Preparation of Laser-Ablated Ag Nanoparticle–MMT Clay-Based Beeswax Antibiofilm Coating
Abstract
:1. Introduction
2. Results and Discussion
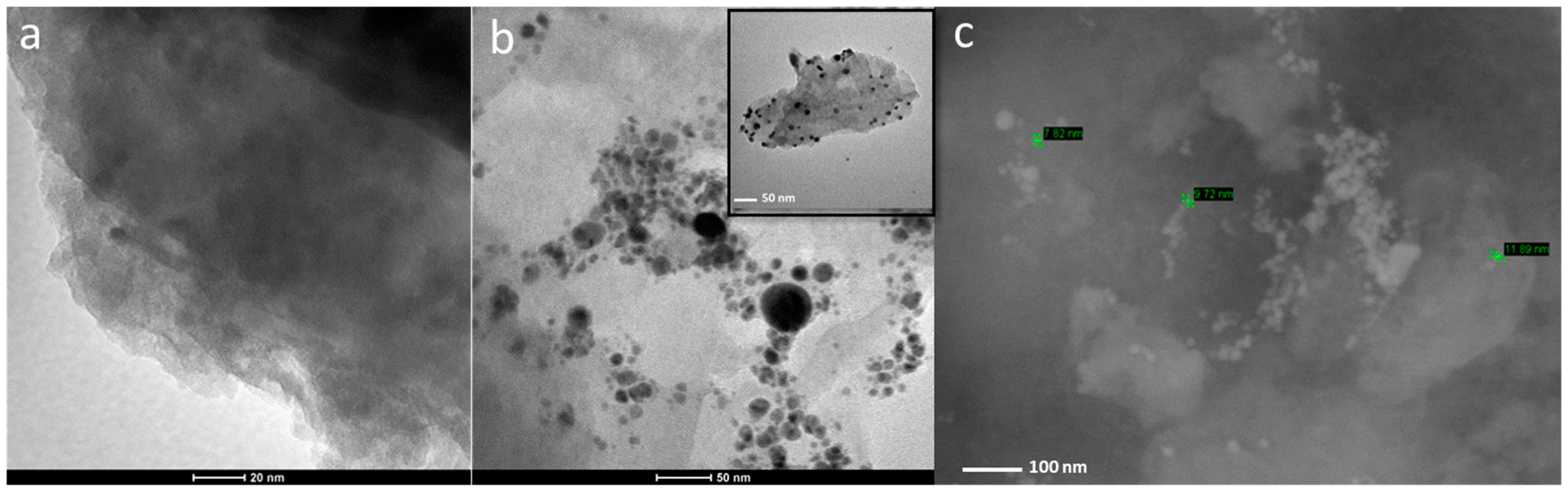
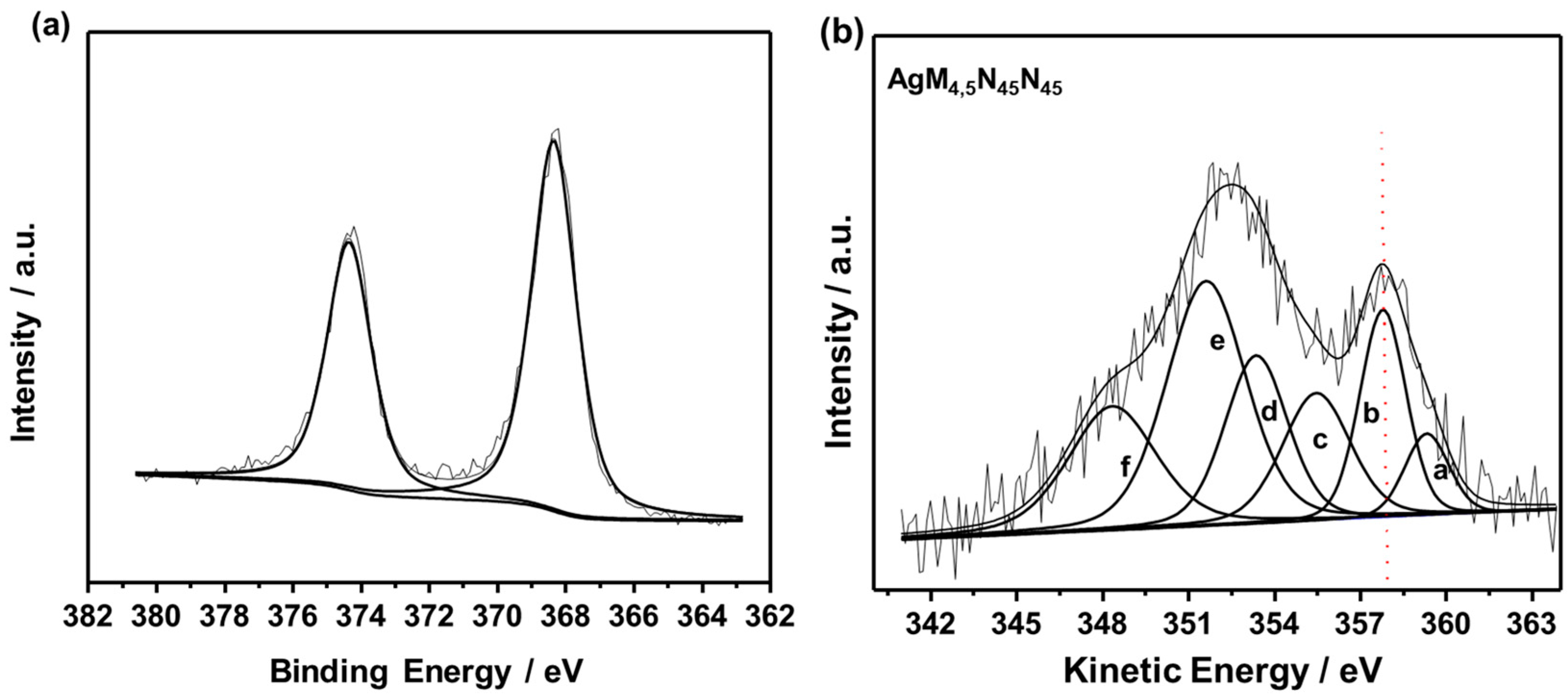
2.1. Characterization of L-Ag/MMT and L-Ag/MMT/Beeswax
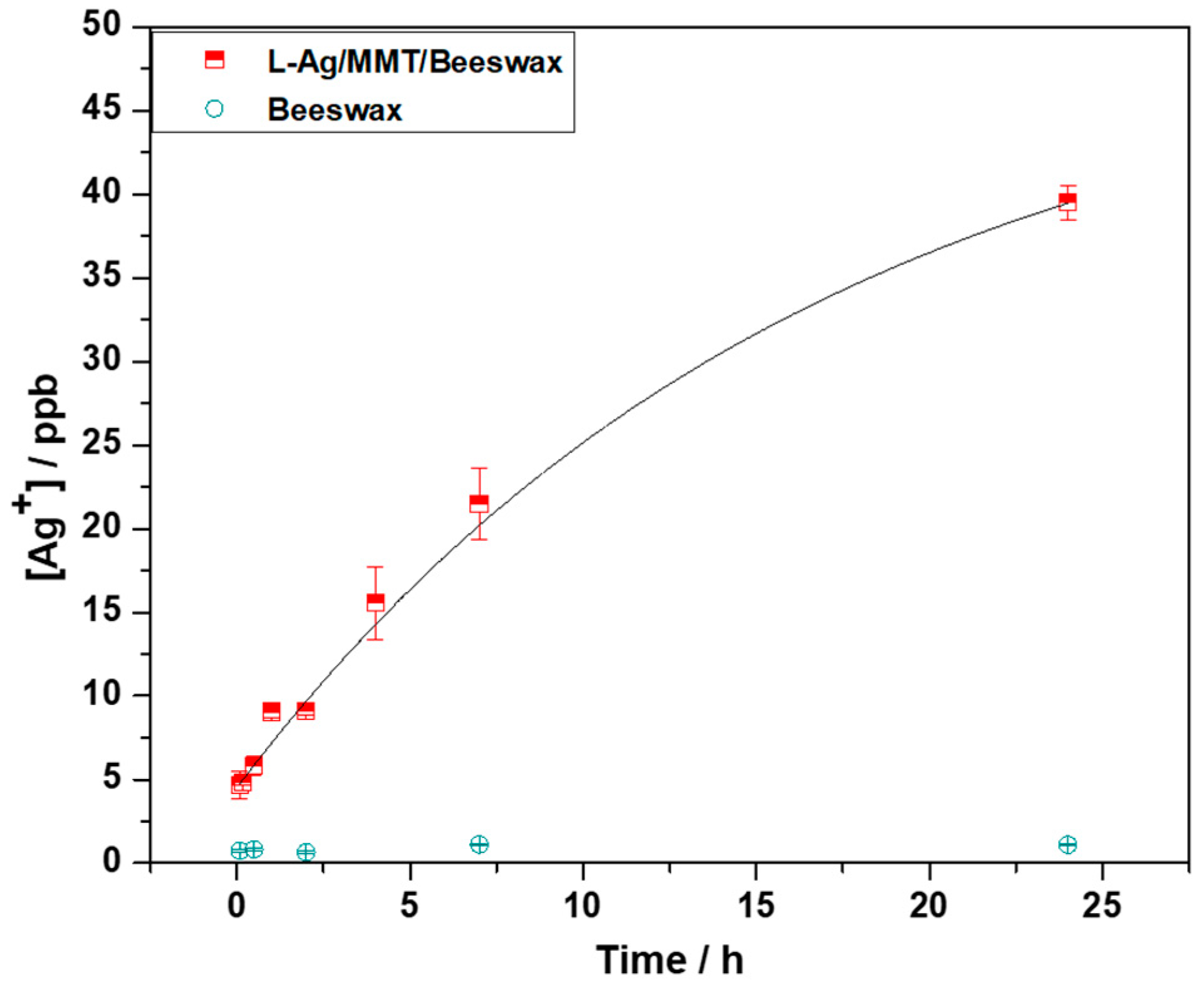
2.2. Kinetics of Silver Release
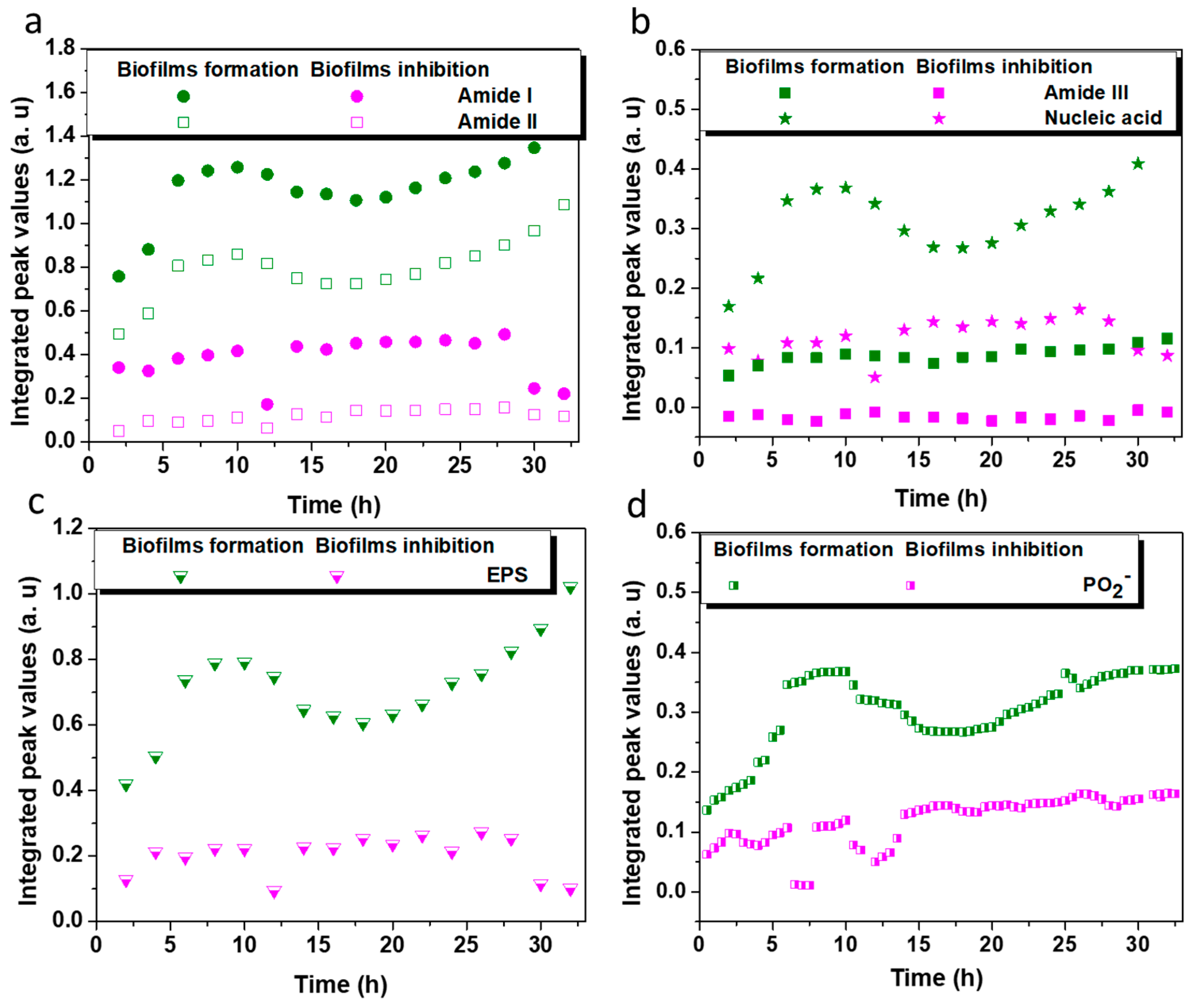
2.3. Biofilm Inhibition Efficiency on the Coated ZnSe Surface
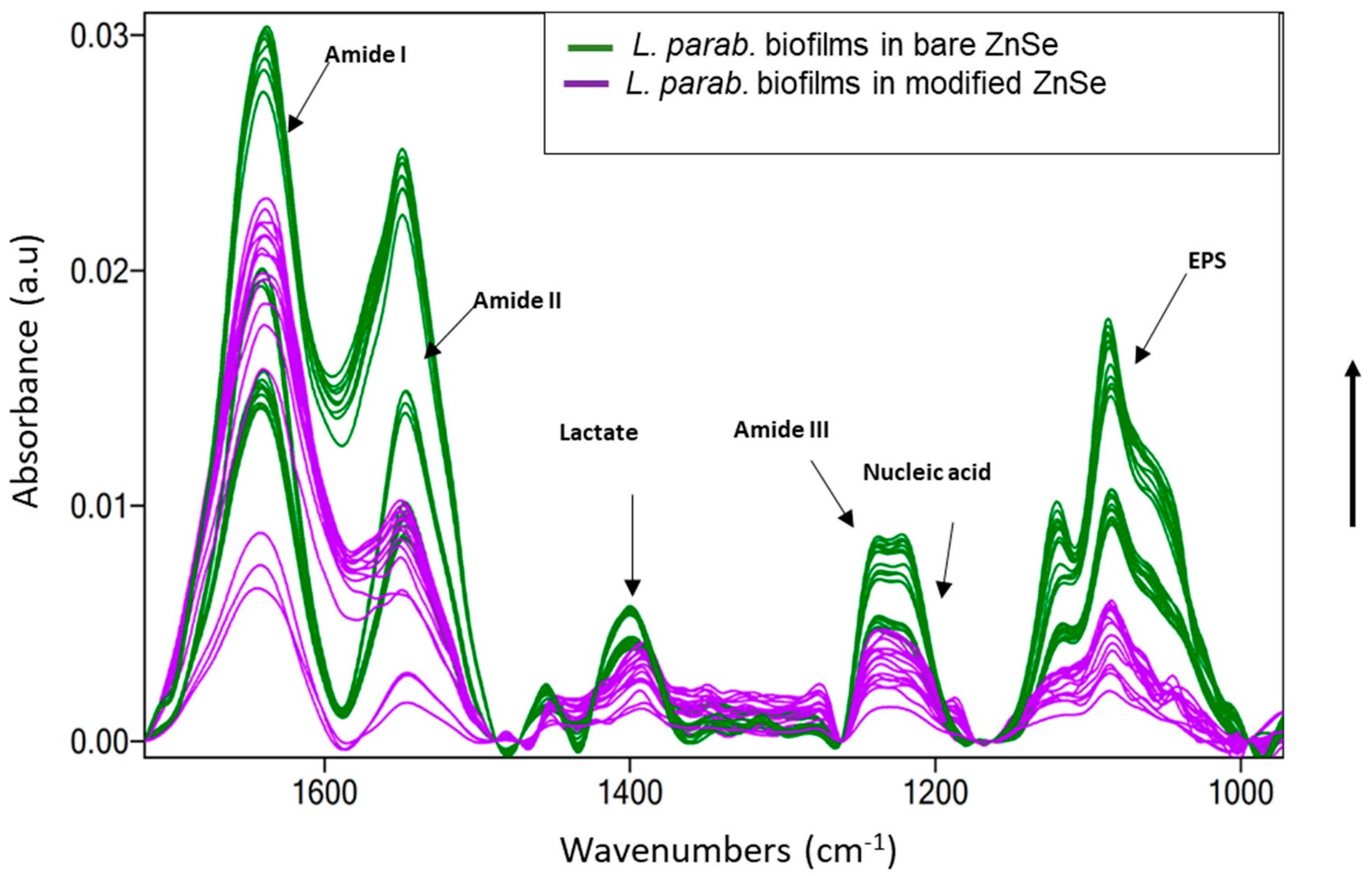
2.4. Optical Density Measurement and Microscopy on L. parabuchneri Biofilms
3. Materials and Methods
3.1. Materials
3.2. Preparation of L-Ag NPs, L-Ag/MMT Nanocomposite, and L-Ag/MMT/Beeswax Coating
3.3. TEM and SEM Morphological Characterization
3.4. XPS and UV–Vis Characterization
3.5. Determination of Silver Release
3.6. Bacterial Growth and Culture Conditions
3.7. Modification of ZnSe Waveguide in Inactive Spots
3.8. In Situ FTIR-ATR Spectroscopic Strategy as a Biofilm Inhibition Study
3.9. Biofilms Grown on Silicon Wafer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance of Antimicrobial Resistance in Europe 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2018 (accessed on 24 March 2022).
- Global Action Plan on Antimicrobial Resistance. Available online: https://apo.who.int/publications/i/item/global-action-plan-on-antimicrobial-resistance (accessed on 6 February 2022).
- Diaz, M.; Ladero, V.; Redruello, B.; Sanchez-Llana, E.; del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. A PCR-DGGE Method for the Identification of Histamine-Producing Bacteria in Cheese. Food Control 2016, 63, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; del Rio, B.; Sanchez-Llana, E.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Histamine-Producing Lactobacillus parabuchneri Strains Isolated from Grated Cheese Can Form Biofilms on Stainless Steel. Food Microbiol. 2016, 59, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Møller,, C.O.d.A.; Castro-Mejía, J.L.; Krych, L.; Rattray, F.P. Histamine-Forming Ability of Lentilactobacillus parabuchneri in Reduced Salt Cheddar Cheese. Food Microbiol. 2021, 98, 103789. [Google Scholar] [CrossRef]
- Bajrami, D.; Fischer, S.; Barth, H.; Hossain, S.I.; Cioffi, N.; Mizaikoff, B. Antimicrobial Efficiency of Chitosan and Its Methylated Derivative against Lentilactobacillus parabuchneri Biofilms. Molecules 2022, 27, 8647. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, R.; Pino, C.; Henríquez, H.; Villanueva, F.; Riquelme, A.; Montealegre, R.; Agostini, D.; Batista-González, A.; Leiva, G.; Contreras, R.A. Elucidation of Antimicrobial and Antioxidant Activities of Selected Plant-Based Mayonnaise-Derived Essential Oils against Lactic Acid Bacteria. J. Food Pro-Cessing Preserv. 2022, 46, e16339. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Nguyen, T.V.; Chu, A.D.; Tran, H.V.; Tran, L.T.; Huynh, C.D. A Label-Free Colorimetric Sensor Based on Silver Nanoparticles Directed to Hydrogen Peroxide and Glucose. Arab. J. Chem. 2018, 11, 1134–1143. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- López-Lorente, A.I.; Picca, R.A.; Izquierdo, J.; Kranz, C.; Mizaikoff, B.; Di Franco, C.; Cárdenas, S.; Cioffi, N.; Palazzo, G.; Valentini, A. Ion Beam Sputtering Deposition of Silver Nanoparticles and TiOx/ZnO Nanocomposites for Use in Surface Enhanced Vibrational Spectroscopy (SERS and SEIRAS). Microchim. Acta 2018, 185, 153. [Google Scholar] [CrossRef]
- Hossain, S.I.; Aziz, M.A.; Han, D.; Selvam, P.; Shanmugam, S. Fabrication of SPAEK–Cerium Zirconium Oxide Nanotube Composite Membrane with Outstanding Performance and Durability for Vanadium Redox Flow Batteries. J. Mater. Chem. A 2018, 6, 20205–20213. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Cioffi, N. Novel Polyethylene Oxide Coatings Implementing Ultra-Stable Laser-Ablated Silver Nanoparticles. Appl. Surf. Sci. 2020, 507, 145156. [Google Scholar] [CrossRef]
- Sun, G.; Jia, S.; Zhang, X.; Kang, Z.; Cui, M.; Wang, B.; Wang, B.; Yang, D.-P. Anchoring Core–Shell Cu@Cu2O Nanoparticles to Two-Dimensional Carbon Nanosheets for Bacterial Disinfection. ACS Appl. Nano Mater. 2021, 4, 9831–9841. [Google Scholar] [CrossRef]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial Silver Resistance Mechanisms: Recent Developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Kvitek, L.; Panacek, A.; Prucek, R.; Hrbac, J.; Vecerova, R.; Zboril, R. Comparative Study of Antimicrobial Activity of AgBr and Ag Nanoparticles (NPs). PLoS ONE 2015, 10, e0119202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Crit. Rev. Nanomater. 2021, 11, 1687. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Ronco, R.; Bonerba, E.; Tantillo, G.; Pollini, M.; Sannino, A.; Valentini, A.; Cataldi, T.R.I.; Cioffi, N. Investigation of Industrial Polyurethane Foams Modified with Antimicrobial Copper Nanoparticles. Materials 2016, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Makvandi, P.; Wang, C.; Zare, E.N.; Borzacchiello, A.; Niu, L.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally Stable Silver Nanoparticles Synthesized by Laser Ablation in Alcoholic Organic Solvent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 148–158. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Dermatas, D.; Dadachov, M.S. Rietveld Quantification of Montmorillonites in Lead-Contaminated Soils. Appl. Clay Sci. 2003, 23, 245–255. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay Minerals and Their Beneficial Effects upon Human Health. A Review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Özdemir, G.; Limoncu, M.H.; Yapar, S. The Antibacterial Effect of Heavy Metal and Cetylpridinium-Exchanged Montmorillonites. Appl. Clay Sci. 2010, 48, 319–323. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. (Eds.) Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-099371-3. [Google Scholar]
- Tulloch, A.P. Beeswax—Composition and Analysis. Bee World 1980, 61, 47–62. [Google Scholar] [CrossRef]
- Pamplona-Zomenhan, L.C.; Pamplona, B.C.; da Silva, C.B.; Marcucci, M.C.; Mimica, L.M.J. Evaluation of the in vitro antimicrobial activity of an ethanol extract of Brazilian classified propolis on strains of Staphylococcus aureus. Braz. J. Microbiol. 2011, 42, 1259–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A Minireview of Its Antimicrobial Activity and Its Application in Medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; Talens, P.; Chiralt, A. Tensile Properties and Water Vapor Permeability of Sodium Caseinate Films Containing Oleic Acid–Beeswax Mixtures. J. Food Eng. 2008, 85, 393–400. [Google Scholar] [CrossRef]
- Jafari, S.M.; Khanzadi, M.; Mirzaei, H.; Dehnad, D.; Chegini, F.K.; Maghsoudlou, Y. Hydrophobicity, Thermal and Micro-Structural Properties of Whey Protein Concentrate–Pullulan–Beeswax Films. Int. J. Biol. Macromol. 2015, 80, 506–511. [Google Scholar] [CrossRef]
- Stenclova, P.; Freisinger, S.; Barth, H.; Kromka, A.; Mizaikoff, B. Cyclic Changes in the Amide Bands Within Escherichia Coli Biofilms Monitored Using Real-Time Infrared Attenuated Total Reflection Spectroscopy (IR-ATR). Appl. Spectrosc. 2019, 73, 424–432. [Google Scholar] [CrossRef]
- Anicuta, S.G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier Transform Infrared (FTIR) Spectroscopy for Characterization of Antimicrobial Films Containing Chitosan. Analele Universității din Oradea, Fascicula: Ecotoxicologie, Zootehnie și Tehnologii de Industrie Alimentară 2010, pp. 815–822. Available online: https://www.cabdirect.org/cabdirect/abstract/20113173121 (accessed on 31 December 2022).
- Sportelli, M.C.; Kranz, C.; Mizaikoff, B.; Cioffi, N. Recent Advances on the Spectroscopic Characterization of Microbial Biofilms: A Critical Review. Anal. Chim. Acta 2022, 1195, 339433. [Google Scholar] [CrossRef]
- Lorite, G.S.; de Souza, A.A.; Neubauer, D.; Mizaikoff, B.; Kranz, C.; Cotta, M.A. On the Role of Extracellular Polymeric Substances during Early Stages of Xylella Fastidiosa Biofilm Formation. Colloids Surf. B Biointerfaces 2013, 102, 519–525. [Google Scholar] [CrossRef]
- Bazán, J.C.; García, N.J.; Dristas, J.A.; Spetter, C.V. Ionic Conductivity in Montmorillonite-Doped Silver Iodide. Solid State Ion. 2004, 170, 57–61. [Google Scholar] [CrossRef]
- Giovannini, G.; Garoli, D.; Rupper, P.; Neels, A.; Rossi, R.M.; Boesel, L.F. Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection. Sensors 2021, 21, 2655. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S.; Yoshimura, K. Wavelength Tuning of Surface Plasmon Resonance Using Dielectric Layers on Silver Island Films. Appl. Phys. Lett. 2003, 82, 3811–3813. [Google Scholar] [CrossRef]
- Beck, F.J.; Polman, A.; Catchpole, K.R. Tunable Light Trapping for Solar Cells Using Localized Surface Plasmons. J. Appl. Phys. 2009, 105, 114310. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Choi, H.; Kim, J.Y.; Lee, T.-W. Silver-Based Nanoparticles for Surface Plasmon Resonance in Organic Optoelectronics. Part. Part. Syst. Charact. 2015, 32, 164–175. [Google Scholar] [CrossRef]
- Bera, S.; Gangopadhyay, P.; Nair, K.G.M.; Panigrahi, B.K.; Narasimhan, S.V. Electron Spectroscopic Analysis of Silver Nanoparticles in a Soda-Glass Matrix. J. Electron. Spectrosc. Relat. Phenom. 2006, 152, 91–95. [Google Scholar] [CrossRef]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface Chemical and Biological Characterization of Flax Fabrics Modified with Silver Nanoparticles for Biomedical Applications. Mater. Sci. Eng. C 2015, 52, 1–10. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)Control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.; Kim, J.D.; Choi, H.C.; Lee, C.W. Antibacterial Activity of CNT-Ag and GO-Ag Nanocomposites Against Gram-Negative and Gram-Positive Bacteria. Bull. Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar] [CrossRef] [Green Version]
- Ediriweera, E.R.H.S.; Premarathna, N.Y. Medicinal and Cosmetic Uses of Bee’s Honey—A Review. AYU 2012, 33, 178–182. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Romero-Gonzalez, M.E.; Pouran, H.M.; Banwart, S.A. In Situ Monitoring of the Biofilm Formation of Pseudomonas Putida on Hematite Using Flow-Cell ATR-FTIR Spectroscopy to Investigate the Formation of Inner-Sphere Bonds between the Bacteria and the Mineral. Mineral. Mag. 2008, 72, 101–106. [Google Scholar] [CrossRef]
- Pousti, M.; Joly, M.; Roberge, P.; Amirdehi, M.A.; Bégin-Drolet, A.; Greener, J. Linear Scanning ATR-FTIR for Chemical Mapping and High-Throughput Studies of Pseudomonas sp. Anal. Chem. 2018, 90, 14475–14483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, F.; Quilès, F. In-Situ Study of Early Stages of Biofilm Formation under Different Environmental Stresses by ATR-FTIR Spectroscopy. Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; HAL Open Science, Formatex Research Center: Badajoz, Spain, 2011; pp. 889–895. [Google Scholar]
- Maquelin, K.; Kirschner, C.; Choo-Smith, L.; van den Braak, N.; Endtz, H.P.; Naumann, D.; Puppels, G. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 2002, 51, 255–271. [Google Scholar] [CrossRef]
- Yunda, E.; Alem, H.; Francius, G.; Gago, R.; Quilès, F. Chemical Functionalization of the Zinc Selenide Surface and Its Impact on Lactobacillus rhamnosus GG Biofilms. ACS Appl. Mater. Interfaces 2020, 12, 14933–14945. [Google Scholar] [CrossRef]
- Oust, A.; Møretrø, T.; Kirschner, C.; Narvhus, J.A.; Kohler, A. FT-IR Spectroscopy for Identification of Closely Related Lactobacilli. J. Microbiol. Methods 2004, 59, 149–162. [Google Scholar] [CrossRef]
- Kudrinskiy, A.A.; Ivanov, A.Y.; Kulakovskaya, E.V.; Klimov, A.I.; Zherebin, P.M.; Khodarev, D.V.; Le, A.-T.; Tam, L.T.; Lisichkin, G.V.; Krutyakov, Y.A. The Mode of Action of Silver and Silver Halides Nanoparticles against Saccharomyces Cerevisiae Cells. J. Nanoparticles 2014, 2014, 568635. [Google Scholar] [CrossRef] [Green Version]
- Ascone, P.; Maurer, J.; Haldemann, J.; Irmler, S.; Berthoud, H.; Portmann, R.; Fröhlich-Wyder, M.T.; Wechsler, D. Prevalence and Diversity of Histamine-Forming Lactobacillus Parabuchneri Strains in Raw Milk and Cheese—A Case Study. Int. Dairy J. 2017, 70, 26–33. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.T.; Guggisberg, D.; Badertscher, R.; Wechsler, D.; Wittwer, A.; Irmler, S. The Effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the Eye Formation of Semi-Hard Cheese. Int. Dairy J. 2013, 33, 120–128. [Google Scholar] [CrossRef]
- Bodmer, S.; Imark, C.; Kneubühl, M. Biogenic Amines in Foods: Histamine and Food Processing. Inflamm. Res. 1999, 48, 296–300. [Google Scholar] [CrossRef]
- Dinić, M.; Pecikoza, U.; Djokić, J.; Stepanović-Petrović, R.; Milenković, M.; Stevanović, M.; Filipović, N.; Begović, J.; Golić, N.; Lukić, J. Exopolysaccharide Produced by Probiotic Strain Lactobacillus Paraplantarum BGCG11 Reduces Inflammatory Hyperalgesia in Rats. Front. Pharmacol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. A Distinct Utility of the Amide III Infrared Band for Secondary Structure Estimation of Aqueous Protein Solutions Using Partial Least Squares Methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Sutherland, I.W. The Biofilm Matrix—An Immobilized but Dynamic Microbial Environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Zhang, T. Surface-Enhanced Raman Scattering (SERS) Revealing Chemical Variation during Biofilm Formation: From Initial Attachment to Mature Biofilm. Anal. Bioanal. Chem. 2012, 404, 1465–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pink, J.; Smith-Palmer, T.; Chisholm, D.; Beveridge, T.J.; Pink, D.A. An FTIR Study of Pseudomonas Aeruginosa PAO1 Biofilm Development: Interpretation of ATR-FTIR Data in the 1500-1180 Cm-1 Region. Biofilms 2005, 2, 165–175. [Google Scholar] [CrossRef]
- Badireddy, A.R.; Farner Budarz, J.; Marinakos, S.M.; Chellam, S.; Wiesner, M.R. Formation of Silver Nanoparticles in Visible Light-Illuminated Waters: Mechanism and Possible Impacts on the Persistence of AgNPs and Bacterial Lysis. Environ. Eng. Sci. 2014, 31, 338–349. [Google Scholar] [CrossRef]
- Fahs, A.; Quilès, F.; Jamal, D.; Humbert, F.; Francius, G. In Situ Analysis of Bacterial Extracellular Polymeric Substances from a Pseudomonas Fluorescens Biofilm by Combined Vibrational and Single Molecule Force Spectroscopies. J. Phys. Chem. B 2014, 118, 6702–6713. [Google Scholar] [CrossRef]
- Caniglia, G.; Sportelli, M.C.; Heinzmann, A.; Picca, R.A.; Valentini, A.; Barth, H.; Mizaikoff, B.; Cioffi, N.; Kranz, C. Silver-Fluoropolymer (Ag-CFX) Films: Kinetic Study of Silver Release, and Spectroscopic-Microscopic Insight into the Inhibition of P. Fluorescens Biofilm Formation. Anal. Chim. Acta 2022, 1212, 339892. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Dittrich, M. Fourier Transform Infrared Spectroscopy for Molecular Analysis of Microbial Cells. Methods Mol. Biol. 2012, 881, 187–211. [Google Scholar] [CrossRef]
- Schmitt, J.; Fringeli, U.P.; Flemming, H.-C. Structural and Temporal Behavior of Biofilms Investigated by FTIR-ATR Spectroscopy. AIP Conf. Proc. 1998, 430, 312. [Google Scholar] [CrossRef]
- Lorite, G.S.; Janissen, R.; Clerici, J.H.; Rodrigues, C.M.; Tomaz, J.P.; Mizaikoff, B.; Kranz, C.; de Souza, A.A.; Cotta, M.A. Surface Physicochemical Properties at the Micro and Nano Length Scales: Role on Bacterial Adhesion and Xylella Fastidiosa Biofilm Development. PLoS ONE 2013, 8, e75247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ding, S.; Wang, G.; Xu, X.; Zhou, G. In Situ Characterization and Analysis of Salmonella Biofilm Formation under Meat Processing Environments Using a Combined Microscopic and Spectroscopic Approach. Int. J. Food Microbiol. 2013, 167, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, G.T.; Mizaikoff, B. Shining New Light on Old Principles: Localization of Evanescent Field Interactions at Infrared-Attenuated Total Reflection Sensing Interfaces. Appl. Spectrosc. 2006, 60, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Bajrami, D.; Fischer, S.; Barth, H.; Sarquis, M.A.; Ladero, V.M.; Fernández, M.; Sportelli, M.C.; Cioffi, N.; Kranz, C.; Mizaikoff, B. In Situ Monitoring of Lentilactobacillus parabuchneri Biofilm Formation via Real-Time Infrared Spectroscopy. npj Biofilms Microbiomes 2022, 8, 92. [Google Scholar] [CrossRef]
- Sharma, G.; Prakash, A. Combined Use of Fourier Transform Infrared and Raman Spectroscopy To Study Planktonic and Biofilm Cells of Cronobacter Sakazakii. J. Microbiol. Biotechnol. Food Sci. 2014, 9, 310–314. [Google Scholar]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N. Inhibiting P. fluorescens Biofilms with Fluoropolymer-Embedded Silver Nanoparticles: An in-Situ Spectroscopic Study. Sci. Rep. 2017, 7, 11870. [Google Scholar] [CrossRef] [Green Version]
- McWhirter, M.J.; Bremer, P.J.; McQuillan, A.J. Direct Infrared Spectroscopic Evidence of PH- and Ionic Strength-Induced Changes in Distance of Attached Pseudomonas Aeruginosa from ZnSe Surfaces. Langmuir 2002, 18, 1904–1907. [Google Scholar] [CrossRef]



| Samples | At% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | Mg | Al | Si | Fe | S | Ag | |
| L-Ag | 92.6 ± 0.8 | 7.1 ± 0.7 | / | / | / | / | / | / | 0.3 ± 0.1 |
| L-Ag/MMT | 16 ± 5 | 55 ± 7 | 0.4 ± 0.2 | 1.0 ± 0.2 | 7.8 ± 1.8 | 17.7 ± 2.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 1.1 ± 0.3 |
| MMT | 26.4 ± 1.6 | 52.6 ± 0.6 | / | 0.6 ± 0.1 | 3.0 ± 0.2 | 17.0 ± 0.7 | 0.4 ± 0.2 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, S.I.; Bajrami, D.; Sportelli, M.C.; Picca, R.A.; Volpe, A.; Gaudiuso, C.; Ancona, A.; Gentile, L.; Palazzo, G.; Ditaranto, N.; et al. Preparation of Laser-Ablated Ag Nanoparticle–MMT Clay-Based Beeswax Antibiofilm Coating. Antibiotics 2023, 12, 194. https://doi.org/10.3390/antibiotics12020194
Hossain SI, Bajrami D, Sportelli MC, Picca RA, Volpe A, Gaudiuso C, Ancona A, Gentile L, Palazzo G, Ditaranto N, et al. Preparation of Laser-Ablated Ag Nanoparticle–MMT Clay-Based Beeswax Antibiofilm Coating. Antibiotics. 2023; 12(2):194. https://doi.org/10.3390/antibiotics12020194
Chicago/Turabian StyleHossain, Syed Imdadul, Diellza Bajrami, Maria Chiara Sportelli, Rosaria Anna Picca, Annalisa Volpe, Caterina Gaudiuso, Antonio Ancona, Luigi Gentile, Gerardo Palazzo, Nicoletta Ditaranto, and et al. 2023. "Preparation of Laser-Ablated Ag Nanoparticle–MMT Clay-Based Beeswax Antibiofilm Coating" Antibiotics 12, no. 2: 194. https://doi.org/10.3390/antibiotics12020194

















