Challenges and Opportunities in Antimicrobial Stewardship among Hematopoietic Stem Cell Transplant and Oncology Patients
Abstract
:1. Introduction
2. Unique Challenges to Antimicrobial Stewardship in Oncology Patients
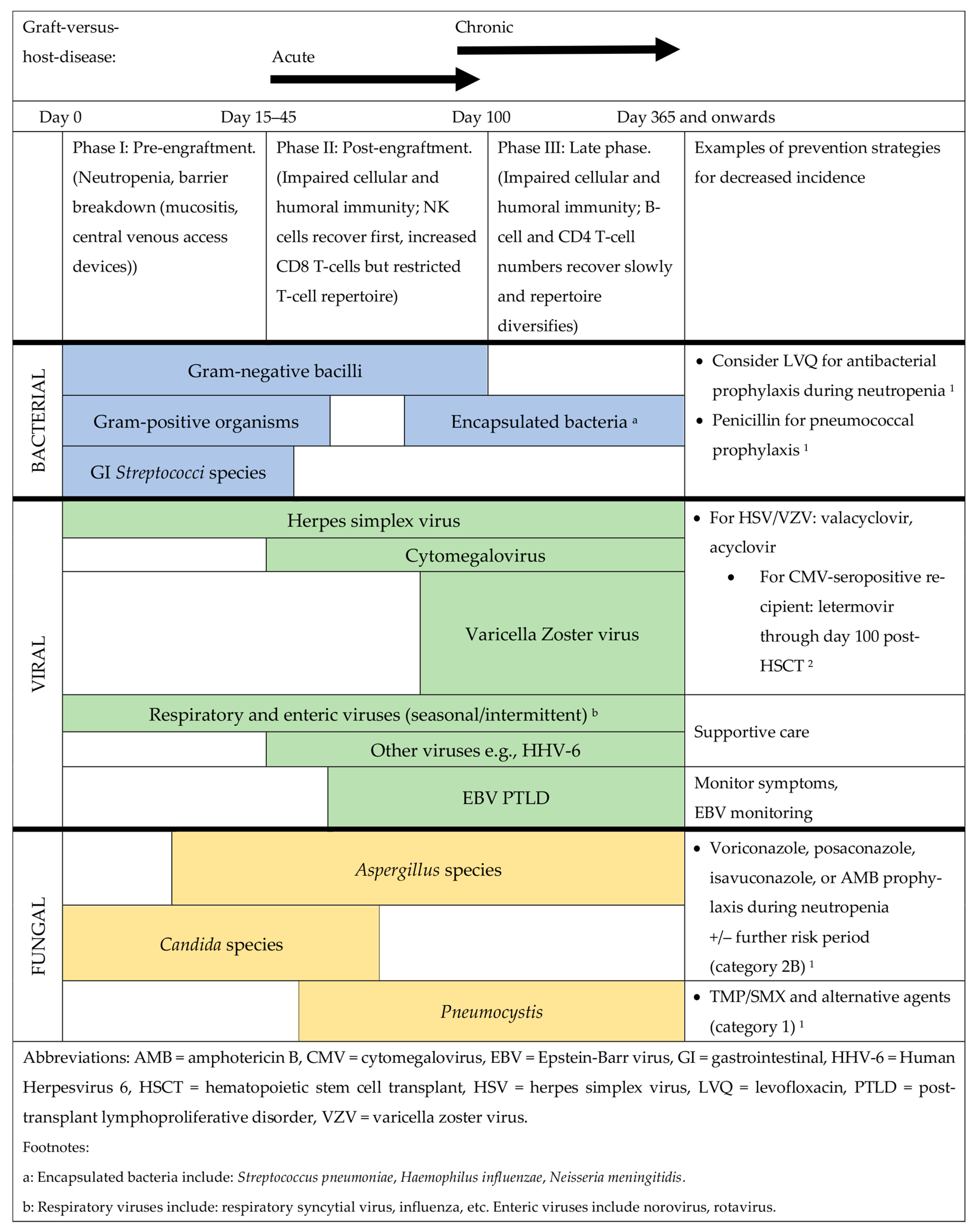
2.1. Understanding Underlying Host Immune Status and Infectious Risks
2.2. Additional Healthcare Considerations and Hematopoietic Stem Cell Transplantation
2.3. Drug–Drug Interactions Affecting Antimicrobial Use
3. Unmet Needs and Opportunities for Antimicrobial Stewardship
3.1. Fever and Neutropenia
3.2. Antibacterial Prophylaxis
3.3. Antifungal Use and Prophylaxis
3.4. Antiviral Use and Prophylaxis
3.5. Adverse Effects of Antimicrobials
3.6. Expansion of Diagnostic and Susceptibility Testing Methods
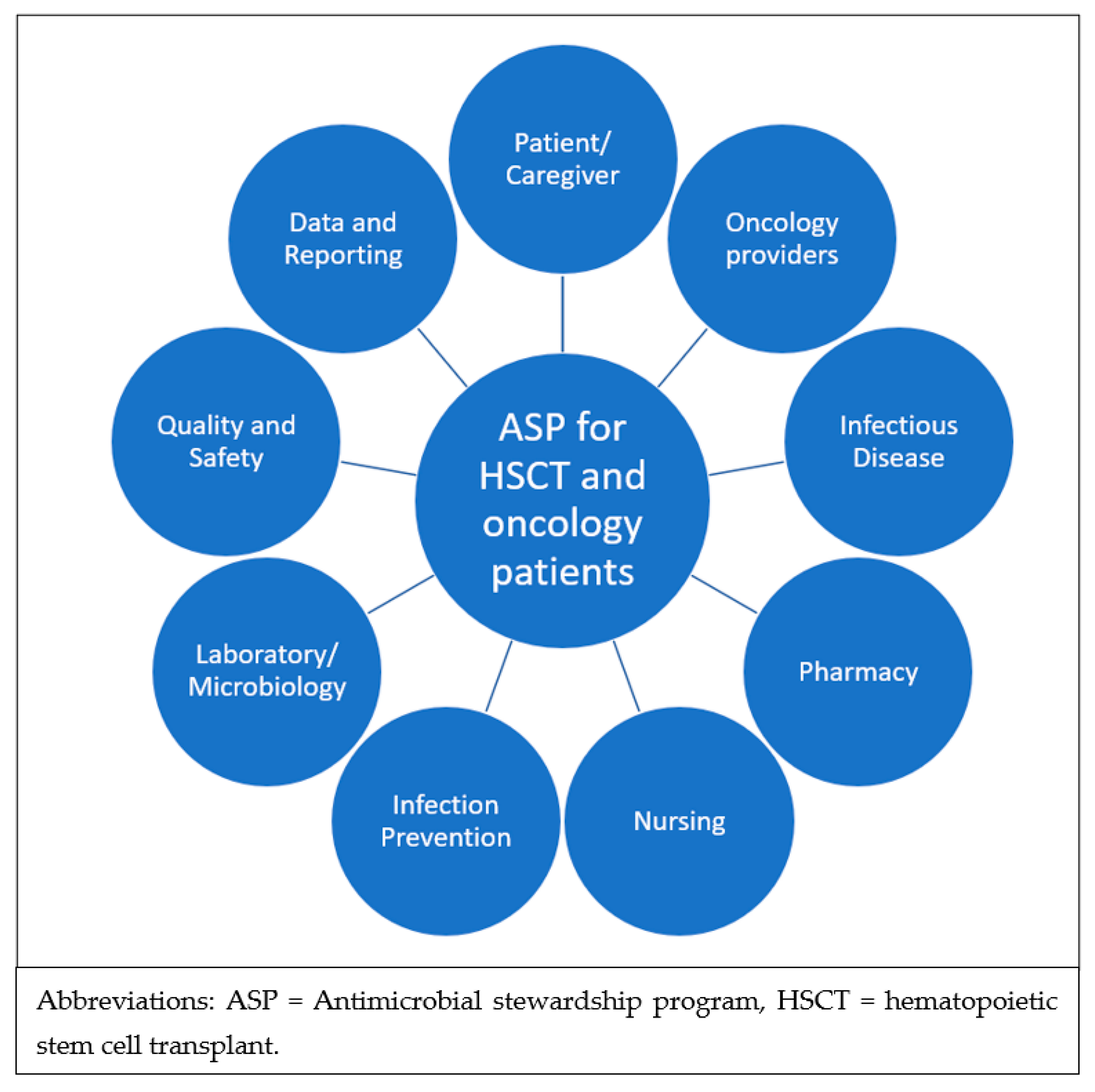
3.7. Lack of Dedicated ASP Guidelines and the Need for Multidisciplinary Implementation
3.8. Summary of Opportunities for ASP Interventions Specific to Oncology and HSCT Patients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Proposals for EU Guidelines on the Prudent Use of Antimicrobials in Humans; ECDC: Stockholm, Sweden, 2017; Available online: http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1643 (accessed on 4 December 2022).
- WHO. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A Practical Toolkit; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- So, M.; Hand, J.; Forrest, G.; Pouch, S.M.; Te, H.; Ardura, M.I.; Bartash, R.M.; Dadhania, D.M.; Edelman, J.; Ince, D.; et al. White Paper on Antimicrobial Stewardship in Solid Organ Transplant Recipients. Am. J. Transplant. 2022, 22, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.; Husain, S. Special Issue: The Urgent Need for Antimicrobial Stewardship in Transplantation. Transpl. Infect. Dis. 2022, 24, e13642. [Google Scholar] [CrossRef]
- Seo, S.K.; Lo, K.; Abbo, L.M. Current State of Antimicrobial Stewardship at Solid Organ and Hematopoietic Cell Transplant Centers in the United States. Infect. Control. Hosp. Epidemiol 2016, 37, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, J.N.; Aitken, S.L.; Krantz, E.M.; Nagel, J.L.; Dadwal, S.S.; Seo, S.K.; Liu, C. Variation in Clinical Practice and Attitudes on Antibacterial Management of Fever and Neutropenia in Patients with Hematologic Malignancy: A Survey of Cancer Centers Across the United States. Open Forum Infect. Dis. 2022, 9, ofac005. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldszmid, R.S.; Dzutsev, A.; Trinchieri, G. Host Immune Response to Infection and Cancer: Unexpected Commonalities. Cell Host Microbe 2014, 15, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Atkins, S.; He, F. Chemotherapy and Beyond. Infect. Dis. Clin. N. Am. 2019, 33, 289–309. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Referenced with Permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prevention and Treatment of Cancer-Related Infections; Version 3.2022; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Lyman, G.H.; Lyman, C.H.; Agboola, O. Anc Study Group Risk Models for Predicting Chemotherapy-Induced Neutropenia. Oncologist 2005, 10, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Tillman, B.F.; Pauff, J.M.; Satyanarayana, G.; Talbott, M.; Warner, J.L. Systematic Review of Infectious Events with the Bruton Tyrosine Kinase Inhibitor Ibrutinib in the Treatment of Hematologic Malignancies. Eur. J. Haematol. 2018, 100, 325–334. [Google Scholar] [CrossRef]
- Safdar, A.; Armstrong, D. Infections in Patients With Hematologic Neoplasms and Hematopoietic Stem Cell Transplantation: Neutropenia, Humoral, and Splenic Defects. Clin. Infect. Dis. 2011, 53, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33, e00035-19. [Google Scholar] [CrossRef] [PubMed]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, S.; Das, A.; Vutthikraivit, W.; Edwards, P.J.; Hardwicke, F.; Short, N.J.; Borthakur, G.; Maiti, A. Risk of Infection Associated With Ibrutinib in Patients With B-Cell Malignancies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2020, 20, 87–97.e5. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernández-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the Safety of Targeted and Biological Therapies: An Infectious Diseases Perspective (Immune Checkpoint Inhibitors, Cell Adhesion Inhibitors, Sphingosine-1-Phosphate Receptor Modulators and Proteasome Inhibitors). Clin. Microbiol. Infect. 2018, 24, S95–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baier, C.; Linke, L.; Eder, M.; Schwab, F.; Chaberny, I.F.; Vonberg, R.-P.; Ebadi, E. Incidence, Risk Factors and Healthcare Costs of Central Line-Associated Nosocomial Bloodstream Infections in Hematologic and Oncologic Patients. PLoS ONE 2020, 15, e0227772. [Google Scholar] [CrossRef]
- Satlin, M.J.; Walsh, T.J. Multidrug-Resistant Enterobacteriaceae, Pseudomonas Aeruginosa and Vancomycin-Resistant Enterococcus: Three Major Threats to Hematopoietic Stem Cell Transplant Recipients. Transpl. Infect. Dis. 2017, 19, e12762. [Google Scholar] [CrossRef]
- Satlin, M.J.; Cohen, N.; Ma, K.C.; Gedrimaite, Z.; Soave, R.; Askin, G.; Chen, L.; Kreiswirth, B.N.; Walsh, T.J.; Seo, S.K. Bacteremia Due to Carbapenem-Resistant Enterobacteriaceae in Neutropenic Patients with Hematologic Malignancies. J. Infect. 2016, 73, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Martirosov, D.M.; Lodise, T.P. Emerging Trends in Epidemiology and Management of Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2016, 85, 266–275. [Google Scholar] [CrossRef]
- Tomblyn, M.; Chiller, T.; Einsele, H.; Gress, R.; Sepkowitz, K.; Storek, J.; Wingard, J.R.; Young, J.-A.H.; Boeckh, M.A. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol. Blood Marrow Transplant. 2009, 15, 1143–1238. [Google Scholar] [CrossRef] [Green Version]
- Hakki, M.; Aitken, S.L.; Danziger-Isakov, L.; Michaels, M.G.; Carpenter, P.A.; Chemaly, R.F.; Papanicolaou, G.A.; Boeckh, M.; Marty, F.M. American Society for Transplantation and Cellular Therapy Series: #3—Prevention of Cytomegalovirus Infection and Disease After Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2021, 27, 707–719. [Google Scholar] [CrossRef]
- Young, J.-A.H.; Logan, B.R.; Wu, J.; Wingard, J.R.; Weisdorf, D.J.; Mudrick, C.; Knust, K.; Horowitz, M.M.; Confer, D.L.; Dubberke, E.R.; et al. Infections after Transplantation of Bone Marrow or Peripheral Blood Stem Cells from Unrelated Donors. Biol. Blood Marrow Transplant. 2016, 22, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.; DeFor, T.; Burns, L.; McGlave, P.; Miller, J.; Wagner, J.; Weisdorf, D. A Comparison of Related Donor Peripheral Blood and Bone Marrow Transplants: Importance of Late-Onset Chronic Graft-versus-Host Disease and Infections. Biol. Blood Marrow Transplant. 2003, 9, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parody, R.; Martino, R.; Rovira, M.; Vazquez, L.; Vázquez, M.J.; de la Cámara, R.; Blazquez, C.; Fernández-Avilés, F.; Carreras, E.; Salavert, M.; et al. Severe Infections after Unrelated Donor Allogeneic Hematopoietic Stem Cell Transplantation in Adults: Comparison of Cord Blood Transplantation with Peripheral Blood and Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2006, 12, 734–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhmedov, M. Infectious Complications in Allogeneic Hematopoietic Cell Transplant Recipients: Review of Transplant-related Risk Factors and Current State of Prophylaxis. Clin. Transplant. 2021, 35, e14172. [Google Scholar] [CrossRef]
- Lussana, F.; Cattaneo, M.; Rambaldi, A.; Squizzato, A. Ruxolitinib-associated Infections: A Systematic Review and Meta-analysis. Am. J. Hematol. 2018, 93, 339–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. Referenced with Permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hematopoietic Cell Transplantation (HCT); Version 2.2022; National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Goldstein, E.J.C.; Owens, R.C., Jr.; Nolin, T.D. Antimicrobial-Associated QT Interval Prolongation: Pointes of Interest. Clin. Infect. Dis. 2006, 43, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Teo, Y.L.; Ho, H.K.; Chan, A. Metabolism-related Pharmacokinetic Drug−drug Interactions with Tyrosine Kinase Inhibitors: Current Understanding, Challenges and Recommendations. Br. J. Clin. Pharmacol. 2015, 79, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Leather, H.L. Drug Interactions in the Hematopoietic Stem Cell Transplant (HSCT) Recipient: What Every Transplanter Needs to Know. Bone Marrow Transplant. 2004, 33, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Glotzbecker, B.; Duncan, C.; Alyea, E.; Campbell, B.; Soiffer, R. Important Drug Interactions in Hematopoietic Stem Cell Transplantation: What Every Physician Should Know. Biol. Blood Marrow Transplant. 2012, 18, 989–1006. [Google Scholar] [CrossRef] [Green Version]
- Klastersky, J. Management of Fever in Neutropenic Patients with Different Risks of Complications. Clin. Infect. Dis. 2004, 39, S32–S37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesher, L.; Rolston, K.V.I. The Current Spectrum of Infection in Cancer Patients with Chemotherapy Related Neutropenia. Infection 2014, 42, 5–13. [Google Scholar] [CrossRef]
- Pillinger, K.E.; Bouchard, J.; Withers, S.T.; Mediwala, K.; McGee, E.U.; Gibson, G.M.; Bland, C.M.; Bookstaver, P.B. Inpatient Antibiotic Stewardship Interventions in the Adult Oncology and Hematopoietic Stem Cell Transplant Population: A Review of the Literature. Ann. Pharmacother. 2020, 54, 594–610. [Google Scholar] [CrossRef]
- Amanati, A.; Sajedianfard, S.; Khajeh, S.; Ghasempour, S.; Mehrangiz, S.; Nematolahi, S.; Shahhosein, Z. Bloodstream Infections in Adult Patients with Malignancy, Epidemiology, Microbiology, and Risk Factors Associated with Mortality and Multi-Drug Resistance. BMC Infect. Dis. 2021, 21, 636. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Lagana, D.; Catford, J.; Shaw, D.; Bak, N. Bloodstream Infections in Neutropenic Patients with Haematological Malignancies. Infect. Dis. Health 2020, 25, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Le Clech, L.; Talarmin, J.-P.; Couturier, M.-A.; Ianotto, J.-C.; Nicol, C.; Le Calloch, R.; Dos Santos, S.; Hutin, P.; Tandé, D.; Cogulet, V.; et al. Early Discontinuation of Empirical Antibacterial Therapy in Febrile Neutropenia: The ANTIBIOSTOP Study. Infect. Dis. 2018, 50, 539–549. [Google Scholar] [CrossRef]
- Aguilar-Guisado, M.; Espigado, I.; Martín-Peña, A.; Gudiol, C.; Royo-Cebrecos, C.; Falantes, J.; Vázquez-López, L.; Montero, M.I.; Rosso-Fernández, C.; de la Luz Martino, M.; et al. Optimisation of Empirical Antimicrobial Therapy in Patients with Haematological Malignancies and Febrile Neutropenia (How Long Study): An Open-Label, Randomised, Controlled Phase 4 Trial. Lancet Haematol. 2017, 4, e573–e583. [Google Scholar] [CrossRef]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European Guidelines for Empirical Antibacterial Therapy for Febrile Neutropenic Patients in the Era of Growing Resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef] [Green Version]
- Satlin, M.J.; Weissman, S.J.; Carpenter, P.A.; Seo, S.K.; Shelburne, S.A. American Society of Transplantation and Cellular Therapy Series, 1: Enterobacterales Infection Prevention and Management after Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2021, 27, 108–114. [Google Scholar] [CrossRef]
- Bucaneve, G.; Micozzi, A.; Menichetti, F.; Martino, P.; Dionisi, M.S.; Martinelli, G.; Allione, B.; D’Antonio, D.; Buelli, M.; Nosari, A.M.; et al. Levofloxacin to Prevent Bacterial Infection in Patients with Cancer and Neutropenia. N. Engl. J. Med. 2005, 353, 977–987. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, J.G.; Perl, T.M. The New Clostridium Difficile—What Does It Mean? N. Engl. J. Med. 2005, 353, 2503–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satlin, M.J.; Chavda, K.D.; Baker, T.M.; Chen, L.; Shashkina, E.; Soave, R.; Small, C.B.; Jacobs, S.E.; Shore, T.B.; van Besien, K.; et al. Colonization With Levofloxacin-Resistant Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae and Risk of Bacteremia in Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. 2018, 67, 1720–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, A.J.; Freifeld, A.G. Optimal Management of Neutropenic Fever in Patients With Cancer. J. Oncol. Pract. 2019, 15, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Flowers, C.R.; Seidenfeld, J.; Bow, E.J.; Karten, C.; Gleason, C.; Hawley, D.K.; Kuderer, N.M.; Langston, A.A.; Marr, K.A.; Rolston, K.V.I.; et al. Antimicrobial Prophylaxis and Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2013, 31, 794–810. [Google Scholar] [CrossRef] [Green Version]
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Vidal, L.; Lawrie, T.A.; van de Wetering, M.D.; Kremer, L.C.; Leibovici, L. Antibiotic Prophylaxis for Bacterial Infections in Afebrile Neutropenic Patients Following Chemotherapy. Cochrane Database Syst. Rev. 2012, 2018, CD004386. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Martino, B.; Specchia, G.; Pastore, D.; Stanzani, M.; Cattaneo, C.; Fanci, R.; et al. Invasive Aspergillosis in Patients with Acute Myeloid Leukemia: A SEIFEM-2008 Registry Study. Haematologica 2010, 95, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.Y.; Lee, D.G.; Yeh, S.P.; Bhurani, D.; Khanh, B.Q.; Low, C.Y.; Norasetthada, L.; Chan, T.; Kwong, Y.L.; Vaid, A.K.; et al. Epidemiology of Invasive Fungal Diseases among Patients with Haematological Disorders in the Asia-Pacific: A Prospective Observational Study. Clin. Microbiol. Infect. 2015, 21, 594.e7–594.e11. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and Outcome of Invasive Fungal Infection in Adult Hematopoietic Stem Cell Transplant Recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uneno, Y.; Imura, H.; Makuuchi, Y.; Tochitani, K.; Watanabe, N. Pre-Emptive Antifungal Therapy versus Empirical Antifungal Therapy for Febrile Neutropenia in People with Cancer. Cochrane Database Syst. Rev. 2022, 2022, 5. [Google Scholar] [CrossRef]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European Guidelines for Primary Antifungal Prophylaxis in Adult Haematology Patients: Summary of the Updated Recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Khanina, A.; Tio, S.Y.; Ananda-Rajah, M.R.; Kidd, S.E.; Williams, E.; Chee, L.; Urbancic, K.; Thursky, K.A.; Australasian Antifungal Guidelines Steering Committee; Slavin, M.A.; et al. Consensus Guidelines for Antifungal Stewardship, Surveillance and Infection Prevention, 2021. Intern. Med. J. 2021, 51, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Lewis, R.E.; Dodds Ashley, E.S.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R.; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; Cornely, O.A.; et al. Core Recommendations for Antifungal Stewardship: A Statement of the Mycoses Study Group Education and Research Consortium. J. Infect. Dis. 2020, 222, S175–S198. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [Green Version]
- Science, M.; Timberlake, K. Antifungal Stewardship: A Budding Branch of Antimicrobial Stewardship. Pediatr. Blood Cancer 2020, 67, e28145. [Google Scholar] [CrossRef]
- Michallet, M.; Sobh, M.; Deray, G.; Gangneux, J.-P.; Pigneux, A.; Larrey, D.; Ribaud, P.; Mira, J.-P.; Nivoix, Y.; Yakoub-Agha, I.; et al. Antifungal Stewardship in Hematology: Reflection of a Multidisciplinary Group of Experts. Clin. Lymphoma Myeloma Leuk. 2021, 21, 35–45. [Google Scholar] [CrossRef]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I Treat CMV Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef]
- Green, M.L.; Leisenring, W.; Xie, H.; Mast, T.C.; Cui, Y.; Sandmaier, B.M.; Sorror, M.L.; Goyal, S.; Özkök, S.; Yi, J.; et al. Cytomegalovirus Viral Load and Mortality after Haemopoietic Stem Cell Transplantation in the Era of Pre-Emptive Therapy: A Retrospective Cohort Study. Lancet Haematol. 2016, 3, e119–e127. [Google Scholar] [CrossRef] [Green Version]
- Merck & Co., Inc. Letermovir [Package Insert]. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209939Orig1s000,209940Orig1s000lbl.pdf (accessed on 22 January 2023).
- Cesaro, S.; Ljungman, P.; Tridello, G.; Mikulska, M.; Wendel, L.; Styczynski, J.; Averbuch, D.; de la Camara, R. New Trends in the Management of Cytomegalovirus Infection after Allogeneic Hematopoietic Cell Transplantation: A Survey of the Infectious Diseases Working Pary of EBMT. Bone Marrow Transplant. 2022, 58, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, S.S.; Papanicolaou, G.; Boeckh, M. How I Prevent Viral Reactivation in High-Risk Patients. Blood 2022. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, A.; Candoni, A.; Gottardi, M.; Facchin, G.; Stella, R.; De Marchi, R.; Michelutti, A.; Cavallin, M.; Rosignoli, C.; Patriarca, F.; et al. Cytomegalovirus Prophylaxis versus Pre-Emptive Strategy: Different CD4+ and CD8+ T Cell Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Transplant. Cell. Ther. 2021, 27, 518.e1–518.e4. [Google Scholar] [CrossRef] [PubMed]
- Takeda Pharmaceuticals, Inc. Maribavir [Package Insert]. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215596lbl.pdf (accessed on 22 January 2023).
- Gilead Sciences, Inc. Cidofovir [Package Insert]. 2000. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/020638s003lbl.pdf (accessed on 22 January 2023).
- Jorgenson, M.R.; Descourouez, J.L.; Kleiboeker, H.; Goldrosen, K.; Schulz, L.; Rice, J.P.; Odorico, J.S.; Mandelbrot, D.A.; Smith, J.A.; Saddler, C.M. Cytomegalovirus Antiviral Stewardship in Solid Organ Transplant Recipients: A New Gold Standard. Transpl. Infect. Dis. 2022, 24, e13864. [Google Scholar] [CrossRef]
- Jorgenson, M.R.; Descourouez, J.L.; Schulz, L.T.; Goldrosen, K.A.; Rice, J.P.; Redfield, R.R.; Saddler, C.M.; Smith, J.A.; Mandelbrot, D.A. The Development and Implementation of Stewardship Initiatives to Optimize the Prevention and Treatment of Cytomegalovirus Infection in Solid-Organ Transplant Recipients. Infect. Control Hosp. Epidemiol. 2020, 41, 1068–1074. [Google Scholar] [CrossRef]
- Anton-Vazquez, V.; Mehra, V.; Mbisa, J.L.; Bradshaw, D.; Basu, T.N.; Daly, M.-L.; Mufti, G.J.; Pagliuca, A.; Potter, V.; Zuckerman, M. Challenges of Aciclovir-Resistant HSV Infection in Allogeneic Bone Marrow Transplant Recipients. J. Clin. Virol. 2020, 128, 104421. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [Green Version]
- Bartash, R.; McCort, M.E.; Cowman, K.; Orner, E.; Szymczak, W.; Nori, P.; Nori, P. 192. A Hematology/Oncology Unit-Specific Antibiogram Emphasizes the Need for Intensified Local Stewardship. Open Forum Infect. Dis. 2020, 7, S101–S102. [Google Scholar] [CrossRef]
- Smith, Z.R.; Tajchman, S.K.; Dee, B.M.; Bruno, J.J.; Qiao, W.; Tverdek, F.P. Development of a Combination Antibiogram for Pseudomonas Aeruginosa Bacteremia in an Oncology Population. J. Oncol. Pharm. Pract. 2016, 22, 409–415. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium Difficile Colitis: Pathogenesis and Host Defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Schuster, M.G.; Cleveland, A.A.; Dubberke, E.R.; Kauffman, C.A.; Avery, R.K.; Husain, S.; Paterson, D.L.; Silveira, F.P.; Chiller, T.M.; Benedict, K.; et al. Infections in Hematopoietic Cell Transplant Recipients: Results From the Organ Transplant Infection Project, a Multicenter, Prospective, Cohort Study. Open Forum Infect. Dis. 2017, 4, ofx050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misch, E.A.; Safdar, N. Clostridioides Difficile Infection in the Stem Cell Transplant and Hematologic Malignancy Population. Infect. Dis. Clin. N. Am. 2019, 33, 447–466. [Google Scholar] [CrossRef]
- Shouval, R.; Waters, N.R.; Gomes, A.L.C.; Zuanelli Brambilla, C.; Fei, T.; Devlin, S.M.; Nguyen, C.L.; Markey, K.A.; Dai, A.; Slingerland, J.B.; et al. Conditioning Regimens Are Associated with Distinct Patterns of Microbiota Injury in Allogeneic Hematopoietic Cell Transplantation. Clin. Cancer Res. 2023, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss of Diversity Is Associated with Use of Systemic Antibiotics and More Pronounced in Gastrointestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Lindner, S.; Gomes, A.L.C.; Devlin, S.M.; Shah, G.L.; Sung, A.D.; Sauter, C.S.; Landau, H.J.; Dahi, P.B.; Perales, M.-A.; et al. Fecal Microbiota Diversity Disruption and Clinical Outcomes after Auto-HCT: A Multicenter Observational Study. Blood 2021, 137, 1527–1537. [Google Scholar] [CrossRef]
- Alonso, C.D.; Maron, G.; Kamboj, M.; Carpenter, P.A.; Gurunathan, A.; Mullane, K.M.; Dubberke, E.R. American Society for Transplantation and Cellular Therapy Series: #5—Management of Clostridioides Difficile Infection in Hematopoietic Cell Transplant Recipients. Transplant. Cell. Ther. 2022, 28, 225–232. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W.; et al. Gut Microbiome Signatures Are Predictive of Infectious Risk Following Induction Therapy for Acute Myeloid Leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Weber, D.; Jenq, R.R.; Peled, J.U.; Taur, Y.; Hiergeist, A.; Koestler, J.; Dettmer, K.; Weber, M.; Wolff, D.; Hahn, J.; et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-Related Mortality with Broad-Spectrum Antibiotic Use after Allogeneic Hematopoietic Stem Cell Transplantation in Human Patients and Mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, J.S.; Young, R.R.; Heston, S.M.; Jenkins, K.; Spees, L.P.; Sung, A.D.; Corbet, K.; Thompson, J.C.; Bohannon, L.; Martin, P.L.; et al. Anaerobic Antibiotics and the Risk of Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Deak, E.; Charlton, C.L.; Bobenchik, A.M.; Miller, S.A.; Pollett, S.; McHardy, I.H.; Wu, M.T.; Garner, O.B. Comparison of the Vitek MS and Bruker Microflex LT MALDI-TOF MS Platforms for Routine Identification of Commonly Isolated Bacteria and Yeast in the Clinical Microbiology Laboratory. Diagn. Microbiol. Infect. Dis. 2015, 81, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Charnot-Katsikas, A.; Tesic, V.; Love, N.; Hill, B.; Bethel, C.; Boonlayangoor, S.; Beavis, K.G. Use of the Accelerate Pheno System for Identification and Antimicrobial Susceptibility Testing of Pathogens in Positive Blood Cultures and Impact on Time to Results and Workflow. J. Clin. Microbiol. 2018, 56, e01166-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacobbe, D.R.; Giani, T.; Bassetti, M.; Marchese, A.; Viscoli, C.; Rossolini, G.M. Rapid Microbiological Tests for Bloodstream Infections Due to Multidrug Resistant Gram-Negative Bacteria: Therapeutic Implications. Clin. Microbiol. Infect. 2020, 26, 713–722. [Google Scholar] [CrossRef]
- Banerjee, R.; Teng, C.B.; Cunningham, S.A.; Ihde, S.M.; Steckelberg, J.M.; Moriarty, J.P.; Shah, N.D.; Mandrekar, J.N.; Patel, R. Randomized Trial of Rapid Multiplex Polymerase Chain Reaction–Based Blood Culture Identification and Susceptibility Testing. Clin. Infect. Dis. 2015, 61, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Komarow, L.; Virk, A.; Rajapakse, N.; Schuetz, A.N.; Dylla, B.; Earley, M.; Lok, J.; Kohner, P.; Ihde, S.; et al. Randomized Trial Evaluating Clinical Impact of RAPid Identification and Susceptibility Testing for Gram-Negative Bacteremia: RAPIDS-GN. Clin. Infect. Dis. 2021, 73, e39–e46. [Google Scholar] [CrossRef]
- Douglas, A.P.; Hall, L.; James, R.S.; Worth, L.J.; Slavin, M.A.; Thursky, K.A. Quality of Inpatient Antimicrobial Use in Hematology and Oncology Patients. Infect. Control. Hosp. Epidemiol. 2021, 42, 1235–1244. [Google Scholar] [CrossRef]
- Aitken, S.L.; Nagel, J.L.; Abbo, L.; Alegria, W.; Barreto, J.N.; Dadwal, S.; Freifeld, A.G.; Jain, R.; Pergam, S.A.; Tverdek, F.P.; et al. Antimicrobial Stewardship in Cancer Patients: The Time Is Now. J. Natl. Compr. Canc. Netw. 2019, 17, 772–775. [Google Scholar] [CrossRef] [Green Version]
| CDC Core Element | Sample Interventions Focused on HSCT/Oncology Patients |
|---|---|
| Hospital Leadership Commitment Dedicate necessary human, financial, and information technology resources. | Accessible information systems (e.g., electronic medical record, surveillance data) Dedicated staff for antimicrobial stewardship |
| Accountability Appoint a leader or co-leaders, such as a physician and pharmacist, responsible for program management and outcomes. | Multidisciplinary approach among hematology/oncology, infectious disease, and pharmacy (“handshake stewardship”) |
| Pharmacy Expertise Appoint a pharmacist, ideally as the co-leader of the stewardship program, to lead implementation efforts to improve antibiotic use. | Antibacterial, antifungal, and antiviral prophylaxis Dose optimization (e.g., extended infusion of beta-lactams) Duration of empiric antimicrobials for febrile neutropenia IV to PO conversion |
| Action Implement interventions, such as prospective auditing and feedback, or preauthorization, to improve antibiotic use. | Development of population specific guidelines Febrile neutropenia Antifungal prophylaxis and treatment Cytomegalovirus prophylaxis Use of microbiology methods to assist with prescribing |
| Tracking Monitor antibiotic prescribing, impact of interventions, and other important outcomes such as C. difficile infection and resistance patterns. | Population- and/or unit-specific antibiograms Prevalence of MDRO Prospective audit and formulary restriction |
| Reporting Regularly report information on antibiotic use and resistance to prescribers, pharmacists, nurses, and hospital leadership. | Tracking and shared reporting of outcomes specific to HSCT/oncology C. difficile Catheter-related infections Prevalence of MDRO |
| Education Educate prescribers, pharmacists, and nurses about adverse reactions to antibiotics, antibiotic resistance, and optimal prescribing. | Population-specific antibiograms Microbiome diversity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumdar, A.; Shah, M.R.; Park, J.J.; Narayanan, N.; Kaye, K.S.; Bhatt, P.J. Challenges and Opportunities in Antimicrobial Stewardship among Hematopoietic Stem Cell Transplant and Oncology Patients. Antibiotics 2023, 12, 592. https://doi.org/10.3390/antibiotics12030592
Majumdar A, Shah MR, Park JJ, Narayanan N, Kaye KS, Bhatt PJ. Challenges and Opportunities in Antimicrobial Stewardship among Hematopoietic Stem Cell Transplant and Oncology Patients. Antibiotics. 2023; 12(3):592. https://doi.org/10.3390/antibiotics12030592
Chicago/Turabian StyleMajumdar, Anjali, Mansi R. Shah, Jiyeon J. Park, Navaneeth Narayanan, Keith S. Kaye, and Pinki J. Bhatt. 2023. "Challenges and Opportunities in Antimicrobial Stewardship among Hematopoietic Stem Cell Transplant and Oncology Patients" Antibiotics 12, no. 3: 592. https://doi.org/10.3390/antibiotics12030592
APA StyleMajumdar, A., Shah, M. R., Park, J. J., Narayanan, N., Kaye, K. S., & Bhatt, P. J. (2023). Challenges and Opportunities in Antimicrobial Stewardship among Hematopoietic Stem Cell Transplant and Oncology Patients. Antibiotics, 12(3), 592. https://doi.org/10.3390/antibiotics12030592




