.04] | 0.93 | ||
| BMI | 0.98 [0.91–1.06] | 0.67 | ||
| Diabetes | 0.55 [0.24–1.22] | 0.18 | 0.69 [0.18–2.62] | 0.58 |
| Arterial hypertension | 0.92 [0.41–2.06] | 0.84 | ||
| Chronic respiratory insufficiency | 3.54 [1.21–13.01] | 0.05 | 3.73 [0.74–22.79] | 0.12 |
| Chronic renal insufficiency | 5.64 [1.00–106.22] | 0.09 | 9.93 [0.84–303.52] | 0.10 |
| Cancer | 0.83 [0.13–0.85] | 1 | ||
| Hemopathy | 0.32 [0.11–0.92] | 0.11 | 0.18 [0.01–2.31] | 0.19 |
| Immunodepression | 0.33 [0.13–0.85] | 0.07 | 0.59 [0.07–4.66] | 0.62 |
| Antibiotic allergy | 0.55 [0.06–4.70] | 0.61 | ||
| COVID + | 0.80 [0.31–1.96] | 0.83 | ||
| Type of pneumonia | ||||
| CAP | Ref. | 0.3 | ||
| VAP | 2.33 [0.73–7.53] | |||
| HAP | 1.67 [0.96–1.00] | |||
| SAPS II | 0.98 [0.96–1.00] | 0.03 | 0.96 [0.93–1.00] | 0.035 * |
| Septic shock | 0.75 [0.31–1.74] | 0.68 | ||
| Receiving antibiotics | 0.23 [0.08–0.56] | <0.01 | 0.38 [0.09–1.47] | 0.17 |
| PaO2/FiO2 | 1.0 [0.99–1.00] | 0.58 | 1.00 [0.99–1.02] | 0.34 |
| Sampling | ||||
| ETA | Ref. | 0.89 | ||
| BAL | 1.15 [0.43–3.31] | |||
| Positive m-PCR | 17.33 [6.52–63.09] | <0.01 | 107.4 [16.04–1727.65] | <0.01 * |
| Ventilation duration | 1.03 [0.97–1.10] | 0.34 | 1.02 [0.93–1.13] | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambe, E.; Bortolotti, P.; Diesnis, R.; Laurans, C.; Héquette-Ruz, R.; Panaget, S.; Herbecq, P.; Vachée, A.; Meybeck, A. Performance and Impact on Antibiotic Prescriptions of a Multiplex PCR in a Real-Life Cohort of Critically Ill Patients with Suspected Ventilated Pneumonia: A Retrospective Monocentric Observational Study. Antibiotics 2023, 12, 1646. https://doi.org/10.3390/antibiotics12121646
Chambe E, Bortolotti P, Diesnis R, Laurans C, Héquette-Ruz R, Panaget S, Herbecq P, Vachée A, Meybeck A. Performance and Impact on Antibiotic Prescriptions of a Multiplex PCR in a Real-Life Cohort of Critically Ill Patients with Suspected Ventilated Pneumonia: A Retrospective Monocentric Observational Study. Antibiotics. 2023; 12(12):1646. https://doi.org/10.3390/antibiotics12121646
Chicago/Turabian StyleChambe, Emma, Perrine Bortolotti, Rémy Diesnis, Caroline Laurans, Rozenn Héquette-Ruz, Sophie Panaget, Patrick Herbecq, Anne Vachée, and Agnès Meybeck. 2023. "Performance and Impact on Antibiotic Prescriptions of a Multiplex PCR in a Real-Life Cohort of Critically Ill Patients with Suspected Ventilated Pneumonia: A Retrospective Monocentric Observational Study" Antibiotics 12, no. 12: 1646. https://doi.org/10.3390/antibiotics12121646






