Piperacillin Steady State Concentrations in Target Tissues Relevant for PJI Treatment—A Randomized Porcine Microdialysis Study Comparing Continuous Infusion with Intermittent Short-Term Infusion
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Microdialysis
4.2. Calibration
4.3. MIC Values
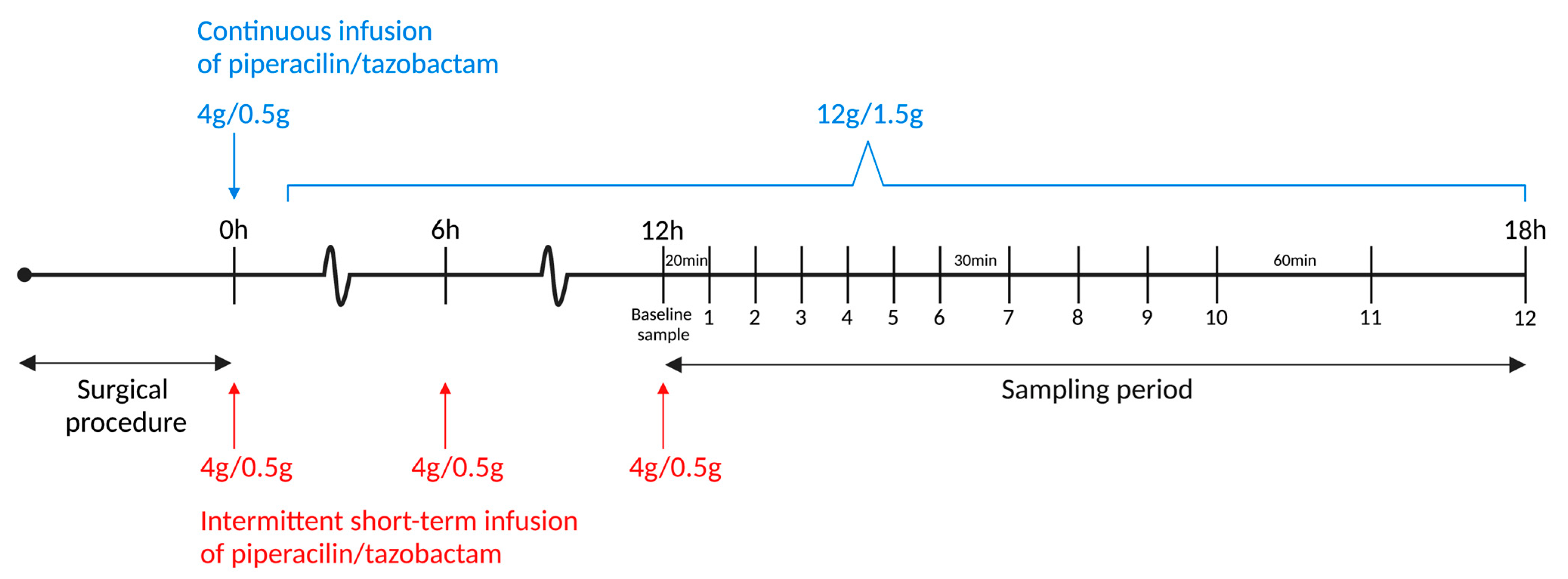
4.4. Randomization and Dosing
4.5. Sampling of Steady State Concentrations
4.6. Target Tissues, Surgical Procedure, and Anaesthesia
4.7. Quantification of Piperacillin Concentrations
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izakovicova, P.; Borens, O.; Trampuz, A. Periprosthetic joint infection: Current concepts and outlook. EFORT Open Rev. 2019, 4, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Bjerke-Kroll, B.T.; Christ, A.B.; McLawhorn, A.S.; Sculco, P.K.; Jules-Elysée, K.M.; Sculco, T.P. Periprosthetic joint infections treated with two-stage revision over 14 years: An evolving microbiology profile. J. Arthroplast. 2014, 29, 877–882. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Randau, T.M.; Franz, A.; Molitor, E.; Hischebeth, G.T.R. Microbiological Profiles of Patients with Periprosthetic Joint Infection of the Hip or Knee. Diagnostics 2022, 12, 1654. [Google Scholar] [CrossRef]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.D.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative prosthetic joint infection: Outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, O911–O919. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.B.; Osmon, D.R.; Steckelberg, J.M.; Sierra, R.J.; Walker, R.C.; Tande, A.J.; Berbari, E.F. Pseudomonas Prosthetic Joint Infections: A Review of 102 Episodes. J. Bone Jt. Infect. 2016, 1, 25–30. [Google Scholar] [CrossRef]
- Rowan, F.E.; Donaldson, M.J.; Pietrzak, J.R.; Haddad, F.S. The Role of One-Stage Exchange for Prosthetic Joint Infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Tannous, E.; Lipman, S.; Tonna, A.; Hector, E.; Hussein, Z.; Stein, M.; Reisfeld, S. Time above the MIC of Piperacillin-Tazobactam as a Predictor of Outcome in Pseudomonas aeruginosa Bacteremia. Antimicrob. Agents Chemother. 2020, 64, e02571-19. [Google Scholar] [CrossRef]
- Burgess, D.S.; Waldrep, T. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam when administered by continuous infusion and intermittent dosing. Clin. Ther. 2002, 24, 1090–1104. [Google Scholar] [CrossRef]
- Knudsen, M.; Bue, M.; Pontoppidan, L.L.; Hvistendahl, M.A.; Søballe, K.; Stilling, M.; Hanberg, P. Evaluation of Benzylpenicillin as an Internal Standard for Measurement of Piperacillin Bone Concentrations Via Microdialysis. J. Pharm. Sci. 2021, 110, 3500–3506. [Google Scholar] [CrossRef]
- Petersen, E.K.; Hanberg, P.; Knudsen, M.; Tøstesen, S.K.; Jørgensen, A.R.; Öbrink-Hansen, K.; Søballe, K.; Stilling, M.; Bue, M. Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis. Antibiotics 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Fawaz, S.; Barton, S.; Nabhani-Gebara, S. Comparing clinical outcomes of piperacillin-tazobactam administration and dosage strategies in critically ill adult patients: A systematic review and meta-analysis. BMC Infect. Dis. 2020, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Su, Y.C.; Wu, K.S.; Wu, T.H.; Yang, C.S. Loading dose and efficacy of continuous or extended infusion of beta-lactams compared with intermittent administration in patients with critical illnesses: A subgroup meta-analysis and meta-regression analysis. J. Clin. Pharm. Ther. 2021, 46, 424–432. [Google Scholar] [CrossRef]
- Roberts, J.A.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Piperacillin penetration into tissue of critically ill patients with sepsis—Bolus versus continuous administration? Crit. Care Med. 2009, 37, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Bue, M.; Sou, T.; Okkels, A.S.L.; Hanberg, P.; Thorsted, A.; Friberg, L.E.; Andersson, T.L.; Öbrink-Hansen, K.; Christensen, S. Population pharmacokinetics of piperacillin in plasma and subcutaneous tissue in patients on continuous renal replacement therapy. Int. J. Infect. Dis. 2020, 92, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.C.P.; Zhao, Q.; Oosterhoff, M.; van Oldenrijk, J.; Abdulla, A.; de Winter, B.C.M.; Bos, K.; Muller, A.E. The mysteries of target site concentrations of antibiotics in bone and joint infections: What is known? A narrative review. Expert Opin. Drug Metab. Toxicol. 2022, 18, 587–600. [Google Scholar] [CrossRef]
- Chaurasia, C.S.; Müller, M.; Bashaw, E.D.; Benfeldt, E.; Bolinder, J.; Bullock, R.; Bungay, P.M.; DeLange, E.C.; Derendorf, H.; Elmquist, W.F.; et al. AAPS-FDA workshop white paper: Microdialysis principles, application and regulatory perspectives. Pharm. Res. 2007, 24, 1014–1025. [Google Scholar] [CrossRef]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Jensen, L.K.; Koch, J.; Henriksen, N.L.; Bue, M.; Tøttrup, M.; Hanberg, P.; Søballe, K.; Jensen, H.E. Suppurative Inflammation and Local Tissue Destruction Reduce the Penetration of Cefuroxime to Infected Bone Implant Cavities. J. Comp. Pathol. 2017, 157, 308–316. [Google Scholar] [CrossRef]
- Tøttrup, M.; Bue, M.; Koch, J.; Jensen, L.K.; Hanberg, P.; Aalbæk, B.; Fuursted, K.; Jensen, H.E.; Søballe, K. Effects of Implant-Associated Osteomyelitis on Cefuroxime Bone Pharmacokinetics: Assessment in a Porcine Model. J. Bone Jt. Surg. Am. 2016, 98, 363–369. [Google Scholar] [CrossRef]
- Bue, M.; Hanberg, P.; Koch, J.; Jensen, L.K.; Lundorff, M.; Aalbaek, B.; Jensen, H.E.; Søballe, K.; Tøttrup, M. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J. Orthop. Res. 2018, 36, 1093–1098. [Google Scholar] [CrossRef]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of β-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Piperacillin-Tazobactam: Rationale for the Clinical Breakpoints, Version 1.0. 2010. Available online: http://www.eucast.org (accessed on 1 November 2022).
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wallemacq, P.E.; Van Bambeke, F.; Tulkens, P.M. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. Anti. Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef]
- Thabet, P.; Joshi, A.; MacDonald, E.; Hutton, B.; Cheng, W.; Stevens, A.; Kanji, S. Clinical and pharmacokinetic/dynamic outcomes of prolonged infusions of beta-lactam antimicrobials: An overview of systematic reviews. PLoS ONE 2021, 16, e0244966. [Google Scholar] [CrossRef]
- Quinton, M.C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of β-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef]
- Chiriac, U.; Richter, D.C.; Frey, O.R.; Röhr, A.C.; Helbig, S.; Preisenberger, J.; Hagel, S.; Roberts, J.A.; Weigand, M.A.; Brinkmann, A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics 2021, 10, 667. [Google Scholar] [CrossRef]
- Mah, G.T.; Mabasa, V.H.; Chow, I.; Ensom, M.H. Evaluating outcomes associated with alternative dosing strategies for piperacillin/tazobactam: A qualitative systematic review. Ann. Pharmacother. 2012, 46, 265–275. [Google Scholar] [CrossRef]
- Incavo, S.J.; Ronchetti, P.J.; Choi, J.H.; Wu, H.; Kinzig, M.; Sörgel, F. Penetration of piperacillin-tazobactam into cancellous and cortical bone tissues. Antimicrob. Agents Chemother. 1994, 38, 905–907. [Google Scholar] [CrossRef]
- Boselli, E.; Breilh, D.; Biot, L.; Bel, J.C.; Saux, M.C.; Allaouchiche, B. Penetration of piperacillin/tazobactam into cancellous and cortical bone tissue. Curr. Ther. Res. 2001, 62, 538–545. [Google Scholar] [CrossRef]
- Meroni, G.; Tsikopoulos, A.; Tsikopoulos, K.; Allemanno, F.; Martino, P.A.; Soares Filipe, J.F. A Journey into Animal Models of Human Osteomyelitis: A Review. Microorganisms 2022, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Joukhadar, C.; Müller, M. Microdialysis: Current applications in clinical pharmacokinetic studies and its potential role in the future. Clin. Pharmacokinet. 2005, 44, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Kho, C.M.; Enche Ab Rahim, S.K.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2017, 54, 3506–3527. [Google Scholar] [CrossRef]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 1 November 2022).
- Sörgel, F.; Kinzig, M. Pharmacokinetic characteristics of piperacillin/tazobactam. Intensive Care Med. 1994, 20 (Suppl. 3), S14–S20. [Google Scholar] [CrossRef]

| Continuous Infusion | Intermittent Short-Term Infusion | ||||||
|---|---|---|---|---|---|---|---|
| [Min] (95%CI) | [%] | [Min] (95%CI) | [%] | p-Value | |||
| MIC = 4 mg/L | |||||||
| Cortical bone | 65 | (−37–166) | 20 | 133 | (1–266) | 040 | <0.270 |
| Cancellous bone | 330 | (330–330) | 100 | 244 | (182–306) | 074 | <0.003 |
| Subcutaneous tissue | 330 | (330–330) | 100 | 253 | (201–305) | 077 | <0.033 |
| Knee joint | 330 | (330–330) | 100 | 272 | (239–304) | 082 | <0.019 |
| Plasma | 330 | (330–330) | 100 | 300 | (273–328) | 091 | <0.001 |
| MIC = 8 mg/L | |||||||
| Cortical bone | 0 | (0–0) | 0 | 55 | (−16–126) | 017 | <0.154 |
| Cancellous bone | 290 | (206–374) | 88 | 187 | (128–246) | 057 | <0.019 |
| Subcutaneous tissue | 330 | (330–330) | 100 | 212 | (137–285) | 064 | <0.008 |
| Knee joint | 307 | (247–367) | 93 | 220 | (160–279) | 067 | <0.021 |
| Plasma | 330 | (330–330) | 100 | 238 | (200–277) | 072 | <0.001 |
| MIC = 16 mg/L | |||||||
| Cortical bone | 0 | (0–0) | 000 | 0 | (0–0) | 0000 | <1.000 |
| Cancellous bone | 217 | (73–361) | 66 | 119 | (78–159) | 036 | <0.176 |
| Subcutaneous tissue | 322 | (302–342) | 98 | 152 | (82–223) | 046 | <0.002 |
| Knee joint | 275 | (134–416) | 83 | 162 | (102–222) | 049 | <0.036 |
| Plasma | 322 | (302–342) | 98 | 163 | (139–187) | 049 | <0.001 |
| MIC = 64 mg/L | |||||||
| Cortical bone | 0 | (0–0) | 0 | 0 | (0–0) | 00 | 1.000 |
| Cancellous bone | 0 | (0–0) | 0 | 7 | (−6–21) | 02 | 0.467 |
| Subcutaneous tissue | 10 | (−15–34) | 3 | 30 | (−10–69) | 09 | 0.392 |
| Knee joint | 0 | (0–0) | 0 | 30 | (1–60) | 09 | 0.056 |
| Plasma | 69 | (−38–175) | 21 | 69 | (58–79) | 021 | 0.099 |
| Continuous Infusion | Intermittent Short-Term Infusion | p-Value | |||
|---|---|---|---|---|---|
| Median AUC12–18 h, [min·mg/mL], (95%CI) | |||||
| Cortical bone | 1394 | (786–2473) | 1649 | (896–3036) | 0.684 |
| Cancellous bone | 12,709 | (7870–2,0523) | 8009 | (5137–12,485) | 0.151 |
| Subcutaneous tissue | 23,230 | (15,557–34,686) | 8612 | (5946–12,485) | 0.002 |
| Knee joint | 17,785 | (11,327–27,925) | 9094 | (6049–13,670) | 0.033 |
| Plasma | 33,573 | (19,006–59,303) | 24,080 | (13,571–42,725) | 0.394 |
| Median Cmax, [mg/L], (95%CI) | |||||
| Cortical bone | 3 | (2–5) | 5 | (3–9) | 0.117 |
| Cancellous bone | 22 | (14–35) | 46 | (30–70) | 0.026 |
| Subcutaneous tissue | 40 | (26–63) | 77 | (51–117) | 0.041 |
| Knee joint | 35 | (20–60) | 88 | (55–141) | 0.016 |
| Plasma | 72 | (35–145) | 499 | (247–1011) | 0.001 |
| Median AUCtissue/AUCplasma,(95%CI) | |||||
| Cortical bone | 0.04 | (0.02–0.08) | 0.07 | (0.03–0.14) | 0.350 |
| Cancellous bone | 0.41 | (0.21–0.78) | 0.33 | (0.18–0.62) | 0.628 |
| Subcutaneous tissue | 0.56 | (0.28–1.14) | 0.34 | (0.17–0.66) | 0.280 |
| Knee joint | 0.45 | (0.22–0.92) | 0.38 | (0.19–0.76) | 0.724 |
| Median Tmax, [min], (95%CI) * | |||||
| Cortical bone | - | 809 | (750–871) | ||
| Cancellous bone | - | 777 | (689–800) | ||
| Subcutaneous tissue | - | 760 | (701–824) | ||
| Knee joint | - | 750 | (689–817) | ||
| Plasma | - | 742 | (689–800) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, H.C.; Hanberg, P.; Knudsen, M.; Tøstesen, S.K.; Jørgensen, A.R.; Petersen, E.K.; Öbrink-Hansen, K.; Søballe, K.; Stilling, M.; Bue, M. Piperacillin Steady State Concentrations in Target Tissues Relevant for PJI Treatment—A Randomized Porcine Microdialysis Study Comparing Continuous Infusion with Intermittent Short-Term Infusion. Antibiotics 2023, 12, 577. https://doi.org/10.3390/antibiotics12030577
Rasmussen HC, Hanberg P, Knudsen M, Tøstesen SK, Jørgensen AR, Petersen EK, Öbrink-Hansen K, Søballe K, Stilling M, Bue M. Piperacillin Steady State Concentrations in Target Tissues Relevant for PJI Treatment—A Randomized Porcine Microdialysis Study Comparing Continuous Infusion with Intermittent Short-Term Infusion. Antibiotics. 2023; 12(3):577. https://doi.org/10.3390/antibiotics12030577
Chicago/Turabian StyleRasmussen, Hans Christian, Pelle Hanberg, Martin Knudsen, Sara Kousgaard Tøstesen, Andrea René Jørgensen, Elisabeth Krogsgaard Petersen, Kristina Öbrink-Hansen, Kjeld Søballe, Maiken Stilling, and Mats Bue. 2023. "Piperacillin Steady State Concentrations in Target Tissues Relevant for PJI Treatment—A Randomized Porcine Microdialysis Study Comparing Continuous Infusion with Intermittent Short-Term Infusion" Antibiotics 12, no. 3: 577. https://doi.org/10.3390/antibiotics12030577
APA StyleRasmussen, H. C., Hanberg, P., Knudsen, M., Tøstesen, S. K., Jørgensen, A. R., Petersen, E. K., Öbrink-Hansen, K., Søballe, K., Stilling, M., & Bue, M. (2023). Piperacillin Steady State Concentrations in Target Tissues Relevant for PJI Treatment—A Randomized Porcine Microdialysis Study Comparing Continuous Infusion with Intermittent Short-Term Infusion. Antibiotics, 12(3), 577. https://doi.org/10.3390/antibiotics12030577








