Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Susceptibility
2.2. Growth
2.3. Virulence Factors
2.4. Motility and Biofilm Formation
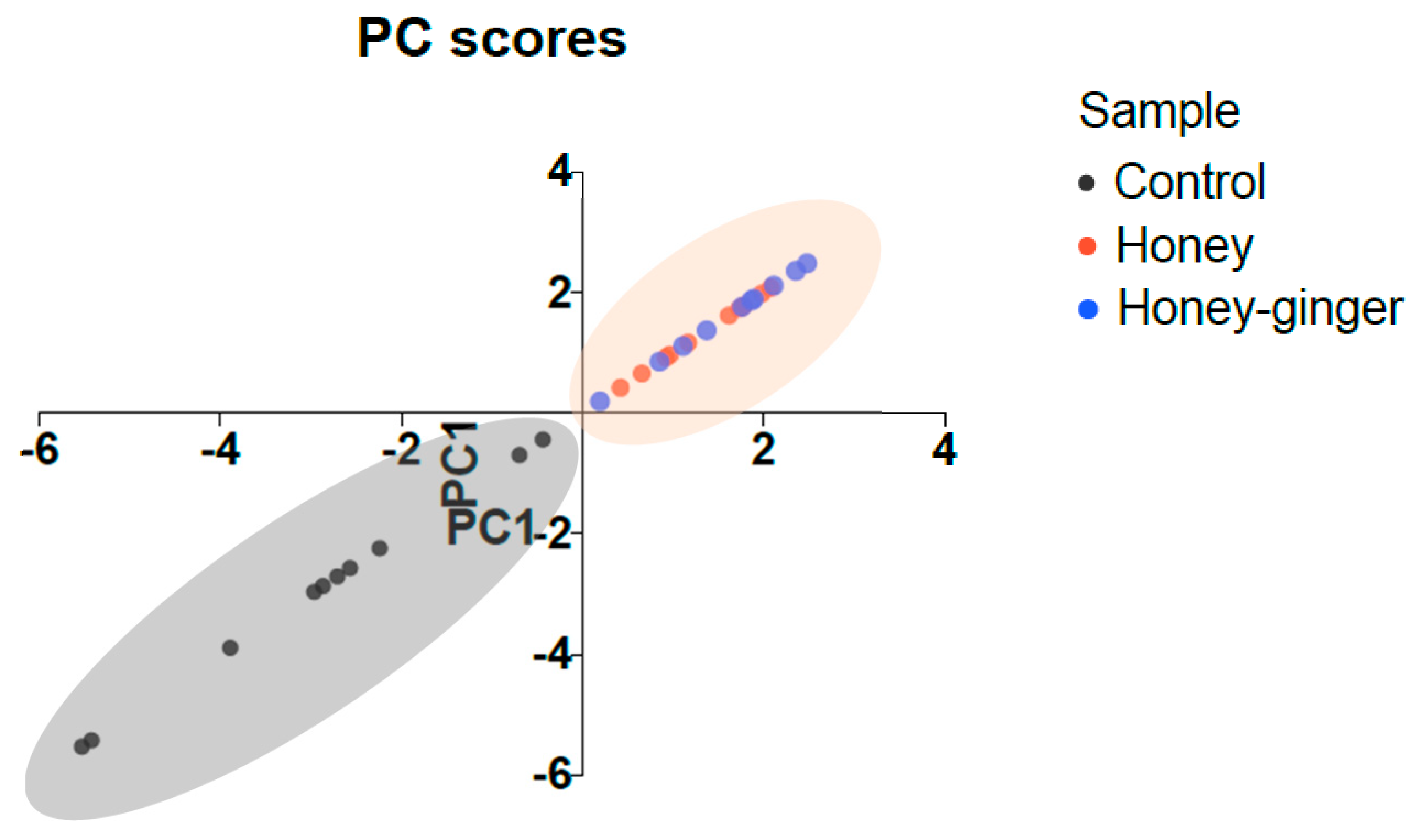
2.5. Chemometric Analysis
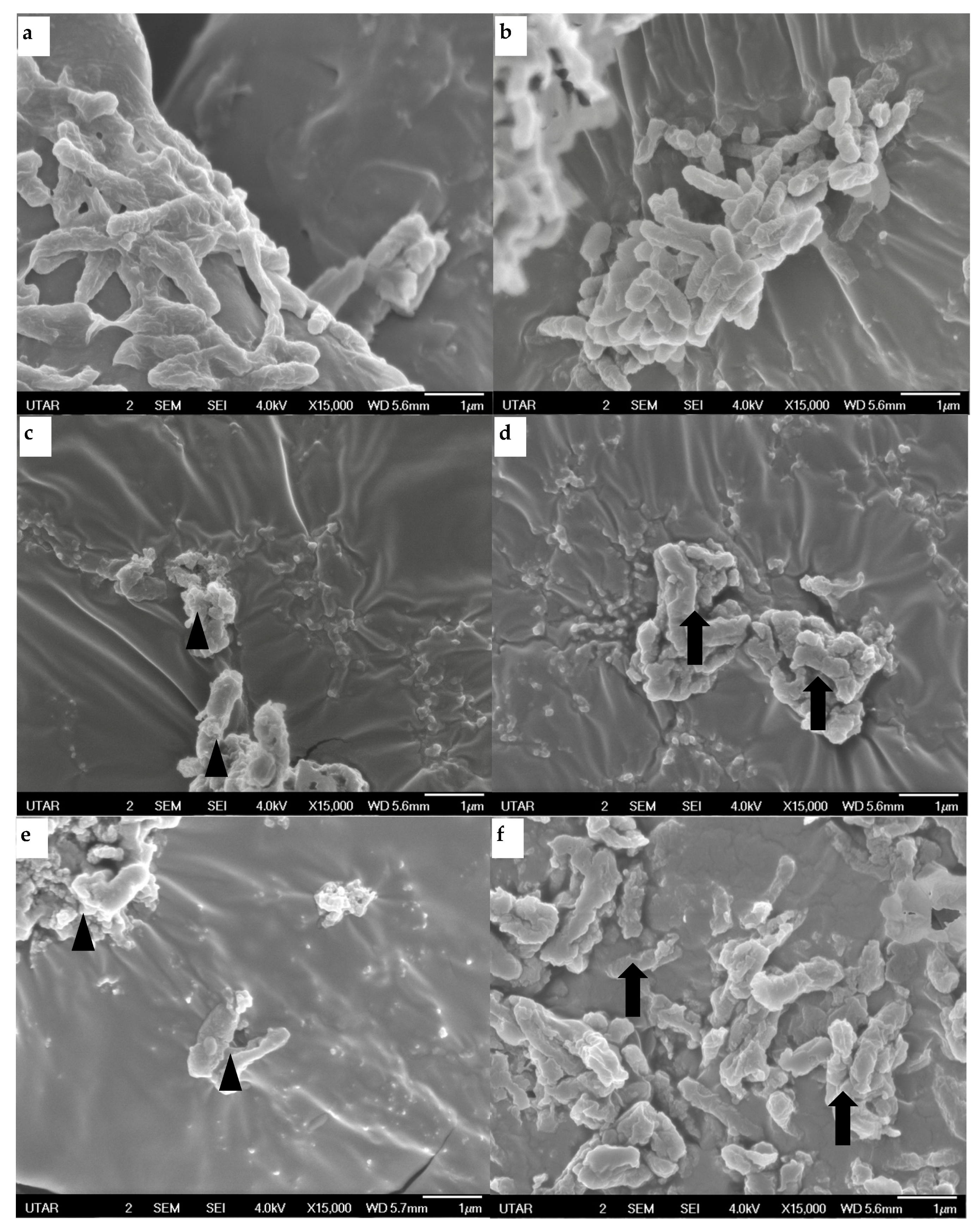
2.6. Morphology
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Bacterial Samples
4.3. Agar Well-Diffusion Assay
4.4. Azocasein Assay
4.5. Pyocyanin Assay
4.6. Exotoxin A Assay
4.7. Swarming and Swimming Motility Assay
4.8. Biofilm Inhibition Assay
4.9. Chemometric Analysis
4.10. Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front. Cell Infect. Microbiol. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 7 May 2022).
- Li, H.; Li, X.; Wang, Z.; Fu, Y.; Ai, Q.; Dong, Y.; Yu, J. Autoinducer-2 regulates Pseudomonas aeruginosa PAO1 biofilm formation and virulence production in a dose-dependent manner. 2015. BMC Microbiol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Antunes, L.C.M.; Ferreira, R.B.; Buckner, M.M.; Finlay, B.B. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L.G. Honey’s ability to counter bacterial infections arises from both bactericidal compounds and QS inhibition. Front. Microbiol. 2012, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, L.; Chhibber, S.; Kumar, R.; Kumar, M.; Harjai, K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 2015, 102, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Gobin, I.; Crnković, G.; Magdalenić, M.; Begić, G.; Babić, A.; Lušić, D.; Vučković, D. Antibacterial potential of Croatian honey against antibiotic resistant pathogenic bacteria. Med. Glas. 2018, 15, 139–144. [Google Scholar]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another alternative in the fight against antibiotic-resistant bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.J.; Sit, N.W.; Ooi, P.A.C.; Ee, K.Y.; Lim, T.M. The antibacterial potential of honeydew honey produced by stingless bee (Heterotrigona itama) against antibiotic resistant bacteria. Antibiotics 2020, 9, 871. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, H.D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE 2013, 8, e76106. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Lee, S.H.; Byun, Y.; Park, H.D. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewnetu, Y.; Lemma, W.; Birhane, N. Synergetic antimicrobial effects of mixtures of Ethiopian honeys and ginger powder extracts on standard and resistant clinical bacteria isolates. Evid. Based Complementary Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczyńska, A.; Newerli-Guz, J.; Szweda, P. Influence of the addition of selected spices on sensory quality and biological activity of honey. J. Food Qual. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Ahmed, A.A.; Salih, F.A. Low concentrations of local honey modulate exotoxin A expression, and quorum sensing related virulence in drug-resistant Pseudomonas aeruginosa recovered from infected burn wounds. Iran. J. Basic Med. Sci. 2018, 22, 568–575. [Google Scholar]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Khan, J.A.; Iqbal, Z.; Rahman, S.U.; Farzana, K.; Khan, A. Prevalence and resistance pattern of Pseudomonas aeruginosa against various antibiotics. Pak. J. Pharm. Sci. 2008, 21, 311–315. [Google Scholar]
- Master, R.N.; Clark, R.B.; Karlowsky, J.A.; Ramirez, J.; Bordon, J.M. Analysis of resistance, cross-resistance and antimicrobial combinations for Pseudomonas aeruginosa isolates from 1997 to 2009. Int. J. Antimicrob. Agents 2011, 38, 291–295. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Tseng, S.P.; Teng, L.J.; Ho, S.W. Pan-drug-resistant Pseudomonas aeruginosa causing nosocomial infection at a university hospital in Taiwan. Clin. Microbiol. Infect. 2005, 11, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Biswal, I.; Arora, B.S.; Kasana, D. Incidence of multidrug resistant Pseudomonas aeruginosa isolated from burn patients and environment of teaching institution. J. Clin. Diagnostic Res. 2014, 8, 26–29. [Google Scholar]
- Defoirdt, T.; Brackman, G.; Coenye, T. Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 2013, 21, 619–624. [Google Scholar] [CrossRef]
- Chu, W.; Zhou, S.; Jiang, Y.; Zhu, W.; Zhuang, X.; Fu, J. Effect of traditional Chinese herbal medicine with antiquorum sensing activity on Pseudomonas aeruginosa. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jayaseelan, S.; Ramaswamy, D.; Dharmaraj, S. Pyocyanin: Production, applications, challenges and new insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Gellatly, S.L.; Hancock, R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Wagner, V.E.; Bushnell, D.; Passador, L.; Brooks, A.I.; Iglewski, B.H. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: Effects of growth phase and environment. J. Bacteriol. 2003, 185, 2080–2095. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.E.; Maddocks, S.E.; Cooper, R.A. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 2015, 70, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazza, N.C.; Merritt, J.H.; Brothers, K.M.; O’Toole, G.A. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 2007, 189, 3603–3612. [Google Scholar] [CrossRef] [Green Version]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Yoon, S.S. Pseudomonas aeruginosa biofilm, a programmed bacterial life for fitness. J. Microbiol. Biotechnol. 2017, 27, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Evaluation of physiological effects induced by Manuka honey upon Staphylococcus aureus and Escherichia coli. Microorganisms 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanis, C.; Stavropoulou, E.; Giorgi, E.; Voidarou, C.; Constantinidis, T.C.; Vrioni, G.; Tsakris, A. Honey’s antioxidant and antimicrobial properties: A bibliometric study. Antioxidants 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; approved standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Coêlho, D.F.; Saturnino, T.P.; Fernandes, F.F.; Mazzola, P.G.; Silveira, E.; Tambourgi, E.B. Azocasein substrate for determination of proteolytic activity: Reexamining a traditional method using bromelain samples. Biomed. Res. Int. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.J.; Lim, K.Y.; Chong, J.Y.; Low, K.L. In vitro screening of honey against Enterococcus spp. biofilm. J. Med. Biol. Eng. 2014, 3, 23–28. [Google Scholar] [CrossRef]


| P. aeruginosa | AMP | GEN | IPM | ATM | CIP |
|---|---|---|---|---|---|
| ATCC 27853 | 0 (R) | 32 (S) | 28 (S) | 32 (S) | 43 (S) |
| Clinical isolate 1 | 0 (R) | 34 (S) | 31 (S) | 15 (R) | 26 (S) |
| Clinical isolate 2 | 0 (R) | 28 (S) | 30 (S) | 18 (I) | 14 (R) |
| Clinical isolate 3 | 0 (R) | 20 (S) | 25 (S) | 28 (S) | 0 (R) |
| Clinical isolate 4 | 0 (R) | 24 (S) | 25 (S) | 19 (I) | 17 (R) |
| P. aeruginosa | Skim Milk Agar | King A Agar | ||||
|---|---|---|---|---|---|---|
| Control | Honey | Honey–Ginger | Control | Honey | Honey–Ginger | |
| ATCC 27853 | 18.7 ± 0.6 * | 10.3 ± 0.6 | 7.0 ± 1.0 ^ | 11.7 ± 0.6 * | 9.3 ± 1.5 | 9.0 ± 0 |
| Clinical isolate 1 | 21.0 ± 1.0 ** | 22.7 ± 6.7 | 17.7 ± 2.1 | 14.0 ± 1.0 * | 9.3 ± 1.5 | 7.3 ± 0.6 |
| Clinical isolate 2 | 20.0 ± 0 ** | 18.7 ± 4.6 | 14.7 ± 1.5 | 10.3 ± 0.6 * | 0 | 0 |
| Clinical isolate 3 | 10.7 ± 0.6 * | 7.0 ± 0 | 0 | 10.7 ± 0.6 * | 8.7 ± 0.6 | 8.3 ± 1.5 |
| Clinical isolate 4 | 22.3 ± 4.9 ** | 16.3 ± 0.6 | 15.0 ± 1.0 | 35.3 ± 4.7 * | 8.0 ± 2.0 | 7.7 ± 0 |
| P. aeruginosa | a Pyocyanin Production | b Exotoxin A Concentration | ||||
|---|---|---|---|---|---|---|
| Control | Honey | Honey–Ginger | Control | Honey | Honey–Ginger | |
| ATCC 27853 | 1.70 ± 0.87 | 0.74 ± 0.05 | 1.05 ± 0.36 | 107.19 ± 11.54 * | 46.52 ± 5.33 | 61.02 ± 3.27 |
| Clinical isolate 1 | 1.78 ± 0.03 * | 0.73 ± 1.07 | 1.07 ± 0.01 ^ | 95.39 ± 0.91 * | 57.44 ± 5.36 | 67.98 ± 2.98 |
| Clinical isolate 2 | 1.70 ± 0.02 * | 0.92 ± 0.01 | 0.76 ± 0.02 ^ | 94.55 ± 9.37 * | 39.20 ± 2.66 | 40.46 ± 4.43 |
| Clinical isolate 3 | 1.72 ± 0.34 * | 0.76 ± 0.03 | 0.80 ± 0.03 | 39.20 ± 2.06 | 43.38 ± 2.66 | 45.27 ± 2.96 |
| Clinical isolate 4 | 2.74 ± 0.53 * | 1.18 ± 0.03 | 1.05 ± 0.02 ^ | 117.97 ± 16.48 | 133.68 ± 38.08 | 117.95 ± 4.89 |
| P. aeruginosa | c Motility | |||||
|---|---|---|---|---|---|---|
| Swarming | Swimming | |||||
| Control | Honey | Honey–Ginger | Control | Honey | Honey–Ginger | |
| ATCC 27853 | 28.3 ± 0.6 * | 0 | 1.7 ± 1.2 ^ | 40.0 ± 10.6 * | 0 | 1.7 ± 0.6 |
| Clinical isolate 1 | 26.3 ± 0.6 * | 3.3 ± 0.6 | 6.0 ± 1.0 ^ | 34.3 ± 0.6 * | 6.0 ± 0 | 0 |
| Clinical isolate 2 | 25.3 ± 5.9 * | 5.3 ± 1.2 | 3.3 ± 0.6 ^ | 24.3 ± 6.8 * | 4.0 ± 1.0 | 0 |
| Clinical isolate 3 | 3.3 ± 1.2 * | 0 | 0 | 5.7 ± 1.5 * | 0 | 0 |
| Clinical isolate 4 | 39.0 ± 1.0 * | 2.3 ± 1.2 | 6.3 ± 2.1 ^ | 34.0 ± 0 * | 0 | 0 |
| Principal Component (PC) Number | PC1 | PC2 |
|---|---|---|
| Eigenvalue | 5.607 | 0.9521 |
| Proportion of variance | 70.09% | 11.90% |
| Loading of variable | ||
| Skim milk agar well diffusion | −0.655 | |
| King A agar well diffusion | −0.704 | |
| Protease activity | −0.894 | |
| Pyocyanin production | −0.958 | |
| Exotoxin A concentration | −0.628 | |
| Swarming motility | −0.929 | |
| Swimming motility | −0.929 | |
| Biofilm formation | −0.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, W.-J.; Hing, C.-L.; Loo, C.-B.; Hoh, E.-K.; Loke, I.-L.; Ee, K.-Y. Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation. Antibiotics 2023, 12, 1123. https://doi.org/10.3390/antibiotics12071123
Ng W-J, Hing C-L, Loo C-B, Hoh E-K, Loke I-L, Ee K-Y. Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation. Antibiotics. 2023; 12(7):1123. https://doi.org/10.3390/antibiotics12071123
Chicago/Turabian StyleNg, Wen-Jie, Chin-Lu Hing, Choon-Boq Loo, Ee-Khang Hoh, Ian-Lung Loke, and Kah-Yaw Ee. 2023. "Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation" Antibiotics 12, no. 7: 1123. https://doi.org/10.3390/antibiotics12071123
APA StyleNg, W.-J., Hing, C.-L., Loo, C.-B., Hoh, E.-K., Loke, I.-L., & Ee, K.-Y. (2023). Ginger-Enriched Honey Attenuates Antibiotic Resistant Pseudomonas aeruginosa Quorum Sensing Virulence Factors and Biofilm Formation. Antibiotics, 12(7), 1123. https://doi.org/10.3390/antibiotics12071123







