Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Citric Peel Extract
2.3. Preparation of Silver Nanoparticles (AgNPs)
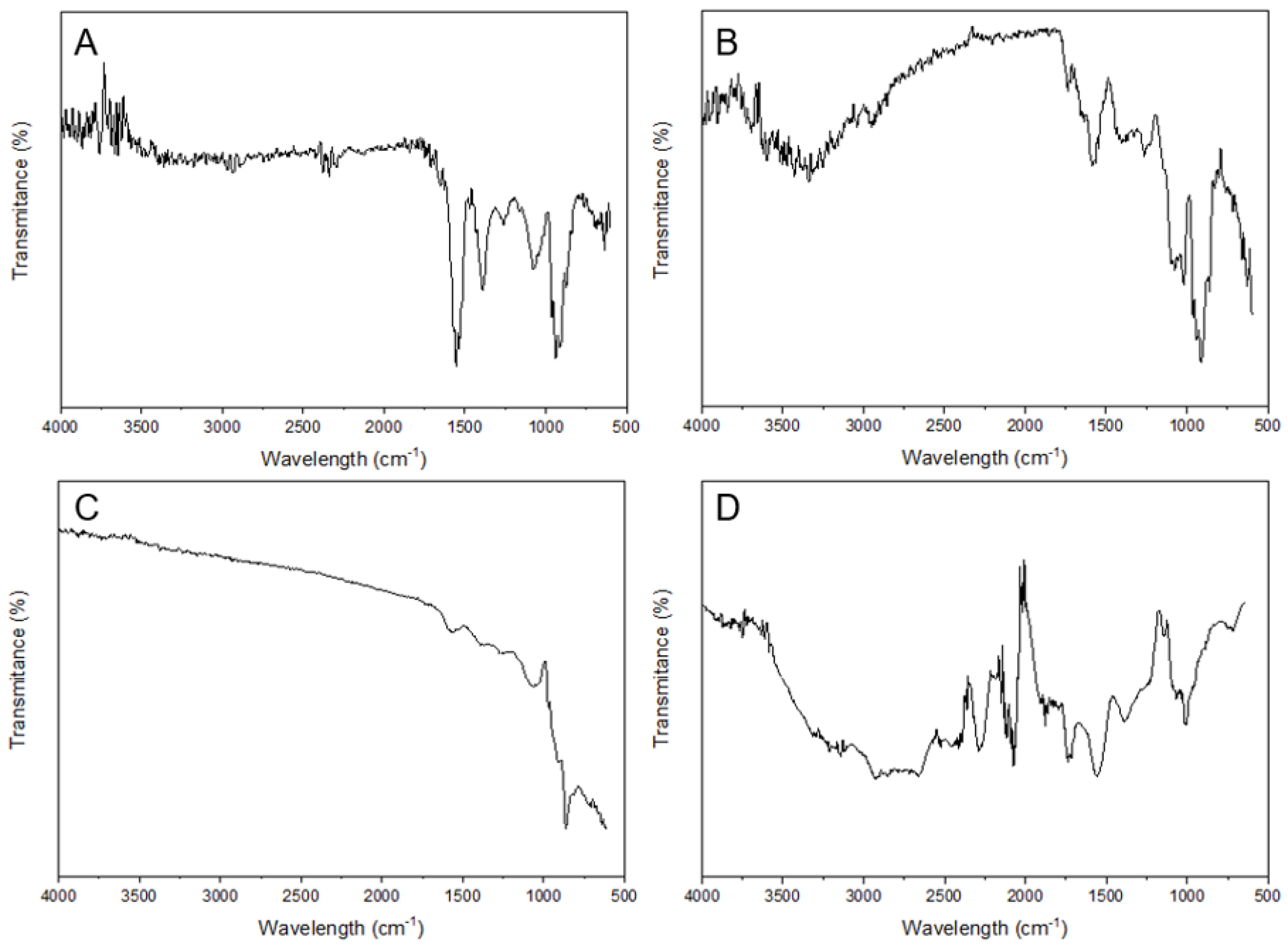
2.4. Characterization of Nanoparticles
2.5. Tested Bacteria
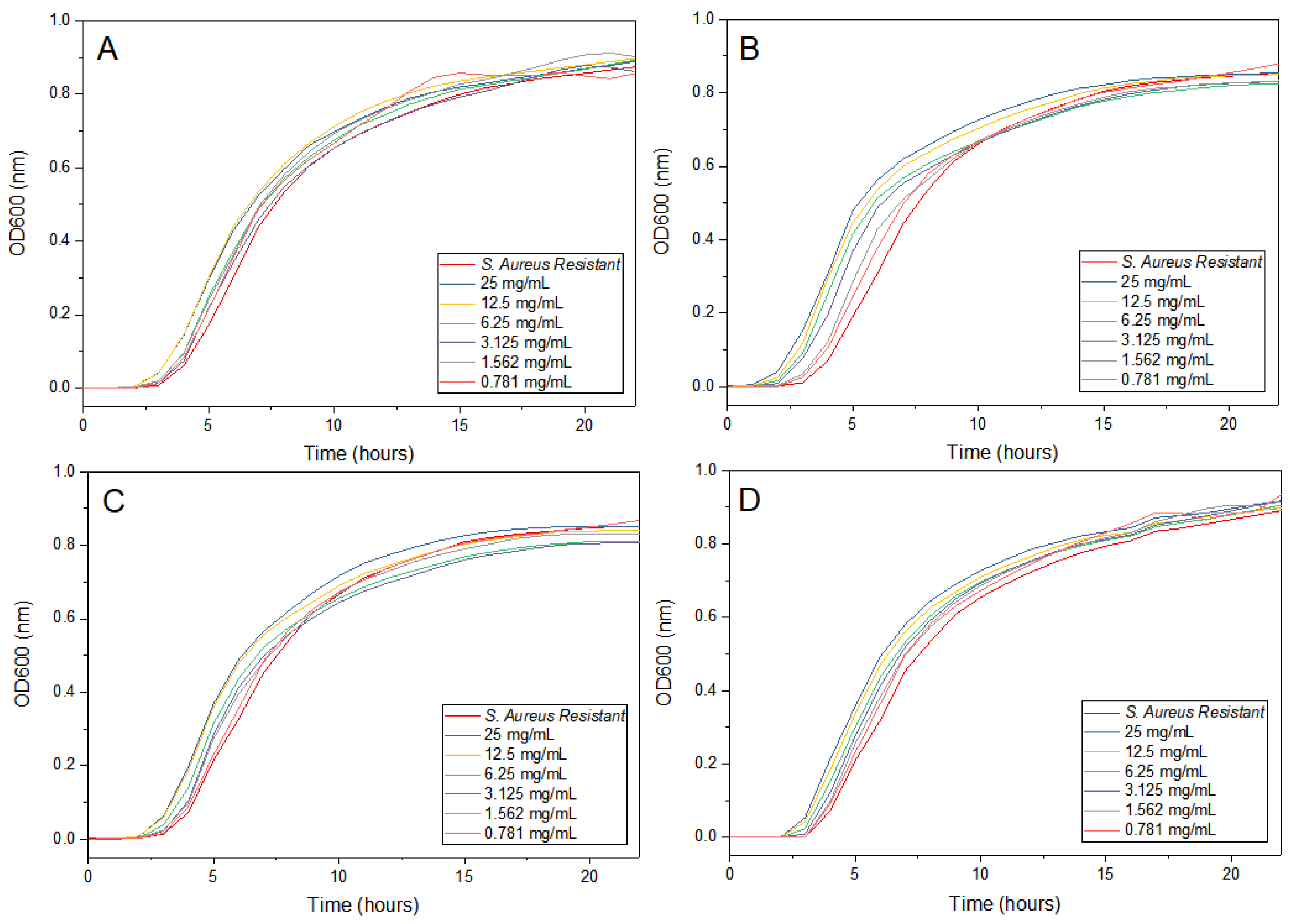
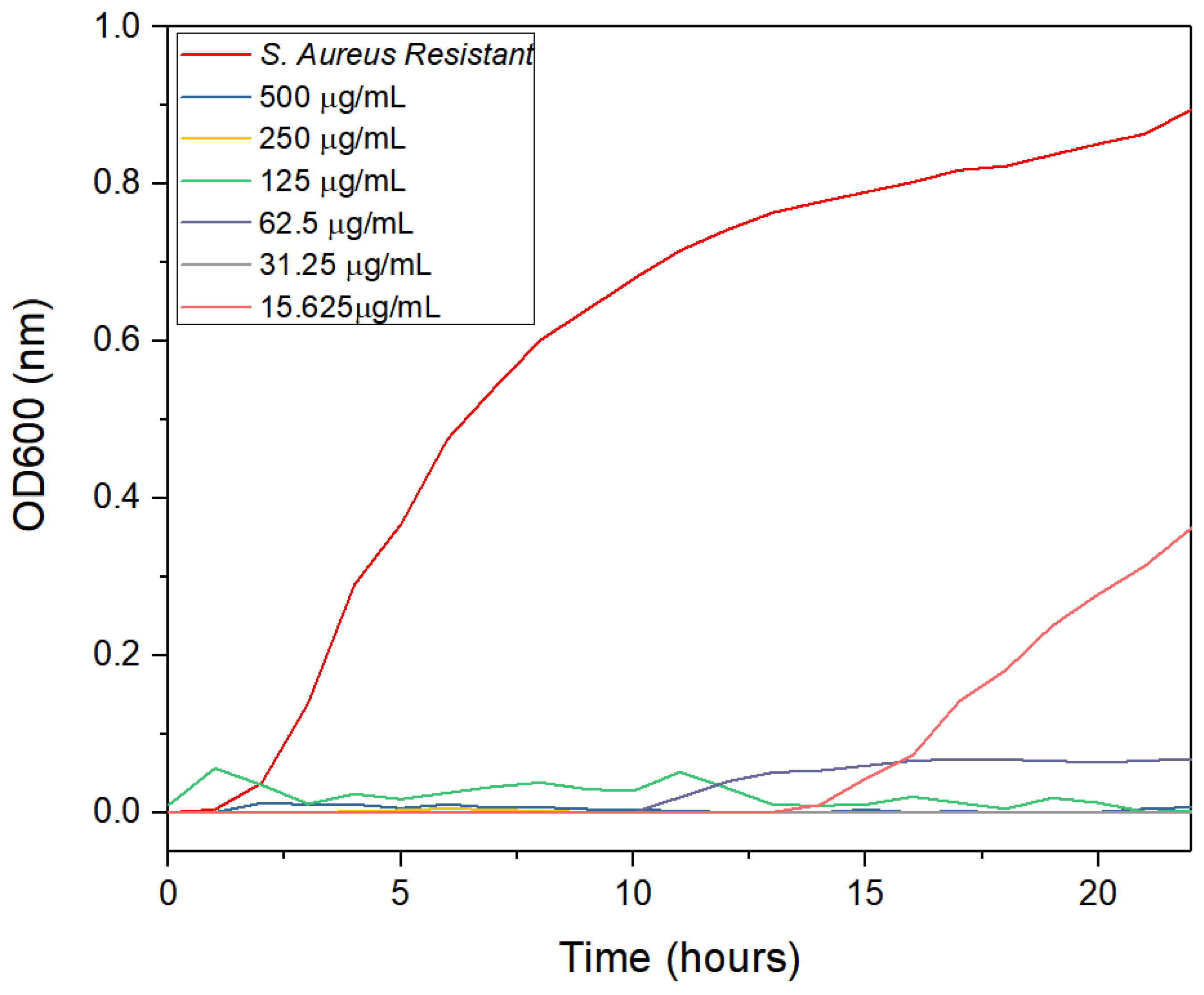
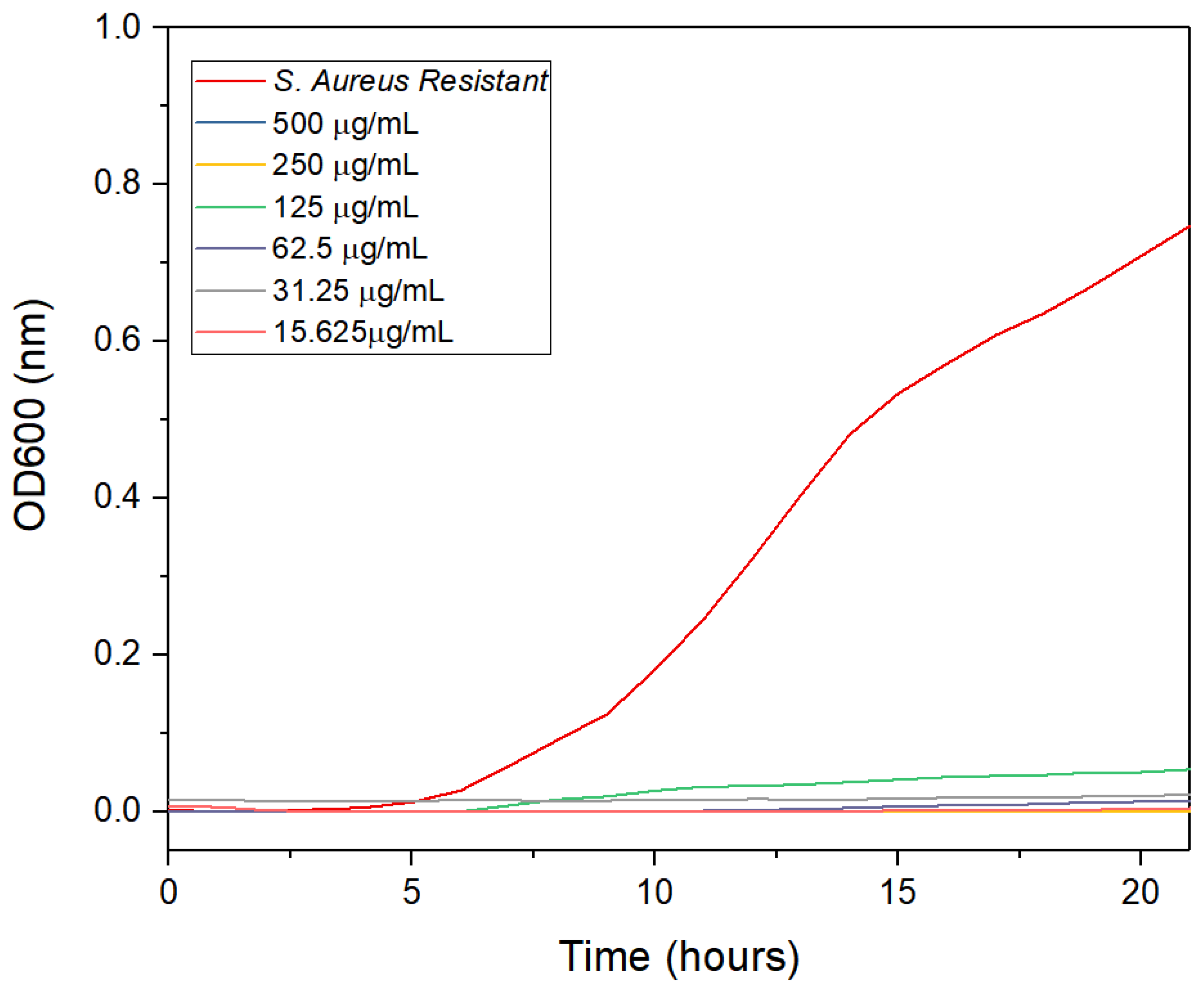
2.6. Antimicrobial Activity of AgNPs
3. Results
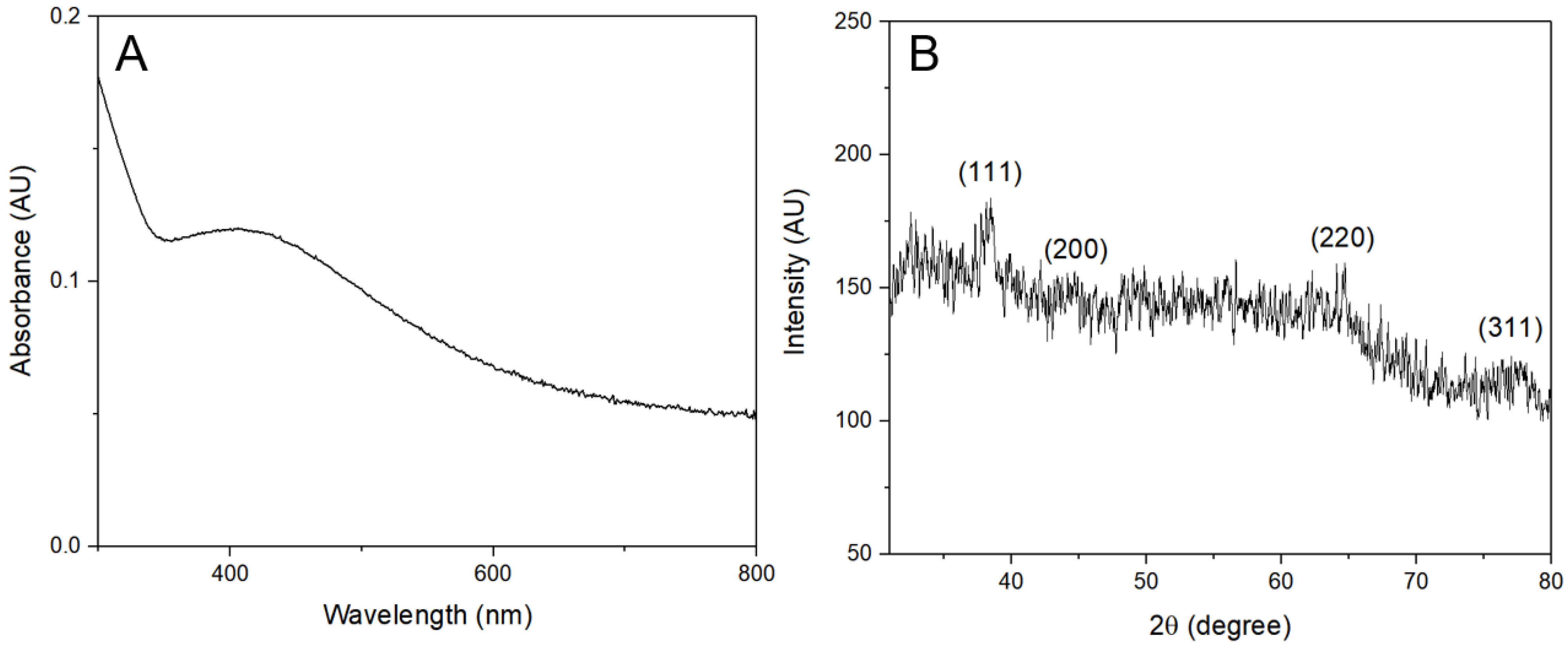
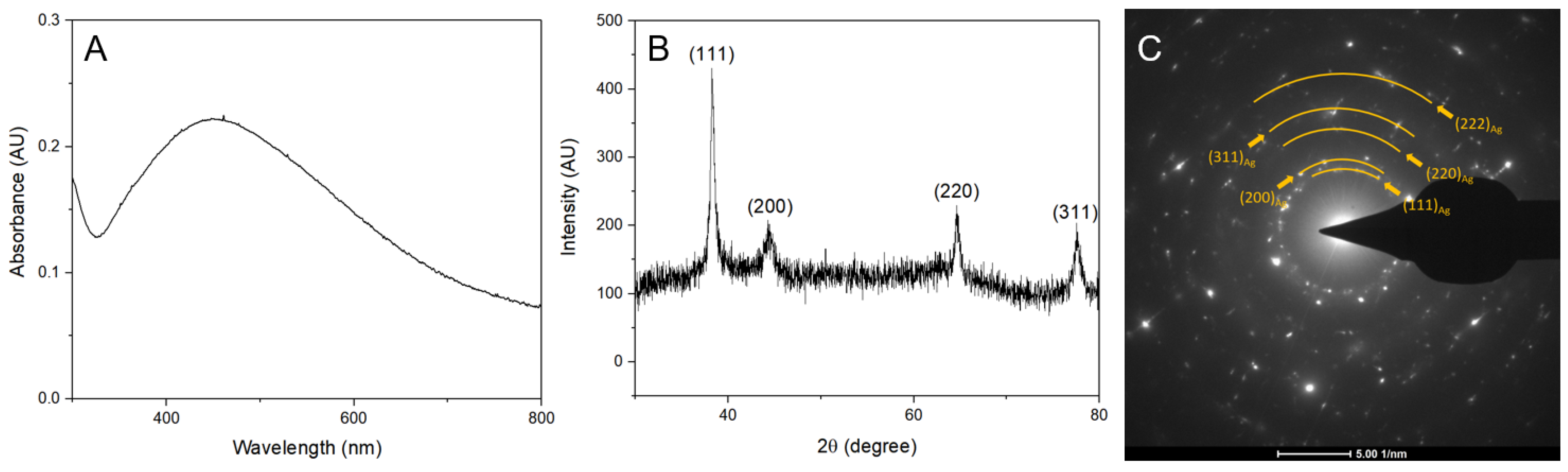
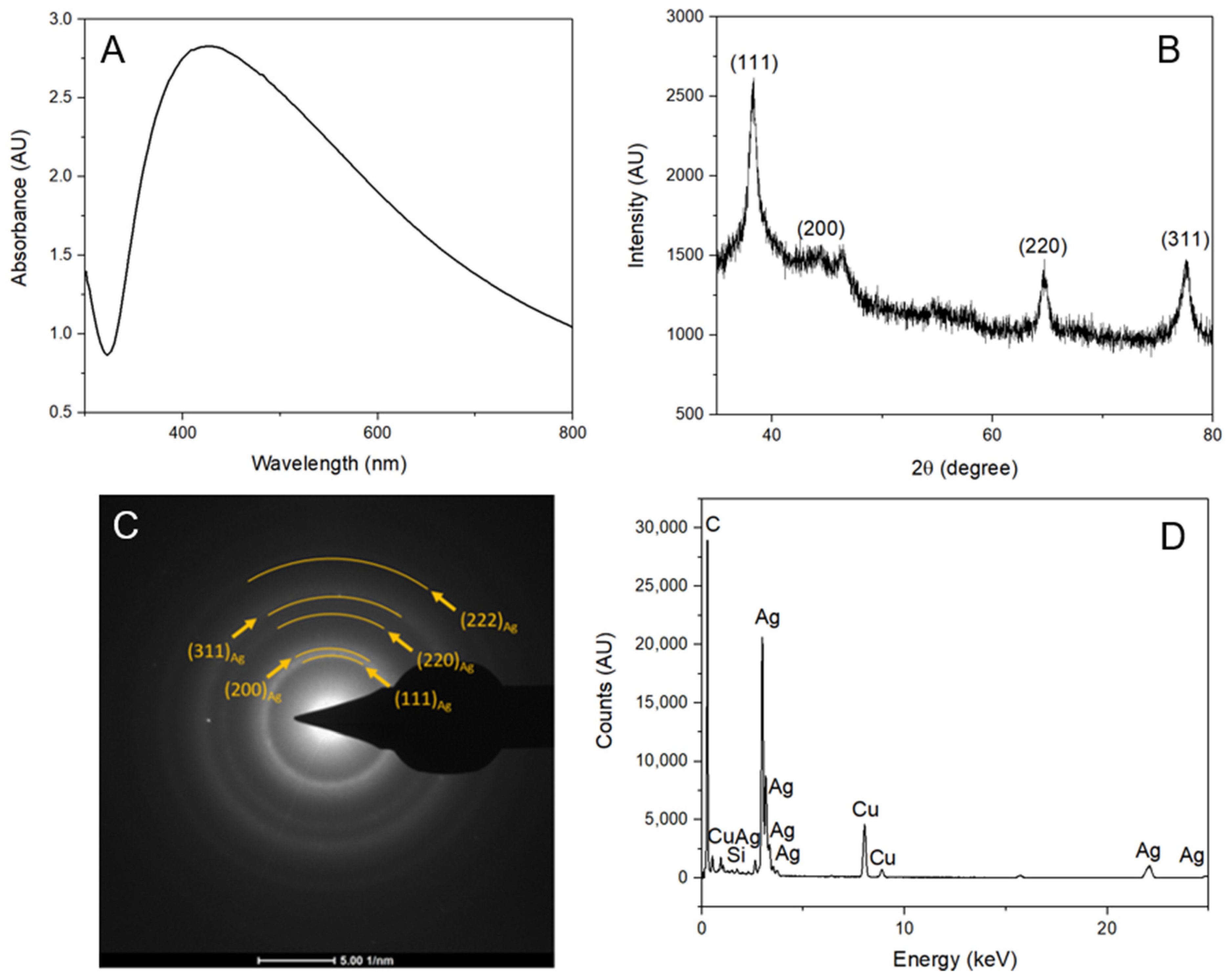
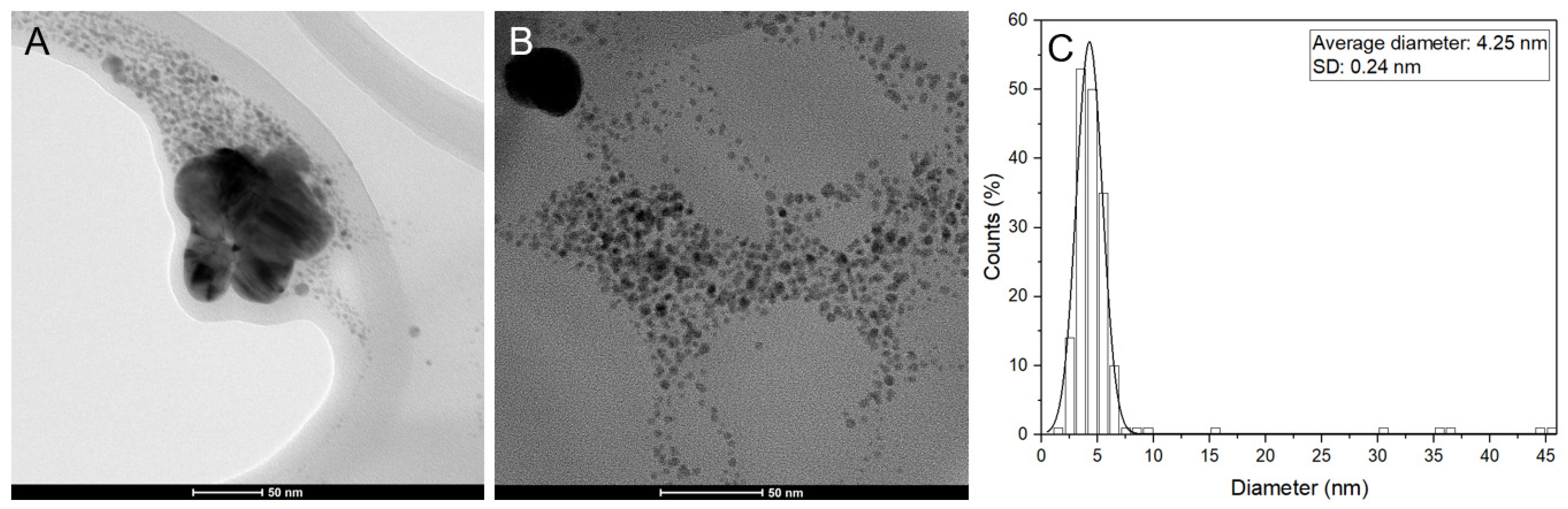
3.1. Characterization of AgNPs
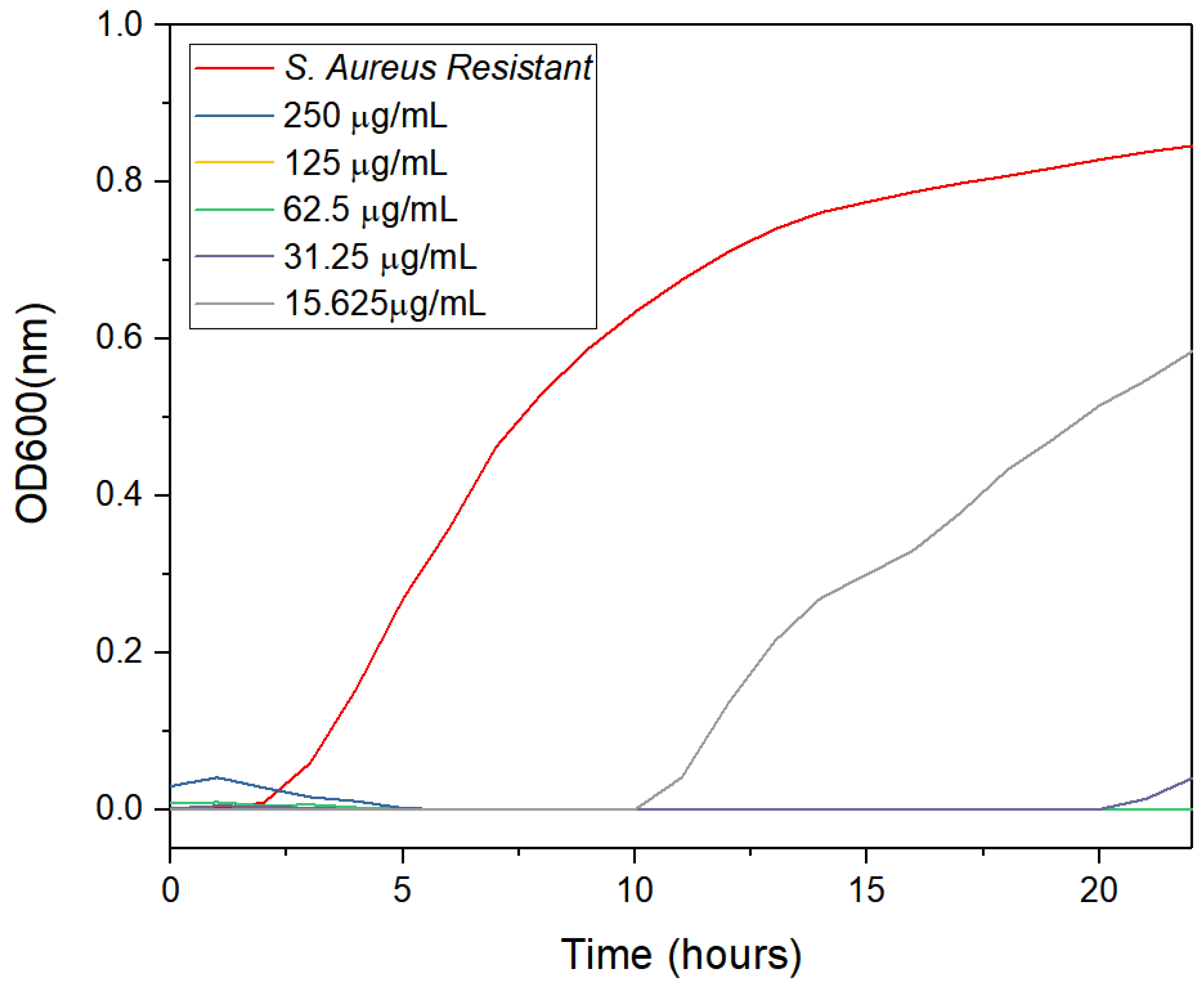
3.2. Antimicrobial Activity
4. Discussion
4.1. Synthesis and Characterization of Ultra-Small AgNPs
4.2. Antimicrobial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef] [PubMed]
- Shabina, S.; Gaurav, S.; Irfan, A.M.; Sarmad, M. Green nanotechnology: A review on nanomedicinal potential and green synthesis of silver nanoparticles (ag-np’s). Res. J. Biotechnol. 2020, 15, 177–187. [Google Scholar]
- Behera, A.; Mittu, B.; Padhi, S.; Patra, N.; Singh, J. Bimetallic nanoparticles: Green synthesis, applications, and future perspectives. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Gatadi, S.; Madhavi, Y.; Chopra, S.; Nanduri, S. Promising antibacterial agents against multidrug resistant Staphylococcus aureus. Bioorg. Chem. 2019, 92, 103252. [Google Scholar] [CrossRef]
- Hegreness, M.; Shoresh, N.; Damian, D.; Hartl, D.; Kishony, R. Accelerated evolution of resistance in multidrug environments. Proc. Natl. Acad. Sci. USA 2008, 105, 13977–13981. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cervantes, J.A.; Meza-Bustillos, J.F.; Resendiz-Hernández, H.; Suárez-Cantú, I.A.; Ortega-Rivera, O.A.; Salinas, E.; Escárcega-Gonzalez, C.E.; Morones-Ramirez, J.R. Re-sensitizing Ampicillin and Kanamycin-Resistant E. coli and S. aureus Using Synergistic Metal Micronutrients-Antibiotic Combinations. Front. Bioeng. Biotechnol. 2020, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Cruz, A.L.; Garza-Cervantes, J.A.; Vasto-Anzaldo, X.G.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J.R. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 5351. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.B.; Sun, D.; Huang, J.; Odoom-Wubah, T.; Li, Q. State of arts on the bio-synthesis of noble metal nanoparticles and their biological application. Chin. J. Chem. Eng. 2021, 30, 272–290. [Google Scholar] [CrossRef]
- Park, J.S.; Ahn, E.-Y.; Park, Y. Asymmetric dumbbell-shaped silver nanoparticles and spherical gold nanoparticles green-synthesized by mangosteen (Garcinia mangostana) pericarp waste extracts. Int. J. Nanomed. 2017, 12, 6895–6908. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Kadja, G.T.; Amalia, D.; Uyun, L.; Rini, W.P.; Hidayat, A.; Fahmi, R.L.; Nasriyanti, D.; Leun, S.G.; Ariyanta, H.A.; et al. A green synthesis of gold–palladium core–shell nanoparticles using orange peel extract through two-step reduction method and its formaldehyde colorimetric sensing performance. Nano-Struct. Nano-Objects 2020, 24, 100535. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2021, 6, 1553–1558. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Shaik, J.P.; Ahamed, A.; Mahmood, A.; Khan, M.; et al. Plant-Extract-Assisted Green Synthesis of Silver Nanoparticles Using Origanum vulgare L. Extract and Their Microbicidal Activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Castro, E.D.B.; Saucedo-Salazar, E.M.; Morales, R.M.C.; Regalado-Soto, D.I.; Treviño-González, F.M.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Tang, R.-C. Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015014. [Google Scholar] [CrossRef]
- Balavijayalakshmi, J.; Ramalakshmi, V. Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J. Appl. Res. Technol. 2017, 15, 413–422. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg2+ biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Bangale, S.; Ghotekar, S. Bio-fabrication of Silver nanoparticles using Rosa chinensis L. extract for antibacterial activities. Int. J. Nano Dimens. 2019, 10, 217–224. [Google Scholar]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Mohan, S.; Panneerselvam, K. An investigation on antibacterial filler property of silver nanoparticles generated from Walnut shell powder by insitu process. Mater. Today Proc. 2021, 39, 368–372. [Google Scholar] [CrossRef]
- Mendoza, E.S.R.; Méndez-Contreras, J.; Aguilar-Lasserre, A.; Vallejo-Cantú, N.; Alvarado-Lassman, A. Evaluation of bioenergy potential from citrus effluents through anaerobic digestion. J. Clean. Prod. 2020, 254, 120128. [Google Scholar] [CrossRef]
- Nasr, H.A.; Nassar, O.M.; El-Sayed, M.H.; Kobisi, A.A. Characterization and antimicrobial activity of lemon peel mediated green synthesis of silver nanoparticles. Int. J. Biol. Chem. 2019, 12, 56–63. [Google Scholar] [CrossRef]
- Ghaffar, A.; Hou, G.A.Y.; Kiran, S.; Rafique, M.A.; Iqbal, S.; Nosheen, S.; Hou, Y.; Afzal, G.; Bashir, M.; Aimun, U. Citrus paradisi fruit peel extract mediated green synthesis of copper nanoparticles for remediation of Disperse Yellow 125 dye. Desalin. Water Treat. 2021, 212, 368–375. [Google Scholar] [CrossRef]
- Çolak, H.; Karaköse, E. Green synthesis and characterization of nanostructured ZnO thin films using Citrus aurantifolia (lemon) peel extract by spin-coating method. J. Alloys Compd. 2017, 690, 658–662. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Gold, Silver and Platinum Nanoparticles Biosynthesized Using Orange Peel Extract. Adv. Mater. Res. 2013, 825, 556–559. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef]
- Jiang, X.; Du, B.; Huang, Y.; Zheng, J. Ultrasmall noble metal nanoparticles: Breakthroughs and biomedical implications. Nano Today 2018, 21, 106–125. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, A.; Maurya, I.K.; Thakur, A.; Kumar, S. Synthesis of ultra small iron oxide and doped iron oxide nanostructures and their antimicrobial activities. J. Taibah Univ. Sci. 2019, 13, 280–285. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Overcoming bacterial physical defenses with molecule-like ultrasmall antimicrobial gold nanoclusters. Bioact. Mater. 2020, 6, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Q.; Zhao, J.; Urmila, K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 2015, 5, srep11033. [Google Scholar] [CrossRef] [PubMed]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.; Yoon, S.-G. Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb. Pathog. 2018, 124, 63–69. [Google Scholar] [CrossRef]
- Suárez-Cerda, J.; Alonso-Nuñez, G.; Espinoza-Gómez, H.; Flores-López, L.Z. Synthesis, kinetics and photocatalytic study of “ultra-small” Ag-NPs obtained by a green chemistry method using an extract of Rosa ‘Andeli’ double delight petals. J. Colloid Interface Sci. 2015, 458, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nouri, A.; Yaraki, M.T.; Lajevardi, A.; Rezaei, Z.; Ghorbanpour, M.; Tanzifi, M. Ultrasonic-assisted green synthesis of silver nanoparticles using Mentha aquatica leaf extract for enhanced antibacterial properties and catalytic activity. Colloid Interface Sci. Commun. 2020, 35, 100252. [Google Scholar] [CrossRef]
- Faghihi, R.; Larijani, K.; Abdossi, V.; Moradi, P. Silver nanoparticles produced by green synthesis using Citrus paradise peel inhibits Botryytis cinerea in vitro. J. Hortic. Postharvest Res. 2020, 3, 151–160. [Google Scholar] [CrossRef]
- Jille, P.D.; Sainte. Chapter 2 The Development of Basic Concepts of Chemical Kinetics in Heterogeneous Catalysis. Compr. Chem. Kinet. 1991, 32, 47–84. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Diez, M.C.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Montanari, A.; Chen, J.; Widmer, W. Citrus Flavonoids: A Review of Past Biological Activity Against Disease. Discovery of new flavonoids from dancy tangerine cold pressed peel oil solids and leaves. Adv. Exp. Med. Biol. 1998, 439, 103–116. [Google Scholar] [CrossRef]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Kim, D.-S.; Kim, D.-Y.; Shin, H.S. Exploiting Fruit Waste Grape Pomace for Silver Nanoparticles Synthesis, Assessing Their Antioxidant, Antidiabetic Potential and Antibacterial Activity Against Human Pathogens: A Novel Approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef]
- Desai, R.; Mankad, V.; Gupta, S.; Jha, P. Size Distribution of Silver Nanoparticles: UV-Visible Spectroscopic Assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Phongtongpasuk, S.; Poadang, S.; Yongvanich, N. Green synthetic approach to prepare silver nanoparticles using longan (Dimocarpus longan) peel extract and evaluation of their antibacterial activities. Mater. Today Proc. 2017, 4, 6317–6325. [Google Scholar] [CrossRef]
- Manal, A.A.; Awatif, A.H.; Khalid, M.O.O.; Dalia, F.A.E.; Nada, E.E.; Lamia, A.A.-L.; Shorog., M.A.-O.; Nada, M.M.; Abdelelah, A.G.A. Silver nanoparticles biogenic synthesized using an orange peel extract and their use as an anti-bacterial agent. Int. J. Phys. Sci. 2014, 9, 34–40. [Google Scholar] [CrossRef]
- Nisha, S.N.; Aysha, O.; Rahaman, J.S.N.; Kumar, P.V.; Valli, S.; Nirmala, P.; Reena, A. Lemon peels mediated synthesis of silver nanoparticles and its antidermatophytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 124, 194–198. [Google Scholar] [CrossRef]
- Makari, H.K.; Ramakrishna, K.; Vaidya, G.; Kulkarni, P.; Balija, S.; Sheikh, H. Green synthesis of silver nanoparticles from muntingia calabura fruit extract and its biological activity. Int. J. Pharm. Sci. Res. 2021, 12, 2957–2965. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.A.; de Cara, S.P.; Diniz, I.M.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Pandey, A.; Kaushik, A.; Tiwari, S.K. Evaluation of antimicrobial activity and phytochemical analysis of Citrus limon. J. Pharm. Biomed. Sci. 2011, 13. Available online: www.jpbms.info (accessed on 8 March 2023).
- Razmjoo, M.; Khaki, P.; Fadaee, N.V. Antimicrobial effect of aqueous extract of orange peel and its effect on the shelf-life of flavored milk. J. Gorgan. Univ. Med. Sci. 2016, 18, 97–102. [Google Scholar]
- Arsène, M.M.J.; Podoprigora, I.V.; Davares, A.K.L.; Razan, M.; Das, M.S.; Senyagin, A.N. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World 2021, 14, 1330–1341. [Google Scholar] [CrossRef] [PubMed]





| Reference | Strain | Treatment | MIC (ppm) | Extract |
|---|---|---|---|---|
| [7] | S. aureus ATCC 6538 | AgNPs | 125 | Gardenia jasminoides leaves |
| Padilla-Cruz, A. L. et al., 2021 [7] | S. aureus ATCC 29,213 | AgNPs | 125 | Gardenia jasminoides leaves |
| Padilla-Cruz, A. L. et al., 2021 [7] | Multidrug-resistant S. aureus | AgNPs | 125 | Gardenia jasminoides leaves |
| [39] | S. aureus | AgNPs | 50 | Galega officinalis |
| Garza-Cervantes, J. A. et al., 2020 [6] | S. aureus-Kan | Kanamycin | 256 | NA |
| Garza-Cervantes, J. A. et al., 2020 [6] | S. aureus-Amp | Ampicillin | 1 | NA |
| [52] | S. aureus | Extract | 14,000 | Lemon peel |
| Razmjoo M. et al., 2016 [53] | S. aureus | Extract | 15,000 | Orange peel |
| Arsène, M et al., 2021 [54] | S. aureus ATCC 6538 | Extract | 6250 | Grapefruit peel |
| This work | Multidrug-resistant S. aureus | AgNPs | 31.25 | Lemon peel |
| This work | Multidrug-resistant S. aureus | AgNPs | 62.50 | Orange peel |
| This work | Multidrug-resistant S. aureus | AgNPs | 15.625 | Grapefruit peel |
| This work | Multidrug-resistant S. aureus | AgNPs | 15.625 | Orange lemon and grapefruit peels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Aude, J.E.; Morones-Ramírez, J.R.; De Haro-Del Río, D.A.; León-Buitimea, A.; Barriga-Castro, E.D.; Escárcega-González, C.E. Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity. Antibiotics 2023, 12, 574. https://doi.org/10.3390/antibiotics12030574
Castañeda-Aude JE, Morones-Ramírez JR, De Haro-Del Río DA, León-Buitimea A, Barriga-Castro ED, Escárcega-González CE. Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity. Antibiotics. 2023; 12(3):574. https://doi.org/10.3390/antibiotics12030574
Chicago/Turabian StyleCastañeda-Aude, Javier Emanuel, José Rubén Morones-Ramírez, David Alejandro De Haro-Del Río, Angel León-Buitimea, Enrique Díaz Barriga-Castro, and Carlos Enrique Escárcega-González. 2023. "Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity" Antibiotics 12, no. 3: 574. https://doi.org/10.3390/antibiotics12030574
APA StyleCastañeda-Aude, J. E., Morones-Ramírez, J. R., De Haro-Del Río, D. A., León-Buitimea, A., Barriga-Castro, E. D., & Escárcega-González, C. E. (2023). Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity. Antibiotics, 12(3), 574. https://doi.org/10.3390/antibiotics12030574









