Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections
Abstract
:1. Introduction
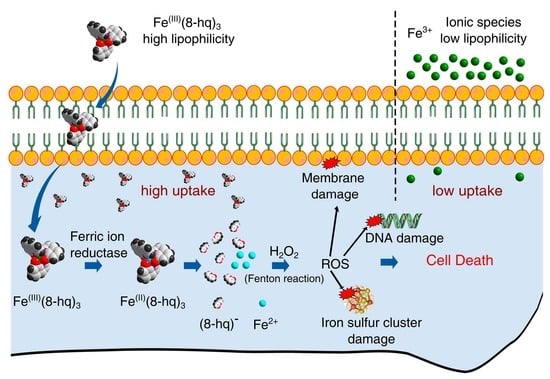
2. Results and Discussion
2.1. Synthesis and Characterization of Fe(8-hq)3
2.2. Evaluation of In Vitro Antibacterial Activity against SA
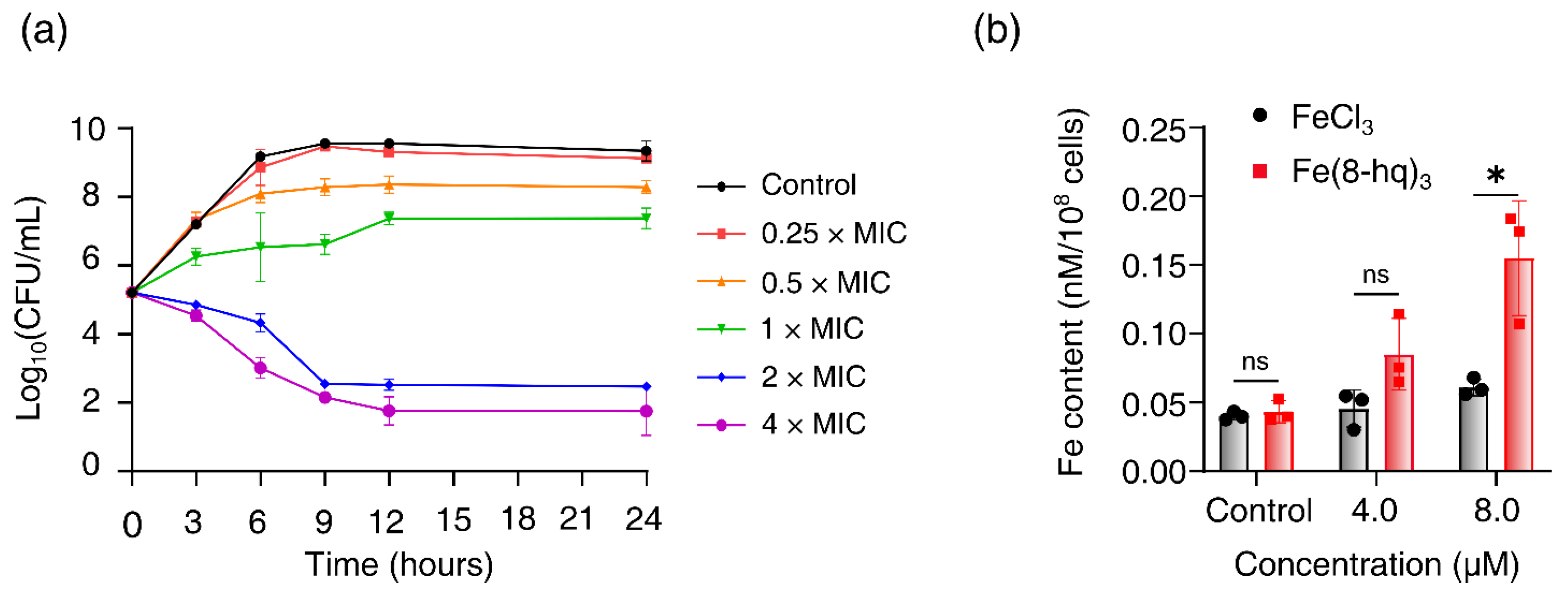
2.3. Time–Kill Assay
2.4. Cellular Uptake Studies
2.5. Inhibition of MRSAα Biofilm Formation
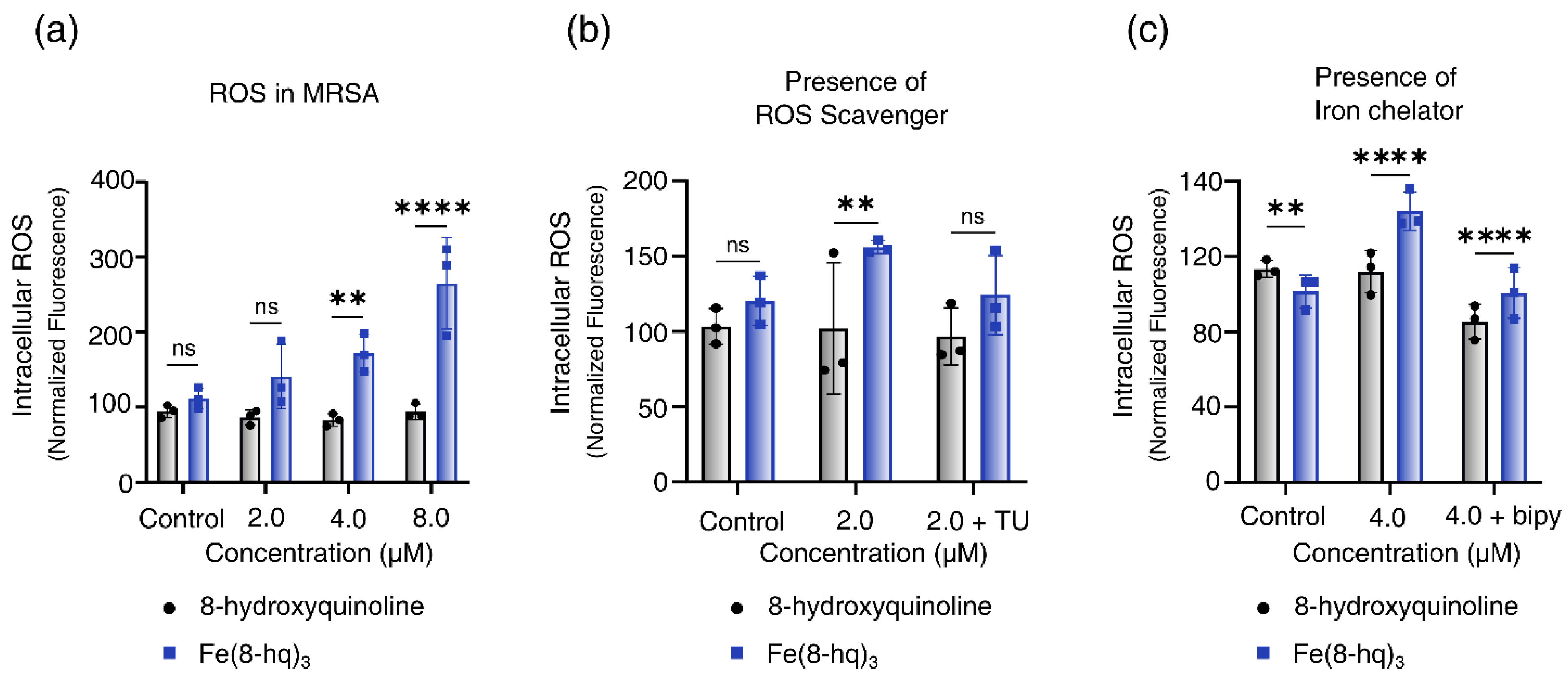
2.6. Measurements of Intracellular Generation of ROS
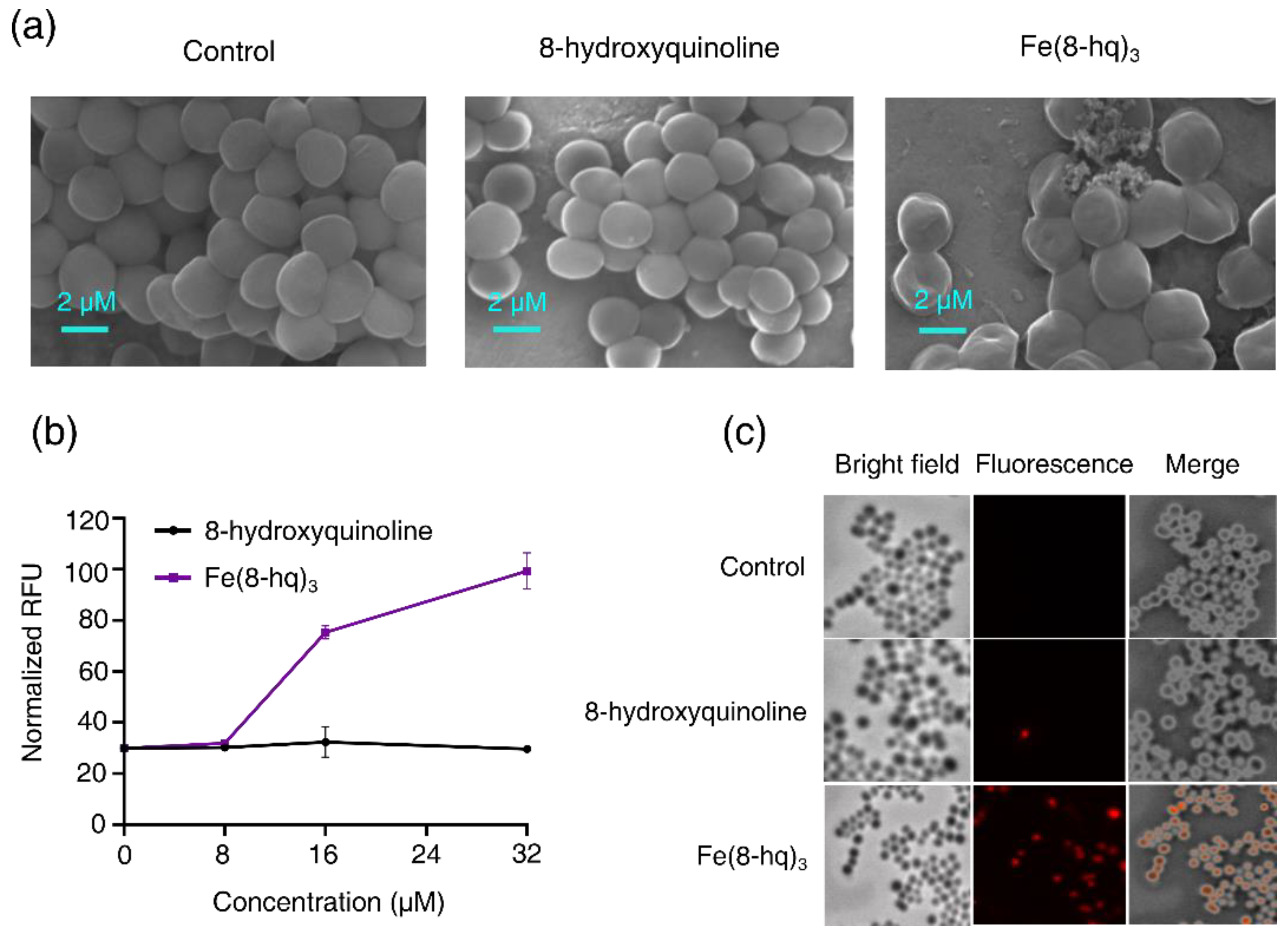
2.7. Imaging Studies of Bacterial Cells by SEM
2.8. The Damaging Effect of Fe(8-hq)3 on Cell Membrane of MRSAα
2.9. Bactericidal Activity of RAW 264.7 Macrophages Promoted by Fe(8-hq)3 against Internalized SA Bacteria
2.10. The In Vitro Evaluation of Resistance Development
2.11. Checkerboard Assays
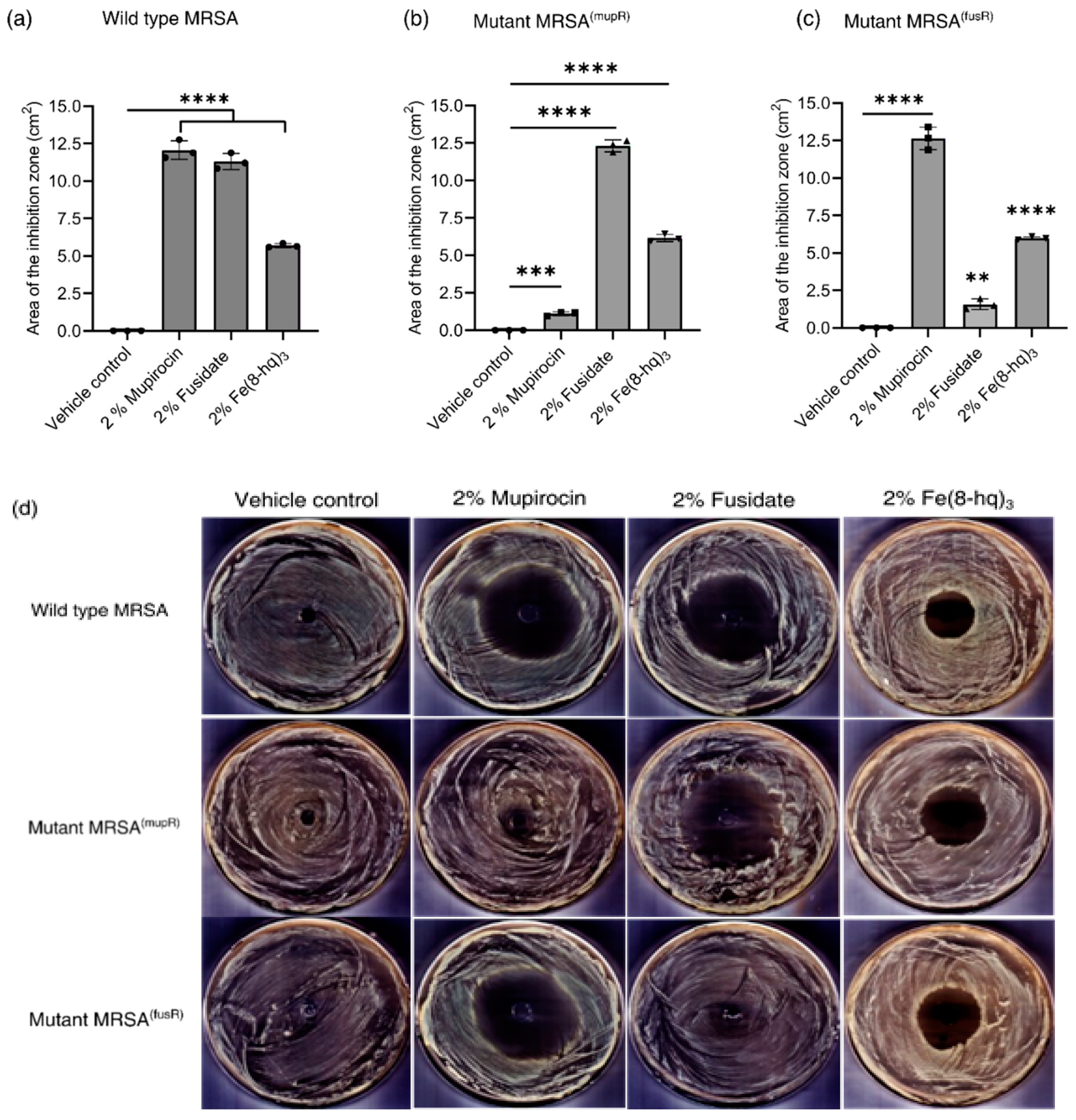
2.12. In Vitro Antimicrobial Effects of 2% Fe(8-hq)3 Ointment
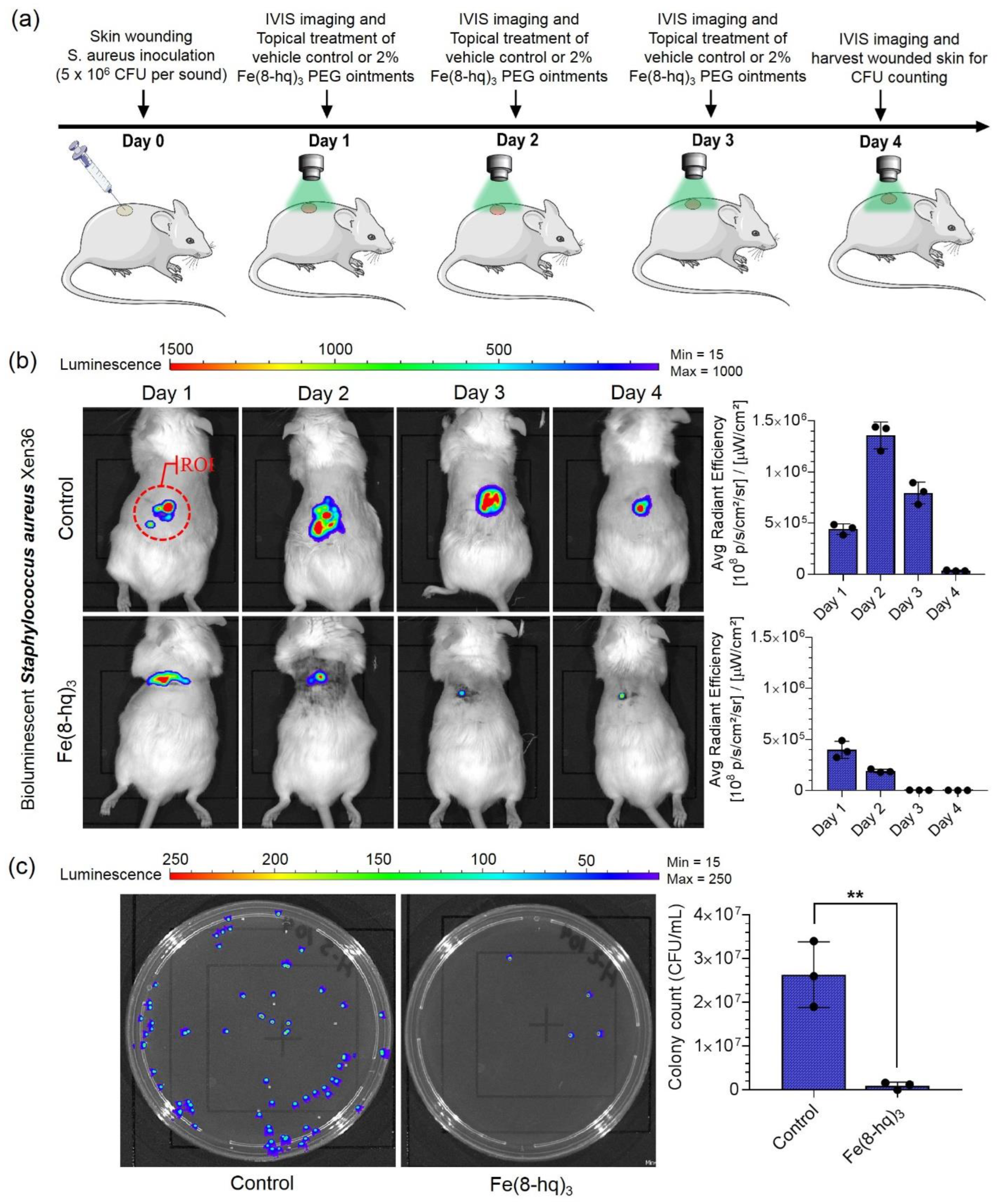
2.13. The In Vivo Validation of Therapeutic Efficacy of 2% Fe(8-hq)3 Ointment
3. Materials and Methods
3.1. Synthesis of Fe(8-hq)3
3.2. Stability of Fe(8-hq)3 in the Bacterial Cell Culture Medium
3.3. Evaluation of Minimum Inhibitory Concentrations (MICs)
3.4. Investigation of Antibacterial Activity of 8-Hydroxyquinoline and Fe(8-hq)3
3.5. Evaluation of Biofilm Inhibition
3.6. Measurements of Intracellular Iron Concentrations
3.7. Time–Kill Assays
3.8. Determination of Intracellular ROS Generation
3.9. Measurements of Intracellular ROS Scavenging Effect
3.10. Measurements of Cellular Membrane Permeabilization
3.11. SEM Imaging Studies of Bacterial Morphology
3.12. The In Vitro Assays of Resistance Development
3.13. Preparation of High-Level Mupirocin- and Fusidate-Resistant MRSAα Strains of SA Bacteria
3.14. Evaluation of Cytotoxicity of Fe(8-hq)3 in RAW 264.7 Cells
3.15. Measurements of Intracellular ROS Generation in Macrophage-like Cells
3.16. Quantitative Measurements of Bactericidal Activity of Macrophages
3.17. Checkerboard Assays
3.18. Preparation of Topical Ointments Containing 2% Mupirocin, 2% Fusidate or 2% Fe(8-hq)3, Respectively
3.19. Validation of In Vitro Antimicrobial Efficacy of the Ointment
3.20. Excisional Murine Skin Wound Infection Model with S. aureus
3.21. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, G.T.; Suaya, J.A.; Baxter, R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a US population: A retrospective population-based study. BMC Infect. Dis. 2013, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Spernovasilis, N.; Psichogiou, M.; Poulakou, G. Skin manifestations of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2021, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A. The fight against antimicrobial resistance is confounded by a global increase in antibiotic usage. ACS Infect. Dis. 2018, 4, 868–870. [Google Scholar] [CrossRef]
- Hajikhani, B.; Goudarzi, M.; Kakavandi, S.; Amini, S.; Zamani, S.; van Belkum, A.; Goudarzi, H.; Dadashi, M. The global prevalence of fusidic acid resistance in clinical isolates of Staphylococcus aureus: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2021, 10, 75. [Google Scholar] [CrossRef]
- Howden, B.P.; Grayson, M.L. Dumb and dumber—The potential waste of a useful antistaphylococcal agent: Emerging fusidic acid resistance in Staphylococcus aureus. Clin. Infect. Dis. 2006, 42, 394–400. [Google Scholar] [CrossRef]
- Brown, E.M.; Thomas, P. Fusidic acid resistance in Staphylococcus aureus isolates. Lancet 2002, 359, 803. [Google Scholar] [CrossRef]
- Mason, B.W.; Howard, A.J.; Magee, J.T. Fusidic acid resistance in community isolates of methicillin-susceptible Staphylococcus aureus and fusidic acid prescribing. J. Antimicrob. Chemother. 2003, 51, 1033–1036. [Google Scholar] [CrossRef]
- Charani, E.; Holmes, A. Antibiotic stewardship—Twenty years in the making. Antibiotics 2019, 8, 7. [Google Scholar] [CrossRef]
- Charani, E.; Castro-Sánchez, E.; Sevdalis, N.; Kyratsis, Y.; Drumright, L.; Shah, N.; Holmes, A. Understanding the determinants of antimicrobial prescribing within hospitals: The role of “prescribing etiquette”. Clin. Infect. Dis. 2013, 57, 188–196. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, B.; Alamri, H.; Yarabarla, S.; Kim, M.H.; Huang, S.D. KCa(H2O)2 [FeIII(CN)6]⋅H2O nanoparticles as an antimicrobial agent against Staphylococcus aureus. Angew. Chem. 2018, 130, 2236–2240. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Benin, B.M.; Yu, B.; Bunge, S.D.; Abeydeera, N.; Huang, S.D.; Kim, M.-H. Lipophilic Ga complex with broad-spectrum antimicrobial activity and the ability to overcome gallium resistance in both Pseudomonas aeruginosa and Staphylococcus aureus. J. Med. Chem. 2021, 64, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, T.M.; Dassanayake, A.C.; Abeydeera, N.; Pant, B.D.; Jaroniec, M.; Kim, M.-H.; Huang, S.D. An aluminum lining to the dark cloud of silver resistance: Harnessing the power of potent antimicrobial activity of γ-alumina nanoparticles. Biomater. Sci. 2021, 9, 7996–8006. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Yu, B.; Pant, B.D.; Kim, M.-H.; Huang, S.D. Harnessing the toxicity of dysregulated iron uptake for killing Staphylococcus aureus: Reality or mirage? Biomater. Sci. 2022, 10, 474–484. [Google Scholar] [CrossRef]
- Pant, B.D.; Benin, B.M.; Abeydeera, N.; Kim, M.-H.; Huang, S.D. Bi2O3 nanoparticles exhibit potent broad-spectrum antimicrobial activity and the ability to overcome Ag-, ciprofloxacin- and meropenem-resistance in P. aeruginosa: The next silver bullet of metal antimicrobials? Biomater. Sci. 2022, 10, 1523–1531. [Google Scholar] [CrossRef]
- Pippi, B.; Joaquim, A.; Lopes, W.; Machado, G.; Bergamo, V.; Giuliani, L.; Abegg, M.; Cruz, L.; Vainstein, M.; Fuentefria, A. 8-Hydroxyquinoline-5-sulfonamides are promising antifungal candidates for the topical treatment of dermatomycosis. J. Appl. Microbiol. 2020, 128, 1038–1049. [Google Scholar] [CrossRef]
- Kassem, E.M.; El-Sawy, E.R.; Abd-Alla, H.I.; Mandour, A.H.; Abdel-Mogeed, D.; El-Safty, M.M. Synthesis, antimicrobial, and antiviral activities of some new 5-sulphonamido-8-hydroxyquinoline derivatives. Arch. Pharmacal Res. 2012, 35, 955–964. [Google Scholar] [CrossRef]
- Pape, V.F.; May, N.V.; Gál, G.T.; Szatmári, I.; Szeri, F.; Fülöp, F.; Szakács, G.; Enyedy, É.A. Impact of copper and iron binding properties on the anticancer activity of 8-hydroxyquinoline derived Mannich bases. Dalton Trans. 2018, 47, 17032–17045. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.A.; Melander, C. Construction and screening of a 2-aminoimidazole library identifies a small molecule capable of inhibiting and dispersing bacterial biofilms across order, class, and phylum. Angew. Chem. Int. Ed. 2008, 47, 5229–5231. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Bey, E.A.; Boothman, D.A.; Gao, J. Superparamagnetic iron oxide nanoparticles: Amplifying ROS stress to improve anticancer drug efficacy. Theranostics 2013, 3, 116. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, Z.; Almutairi, L.; Huang, S.; Kim, M.-H. Harnessing iron-oxide nanoparticles towards the improved bactericidal activity of macrophage against Staphylococcus aureus. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102158. [Google Scholar] [CrossRef]
- Złoch, M.; Pomastowski, P.; Maślak, E.; Monedeiro, F.; Buszewski, B. Study on molecular profiles of Staphylococcus aureus strains: Spectrometric approach. Molecules 2020, 25, 4894. [Google Scholar] [CrossRef]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh, T.J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Divakara, S. L-Lysine based lipidated biphenyls as agents with anti-biofilm and anti-inflammatory properties that also inhibit intracellular bacteria. Chem. Commun. 2017, 53, 8427–8430. [Google Scholar]
- Shrestha, S.K.; Fosso, M.Y.; Green, K.D.; Garneau-Tsodikova, S. Amphiphilic tobramycin analogues as antibacterial and antifungal agents. Antimicrob. Agents Chemother. 2015, 59, 4861–4869. [Google Scholar] [CrossRef]
- Song, R.; Yu, B.; Friedrich, D.; Li, J.; Shen, H.; Krautscheid, H.; Huang, S.D.; Kim, M.-H. Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant S. aureus. Commun. Biol. 2020, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Abeydeera, N.; Dubadi, R.; Kim, M.-H.; Huang, S.D. Broad-spectrum antimicrobial activity of ultrafine (BiO)2CO3 NPs functionalized with PVP that can overcome the resistance to ciprofloxacin, AgNPs and meropenem in Pseudomonas aeruginosa. Antibiotics 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Alboslemy, T.; Yu, B.; Rogers, T.; Kim, M.-H. Staphylococcus aureus biofilm-conditioned medium impairs macrophage-mediated antibiofilm immune response by upregulating KLF2 expression. Infect. Immun. 2019, 87, e00643-18. [Google Scholar] [CrossRef] [PubMed]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef]
- Blanchard, C.; Brooks, L.; Beckley, A.; Colquhoun, J.; Dewhurst, S.; Dunman, P.M. Neomycin sulfate improves the antimicrobial activity of mupirocin-based antibacterial ointments. Antimicrob. Agents Chemother. 2016, 60, 862–872. [Google Scholar] [CrossRef]

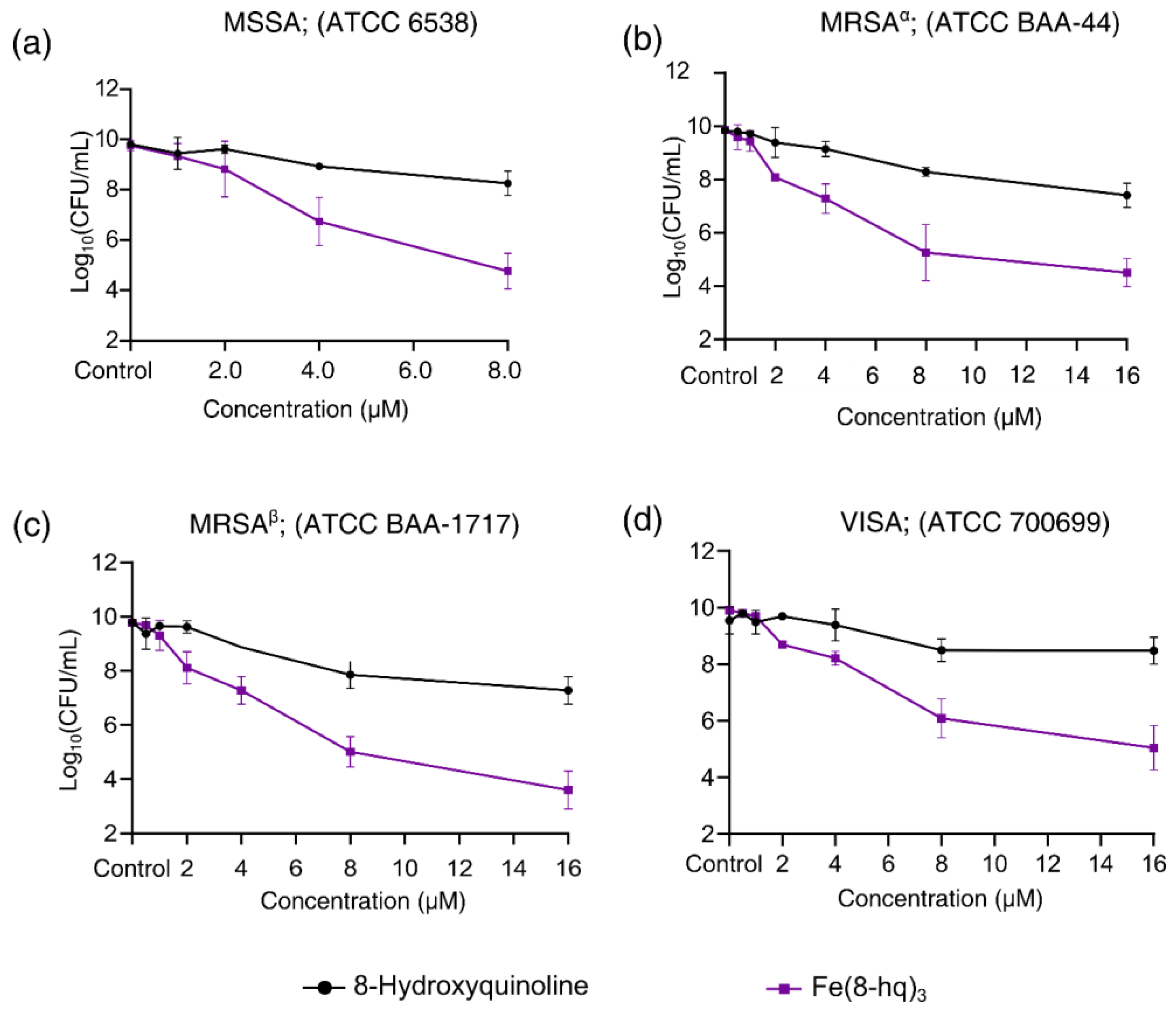

| Bacteria Stains | MIC (in µM) | |
|---|---|---|
| Fe(8-hq)3 | 8-hq | |
| MSSA (ATCC 6538) | 4 µM | 16 µM |
| MRSAα (ATCC BAA-44) | 4 µM | 16 µM |
| MRSAβ (USA 300, ATCC BAA-1717) | 4 µM | 32 µM |
| VISA (ATCC 700699) | 4 µM | 16 µM |
| MICs against MRSAα (ATCC BAA-44) | FIC Index | ||||
|---|---|---|---|---|---|
| (a) | Ciprofloxacin only | Ciprofloxacin with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Ciprofloxacin | |
| 48.0 µM | 6.0 µM | 4.0 µM | 1.0 µM | 0.375 | |
| (b) | MICs against MRSAα (ATCC BAA-44) | FIC Index | |||
| Imipenem only | Imipenem with Fe(8-hq)3 | Fe(8-hq)3 only | Fe(8-hq)3 with Imipenem | ||
| 50.0 µM | 6.25µM | 4.0 µM | 1.0 µM | 0.375 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeydeera, N.; Benin, B.M.; Mudarmah, K.; Pant, B.D.; Chen, G.; Shin, W.S.; Kim, M.-H.; Huang, S.D. Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics 2023, 12, 886. https://doi.org/10.3390/antibiotics12050886
Abeydeera N, Benin BM, Mudarmah K, Pant BD, Chen G, Shin WS, Kim M-H, Huang SD. Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics. 2023; 12(5):886. https://doi.org/10.3390/antibiotics12050886
Chicago/Turabian StyleAbeydeera, Nalin, Bogdan M. Benin, Khalil Mudarmah, Bishnu D. Pant, Guanyu Chen, Woo Shik Shin, Min-Ho Kim, and Songping D. Huang. 2023. "Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections" Antibiotics 12, no. 5: 886. https://doi.org/10.3390/antibiotics12050886
APA StyleAbeydeera, N., Benin, B. M., Mudarmah, K., Pant, B. D., Chen, G., Shin, W. S., Kim, M.-H., & Huang, S. D. (2023). Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics, 12(5), 886. https://doi.org/10.3390/antibiotics12050886