How Did COVID-19 Impact the Antimicrobial Consumption and Bacterial Resistance Profiles in Brazil?
Abstract
:1. Introduction
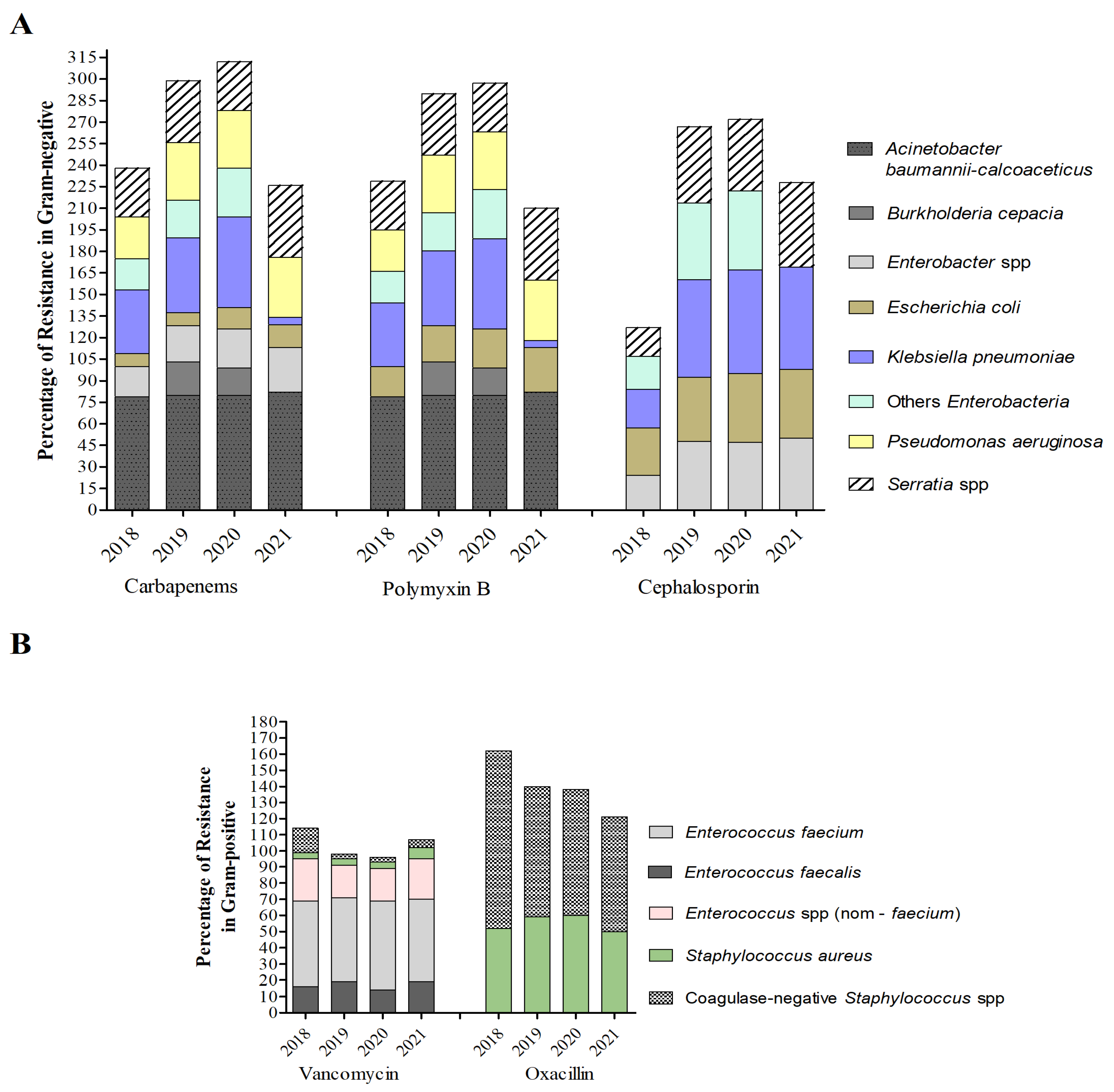
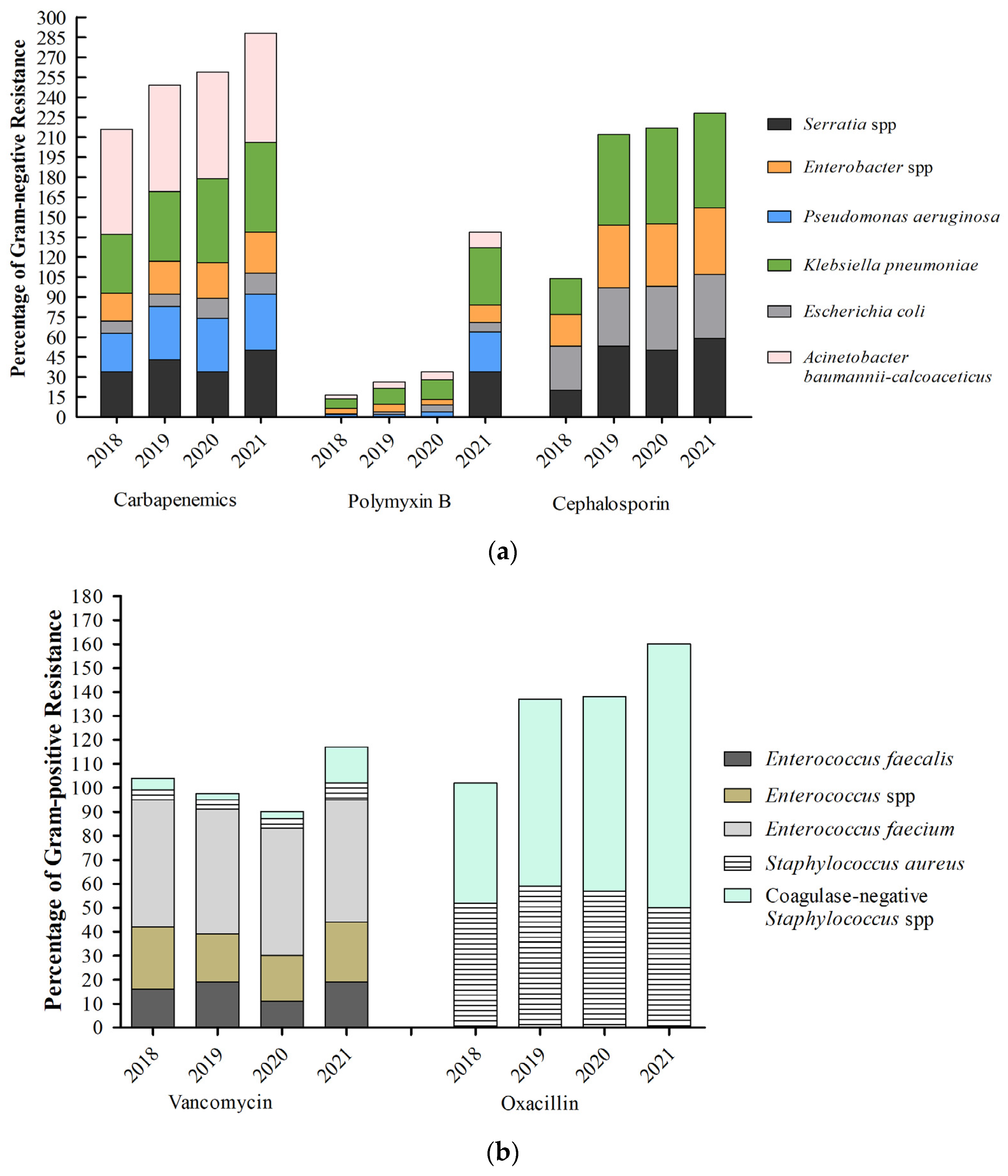
2. Results
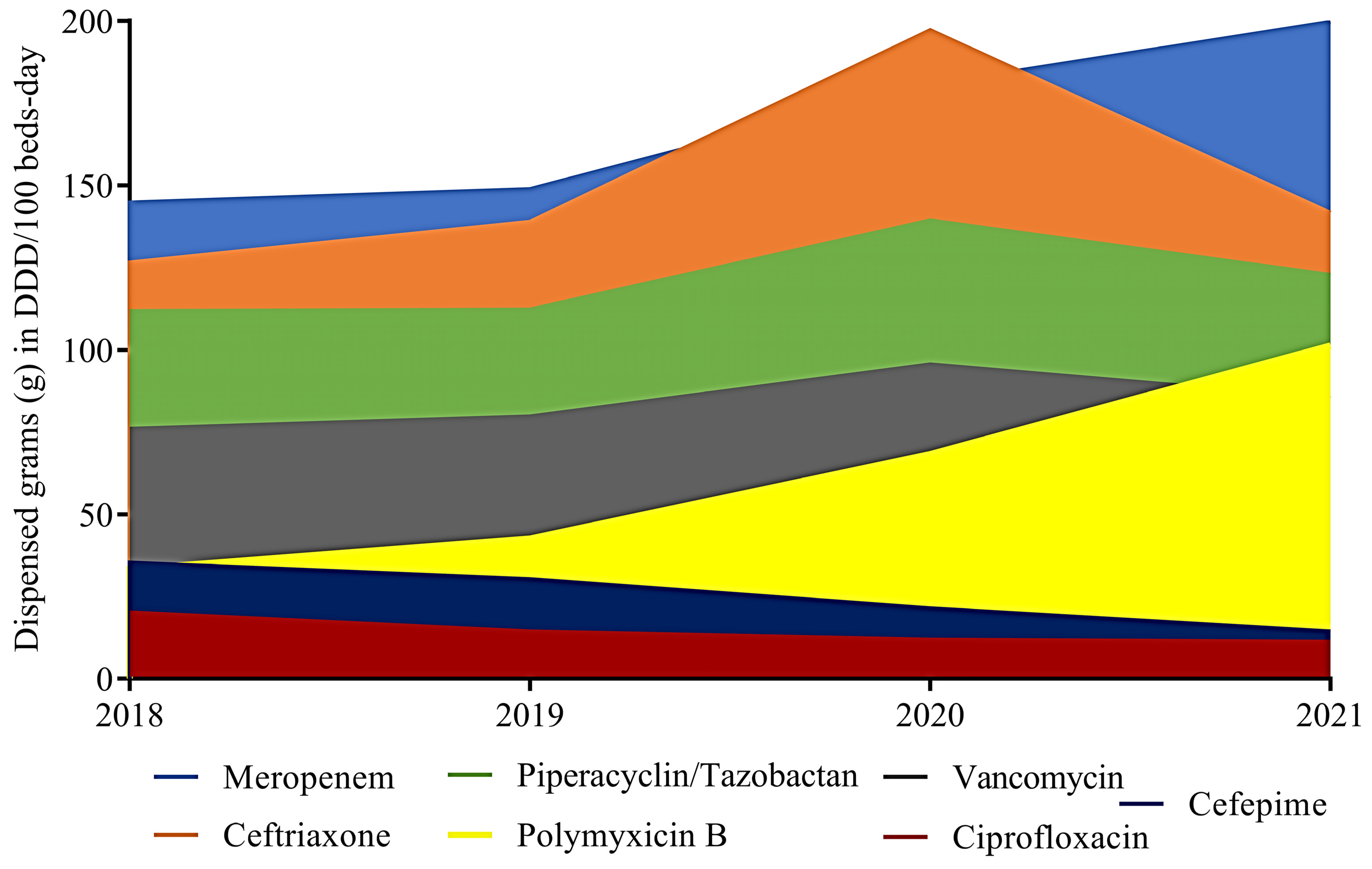
2.1. Antimicrobial Consumption in Hospitals
2.2. Antimicrobial Consumption in the Community
3. Discussion
4. Materials and Methods
4.1. Study Design
Hospital Setting
4.2. Antibiotics
4.3. Data Analysis
4.3.1. Defined Daily Dose—DDD
4.3.2. The Community Consumption of AZM, LEX, and CIP in Relation to the Occurrence of COVID-19
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 2 August 2023).
- O’Neill, J. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 2 September 2021).
- World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. 2014. Available online: https://reliefweb.int/report/world/antimicrobial-resistance-global-report-surveillance-2014?gclid=Cj0KCQjw6KunBhDxARIsAKFUGs8dKpbW8duQSg0MljsU66CsrSQC04QDqxq2vo7SnQKKfTjQYYfJ20AaAgfsEALw_wcB (accessed on 2 August 2023).
- Rossato, L.; Negrão, F.J.; Simionatto, S. Could the COVID-19 Pandemic Aggravate Antimicrobial Resistance? Am. J. Infect. Control 2020, 48, 1129–1130. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial Use, Drug-Resistant Infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Furlan, L.; Caramelli, B. The Regrettable Story of the “COVID Kit” and the “Early Treatment of COVID-19” in Brazil. Lancet Reg. Health—Am. 2021, 4, 100089. [Google Scholar] [CrossRef]
- Gangat, M.A.; Hsu, J.L. Antibiotic Stewardship: A Focus on Ambulatory Care. S D Med. 2015, Spec No, 44–48. [Google Scholar]
- Wise, R.; Hart, T.; Cars, O.; Streulens, M.; Helmuth, R.; Huovinen, P.; Sprenger, M. Antimicrobial Resistance. BMJ 1998, 317, 609–610. [Google Scholar] [CrossRef]
- Auta, A.; Hadi, M.A.; Oga, E.; Adewuyi, E.O.; Abdu-Aguye, S.N.; Adeloye, D.; Strickland-Hodge, B.; Morgan, D.J. Global Access to Antibiotics without Prescription in Community Pharmacies: A Systematic Review and Meta-Analysis. J. Infect. 2019, 78, 8–18. [Google Scholar] [CrossRef]
- Brazil. Ministry of Health. Agência Nacional de Vigilância Sanitária (ANVISA). Resolução de Diretoria Colegiada—RDC No 20 de Maio de 2011; 2011. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/rdc0020_05_05_2011.html (accessed on 2 August 2023).
- Santos-Pinto, C.D.B.; do Rosário Costa, N.; Osorio-de-Castro, C.G.S. Quem Acessa o Programa Farmácia Popular Do Brasil? Aspectos Do Fornecimento Público de Medicamentos. Ciencia e Saude Coletiva 2011, 16, 2963–2973. [Google Scholar] [CrossRef]
- Garcia, M.M.; Guerra Júnior, A.A.; Acúrcio, F.D.A. Avaliação Econômica Dos Programas Rede Farmácia de Minas Do SUS versus Farmácia Popular Do Brasil. Ciênc. Saúde Coletiva 2017, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.L.; Boszczowski, I.; Mortari, N.; Barrozo, L.V.; Neto, F.C.; Lobo, R.D.; Pedroso De Lima, A.C.; Levin, A.S. The Impact of Restricting Over-the-Counter Sales of Antimicrobial Drugs: Preliminary Analysis of National Data. Medicine 2015, 94, e1605. [Google Scholar] [CrossRef]
- PAHO. Americas Report Surge in Drug-Resistant Infections due to Misuse of Antimicrobials during COVID-19: Remarks by Dr. Carissa F. Etienne, Director of the Pan American Health Organization. 2021. Available online: https://www.paho.org/en/news/17-11-2021-americas-report-surge-drug-resistant-infections-due-misuse-antimicrobials-during (accessed on 2 August 2023).
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Del Fiol, F.D.S.; Bergamaschi, C.D.C.; De Andrade, I.P.; Lopes, L.C.; Silva, M.T.; Barberato-Filho, S. Consumption Trends of Antibiotics in Brazil During the COVID-19 Pandemic. Front. Pharmacol. 2022, 13, 844818. [Google Scholar] [CrossRef]
- Bruyndonckx, R.; Hoxha, A.; Quinten, C.; Ayele, G.M.; Coenen, S.; Versporten, A.; Adriaenssens, N.; Muller, A.; Heuer, O.; Monnet, D.L.; et al. Change-Points in Antibiotic Consumption in the Community, European Union/European Economic Area, 1997–2017. J. Antimicrob. Chemother. 2021, 76, ii68–ii78. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Antunes, B.B.P.; Silva, A.A.B.; Nunes, P.H.C.; Martin-Loeches, I.; Kurtz, P.; Hamacher, S.; Bozza, F.A. Antimicrobial Consumption and Drug Utilization Patterns among COVID-19 and Non-COVID-19 Patients. J. Antimicrob. Chemother. 2023, 78, 840–849. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Buchler, A.C.; Gehringer, C.; Widmer, A.F.; Egli, A.; Tschudin-Sutter, S. Risk Factors for Colistin-Resistant Enterobacteriaceae in a Low-Endemicity Setting for Car- Bapenem Resistance—A Matched Case–Control Study. Eurosurveiillance 2018, 1–8. [Google Scholar] [CrossRef]
- Da Silva, K.E.; Baker, S.; Croda, J.; Nguyen, T.N.T.; Boinett, C.J.; Barbosa, L.S.; Tetila, A.; Simionatto, S. Risk Factors for Polymyxin-Resistant Carbapenemase-Producing Enterobacteriaceae in Critically Ill Patients: An Epidemiological and Clinical Study. Int. J. Antimicrob. Agents 2020, 55, 105882. [Google Scholar] [CrossRef]
- Kurihara, M.N.L.; Sales, R.O.D.; Silva, K.E.D.; Maciel, W.G.; Simionatto, S. Multidrug-Resistant Acinetobacter Baumannii Outbreaks: A Global Problem in Healthcare Settings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200248. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, J.L.M.; Gales, A.C. Antimicrobial Resistance in Enterobacteriaceae in Brazil: Focus on β-Lactams and Polymyxins. Braz. J. Microbiol. 2016, 47, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; 2013. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 2 August 2023).
- Santella, B.; Folliero, V.; Pirofalo, G.M.; Serretiello, E.; Zannella, C.; Moccia, G.; Santoro, E.; Sanna, G.; Motta, O.; De Caro, F.; et al. Sepsis—A Retrospective Cohort Study of Bloodstream Infections. Antibiotics 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? JCM 2020, 9, 2744. [Google Scholar] [CrossRef]
- Farfour, E.; Lecuru, M.; Dortet, L.; Le Guen, M.; Cerf, C.; Karnycheff, F.; Bonnin, R.A.; Vasse, M.; Lesprit, P. Carbapenemase-Producing Enterobacterales Outbreak: Another Dark Side of COVID-19. Am. J. Infect. Control. 2020, 48, 1533–1536. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the Potential Long-Term Impact on Antimicrobial Resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Gubbels, S.; Nielsen, J.; Voldstedlund, M.; Kristensen, B.; Schønheyder, H.C.; Vandenbroucke-Grauls, C.M.J.E.; Arpi, M.; Björnsdóttir, M.K.; Knudsen, J.D.; Dessau, R.B.; et al. Utilization of Blood Cultures in Danish Hospitals: A Population-Based Descriptive Analysis. Clin. Microbiol. Infect. 2015, 21, 344.e13–344.e21. [Google Scholar] [CrossRef]
- Panday, R.S.N.; Wang, S.; Van De Ven, P.M.; Hekker, T.A.M.; Alam, N.; Nanayakkara, P.W.B. Evaluation of Blood Culture Epidemiology and Efficiency in a Large European Teaching Hospital. PLoS ONE 2019, 14, e0214052. [Google Scholar] [CrossRef]
- Neves e Castro, P.B.; Da Silva Rodrigues, D.A.; Roeser, H.M.P.; Da Fonseca Santiago, A.; De Cássia Franco Afonso, R.J. Antibiotic Consumption in Developing Countries Defies Global Commitments: An Overview on Brazilian Growth in Consumption. Environ. Sci. Pollut. Res. 2020, 27, 21013–21020. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Jimoh, N.D.; Ogunkola, I.O.; Uwizeyimana, T.; Olayemi, A.H.; Ukor, N.A.; Lucero-Prisno, D.E. The Use of Antibiotics in COVID-19 Management: A Rapid Review of National Treatment Guidelines in 10 African Countries. Trop. Med. Health 2021, 49, 51. [Google Scholar] [CrossRef]
- Högberg, L.D.; Vlahović-Palčevski, V.; Pereira, C.; Weist, K.; Monnet, D.L.; ESAC-Net study group. Decrease in Community Antibiotic Consumption during the COVID-19 Pandemic, EU/EEA, 2020. Eurosurveillance 2021, 26. [Google Scholar] [CrossRef]
- Mamun, A.A.; Saatchi, A.; Xie, M.; Lishman, H.; Blondel-Hill, E.; Marra, F.; Patrick, D.M. Community Antibiotic Use at the Population Level During the SARS-CoV-2 Pandemic in British Columbia, Canada. Open Forum Infect. Dis. 2021, 8, ofab185. [Google Scholar] [CrossRef] [PubMed]
- King, L.M.; Lovegrove, M.C.; Shehab, N.; Tsay, S.; Budnitz, D.S.; Geller, A.I.; Lind, J.N.; Roberts, R.M.; Hicks, L.A.; Kabbani, S. Trends in US Outpatient Antibiotic Prescriptions During the Coronavirus Disease 2019 Pandemic. Clin. Infect. Dis. 2021, 73, e652–e660. [Google Scholar] [CrossRef]
- Knight, B.D.; Shurgold, J.; Smith, G.; MacFadden, D.R.; Schwartz, K.L.; Daneman, N.; Gravel Tropper, D.; Brooks, J. The Impact of COVID-19 on Community Antibiotic Use in Canada: An Ecological Study. Clin. Microbiol. Infect. 2022, 28, 426–432. [Google Scholar] [CrossRef]
- Hsu, J. How COVID-19 Is Accelerating the Threat of Antimicrobial Resistance. BMJ 2020, m1983. [Google Scholar] [CrossRef] [PubMed]
- Laopaiboon, M.; Panpanich, R.; Swa Mya, K. Azithromycin for Acute Lower Respiratory Tract Infections. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Lalwani, P.; Salgado, B.B.; Filho, I.V.P.; Da Silva, D.S.S.; De Morais, T.B.D.N.; Jordão, M.F.; Barbosa, A.R.C.; Cordeiro, I.B.; Neto, J.N.D.S.; De Assunção, E.N.; et al. SARS-CoV-2 Seroprevalence and Associated Factors in Manaus, Brazil: Baseline Results from the DETECTCoV-19 Cohort Study. Int. J. Infect. Dis. 2021, 110, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Abotsi, R.E.; Nicol, M.P.; McHugh, G.; Simms, V.; Rehman, A.M.; Barthus, C.; Ngwira, L.G.; Kwambana-Adams, B.; Heyderman, R.S.; Odland, J.Ø.; et al. The Impact of Long-Term Azithromycin on Antibiotic Resistance in HIV-Associated Chronic Lung Disease. ERJ Open Res. 2022, 8, 00491–02021. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased Antimicrobial Resistance during the COVID-19 Pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Censo Demográfico 2022; 2022. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv102011.pdf (accessed on 2 August 2023).
- Brazil. Ministry of Health. Agência Nacional de Vigilância Sanitária (ANVISA). Relatório Dos Estados: Infecção Relacionada a Assistencia á Saúde. Available online: https://www.gov.br/anvisa/pt-br/assuntos/servicosdesaude/prevencao-e-controle-de-infeccao-e-resistencia-microbiana/copy_of_infeccao-relacionada-a-assistencia-a-saude (accessed on 2 August 2023).
- World Health Organization (WHO). ATC/DDD Index 2023. 2023. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 2 August 2023).
- Brazil. Ministry of Health. Agência Nacional de Vigilância Sanitária (ANVISA). Resolução de Diretoria Colegiada—RDC No 44 de 26 de Outubro de 2010. 2010. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2010/res0044_26_10_2010.html (accessed on 2 August 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massarine, N.C.M.; de Souza, G.H.d.A.; Nunes, I.B.; Salomé, T.M.; Barbosa, M.d.S.; Faccin, I.; Rossato, L.; Simionatto, S. How Did COVID-19 Impact the Antimicrobial Consumption and Bacterial Resistance Profiles in Brazil? Antibiotics 2023, 12, 1374. https://doi.org/10.3390/antibiotics12091374
Massarine NCM, de Souza GHdA, Nunes IB, Salomé TM, Barbosa MdS, Faccin I, Rossato L, Simionatto S. How Did COVID-19 Impact the Antimicrobial Consumption and Bacterial Resistance Profiles in Brazil? Antibiotics. 2023; 12(9):1374. https://doi.org/10.3390/antibiotics12091374
Chicago/Turabian StyleMassarine, Natália Cassago Marcos, Gleyce Hellen de Almeida de Souza, Isadora Batista Nunes, Túlio Máximo Salomé, Marcelo dos Santos Barbosa, Izadora Faccin, Luana Rossato, and Simone Simionatto. 2023. "How Did COVID-19 Impact the Antimicrobial Consumption and Bacterial Resistance Profiles in Brazil?" Antibiotics 12, no. 9: 1374. https://doi.org/10.3390/antibiotics12091374
APA StyleMassarine, N. C. M., de Souza, G. H. d. A., Nunes, I. B., Salomé, T. M., Barbosa, M. d. S., Faccin, I., Rossato, L., & Simionatto, S. (2023). How Did COVID-19 Impact the Antimicrobial Consumption and Bacterial Resistance Profiles in Brazil? Antibiotics, 12(9), 1374. https://doi.org/10.3390/antibiotics12091374






