Antioxidant, Anti-Tyrosinase, and Anti-Skin Pathogenic Bacterial Activities and Phytochemical Compositions of Corn Silk Extracts, and Stability of Corn Silk Facial Cream Product
Abstract
:1. Introduction
2. Results
2.1. Corn Silk (CS) Extract Preparation
2.2. Total Phenolic and Flavonoid Contents of CS Extract
2.3. Phytochemical Profiling
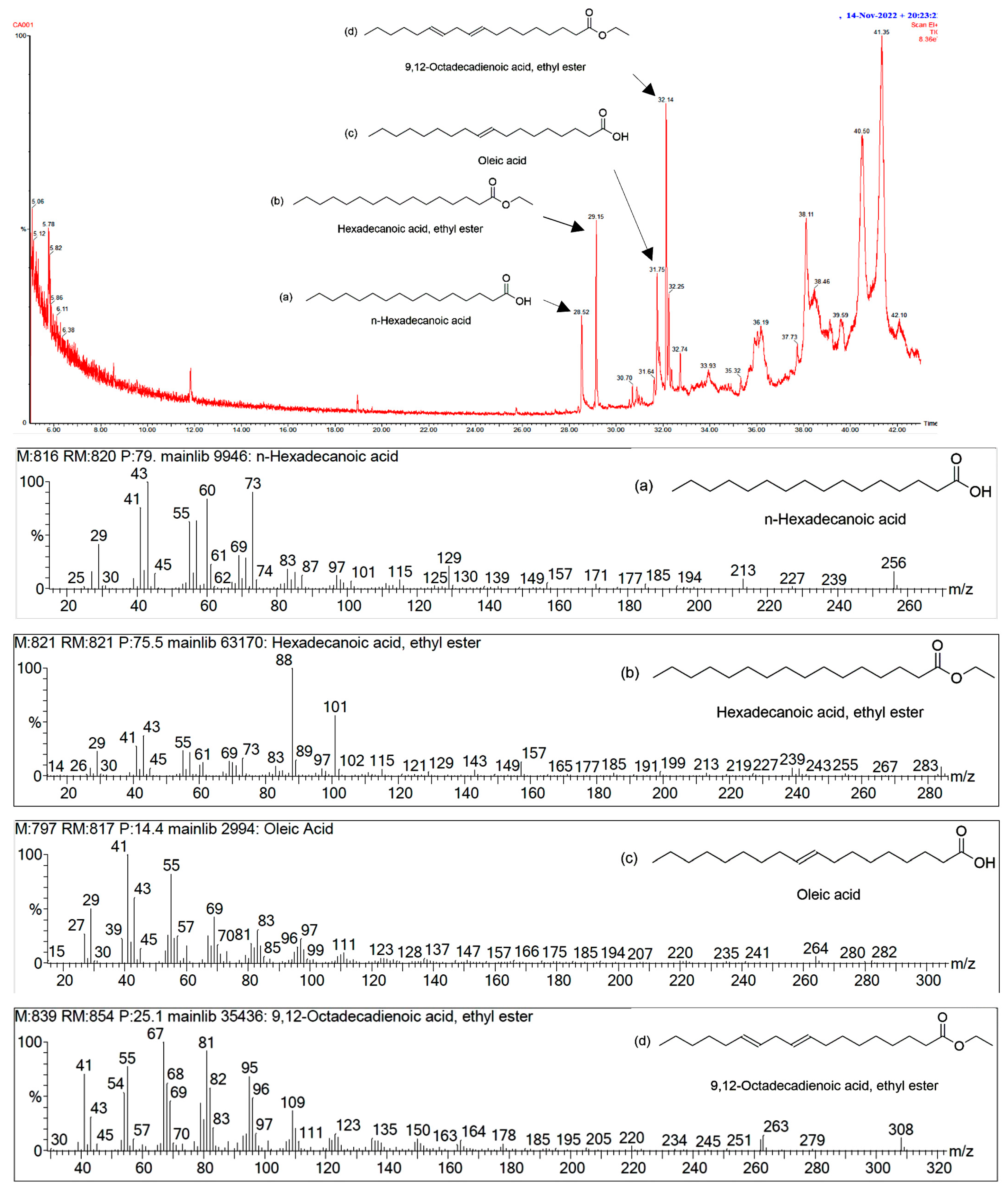
2.4. GC/MS Analysis
2.5. Antioxidant Activity Using DPPH and Reducing Power Assays of CS Extract
2.6. Anti-Tyrosinase Activity of Ethanolic Extract of Corn Silk
2.7. Antibacterial Activities of Ethanolic Extract of Corn Silk
2.8. Stability Test of Nourishing Corn Silk Cream
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Corn Silk (CS) Extract Preparation
4.2. Determination of Total Phenolic Content
4.3. Determination of Total Flavonoid Content
4.4. Phytochemical Profile of CS Extract
4.5. GC/MS Analysis of CS Extract
4.6. Antioxidant Activity
4.6.1. DPPH Radical Scavenging Activity Assay
4.6.2. Reducing Power Assay
4.7. Determination of Corn Silk’s Anti-Tyrosinase Activity
4.8. Antibacterial Activity
4.9. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bacterial Concentration (MBC)
4.10. Formulation of Nourishing Corn Silk Cream Preparation
4.11. Stability Studies of Corn Silk Cream Product
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanokkanjana, K.; Vheewaphongphan, P.; Garivait, S. Black carbon emission from paddy field open burning in Thailand. Environ. Sci. Technol. 2011, 6, 88–92. [Google Scholar]
- Pasukphun, N. Environmental health burden of open burning in northern Thailand: A review. PSRU J. Sci. Technol. 2018, 3, 11–28. [Google Scholar]
- Nurhanan, A.R.; Wan Rosli, W.I. Evaluation of polyphenol content and antioxidant activities of some selected organic and aqueous extracts of cornsilk (Zea mays Hairs). J. Med. Bioeng. 2012, 1, 48–51. [Google Scholar]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Aslam, M.; Muntaha, S.T. Effect of solvent polarity and extraction method on phytochemical composition and antioxidant potential of corn silk. Free Radic. Antioxid. 2019, 9, 5–11. [Google Scholar] [CrossRef]
- Sarepoua, E.; Tangwongchai, R.; Suriharn, B.; Lertrat, K. Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chem. 2015, 169, 424–429. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Hafe, S. Antioxidant activities of Iranian corn silk. Turk. J. Biol. 2008, 32, 43–49. [Google Scholar]
- Liu, J.; Wang, C.; Wang, Z.; Zhang, C.; Lu, S.; Liu, J. The antioxidant and free-radical scavenging activities of extract and fraction from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011, 126, 261–269. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, A.; Kwon, S.A.; Kim, M.; Song, M.; Han, H.W.; Shin, E.J.; Park, E.; Lee, S.M. Potential photoprotective effect of dietary corn silk extracts on ultraviolet B-induced skin damage. Molecules 2019, 24, 2587. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.; Kim, S.S.; Ju, H.M.; Baek, J.H.; Park, C.S.; Lee, D.H. Inhibitory effect of corn silk on skin pigmentation. Molecules 2014, 19, 2808–2818. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Deliencourt-Godefroy, G.; Lopes, L. An effective hydroquinone alternative for topical skin lightening. J. Cosmet. Dermatol. 2020, 19, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The Top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Ismail, I.S. Cosmetic potential of Southeast Asian herbs: An overview. Phytochem. Rev. 2015, 14, 419–428. [Google Scholar] [CrossRef]
- Helmy, A.; El-shazly, M.; Seleem, A.; Abdelmohsen, U.; Salem, M.A.; Samir, A.; Rabeh, M.; Elshamy, A.; Singab, A.N.B. The synergistic effect of biosynthesized silver nanoparticle from a combined extract of parsley, corn silk, and gum Arabic: In vivo antioxidant, anti-inflammatory and antimicrobial activities. Mater. Res. Express 2020, 7, 025002. [Google Scholar] [CrossRef]
- Roger, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar] [CrossRef]
- Ziebuhr, W.; Krimmer, V.; Rachid, S.; Lössner, I.; Götz, F.; Hacker, J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: Evidence for control of the polysaccharide intercellular adhesion synthesis by alternating insertion and excision of insertion sequence element IS256. Mol. Microbiol. 1999, 32, 345–346. [Google Scholar] [CrossRef]
- Chessa, D.; Ganau, G.; Mazzarello, V. An overview of Staphylococcus epidermidis and Staphylococcus aureus with a focus on development countries. J. Infect. Dev. Ctries 2015, 9, 547–550. [Google Scholar] [CrossRef]
- Jeon, J.; Park, S.C.; Her, J.; Lee, J.W.; Han, J.K.; Kim, Y.K.; Kim, K.P.; Ban, C. Comparative lipidomic profiling of the human commensal bacterium Propionibacterium acnes and its extracellular vesicles. RSC Adv. 2018, 8, 15241–15247. [Google Scholar] [CrossRef]
- Dreno, B.; Thiboutot, D.; Gollnick, H.; Bettoli, V.; Kang, S.; Leyden, J.J.; Shalita, A. Antibiotic stewardship in dermatology: Limiting antibiotic use in acne. Eur. J. Dermatol. 2014, 24, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an opportunistic pathogen: An updated of its virulence-associated factors. Microorganism 2021, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Boon, L.K.; Fern, Y.S.; Chee, L.H. Generation Y’s purchase intention towards natural skincare products: A PLS-SEM analysis. Glob. Bus. Manag. Res. Int. J. 2020, 12, 61–77. [Google Scholar]
- Emerald, M.; Emerald, A.; Emerald, L.; Kumar, V. Perspective of natural products in skincare. Pharm. Pharmacol. Int. 2016, 4, 1–3. [Google Scholar]
- Hoang, H.T.; Moon, J.M.; Lee, Y.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Salerno, T.M.G.; Emanuela, T.; Cafeo, G.; Vento, F.; Zoccali, M.; Donato, P.; Dugo, P.; Mondello, L. Hidden threat lurking in extensive hand hygiene during the COVID-19 pandemic: Investigation of sensitizing molecules in gel products by hyphenated chromatography techniques. Anal. Bioanal. Chem. 2023, 415, 3327–3340. [Google Scholar] [CrossRef]
- Alam, E.A. Evaluation of antioxidant and antibacterial activities of Egyptian Maydis stigma (Zea mays hairs) rich in some bioactive constituents. J. Am. Sci. 2011, 7, 726–729. [Google Scholar]
- El-Ghorab, A.; El-Massry, K.F.; Shibamoto, T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of Egyptian corn silk (Zea mays L.). J. Agric. Food Chem. 2007, 55, 9124–9127. [Google Scholar] [CrossRef]
- Kan, A.; Orhan, I.; Coksari, G.; Sener, B. In-vitro neuroprotective properties of the Maydis stigma extracts from four corn varieties. Int. J. Food Sci. Nutr. 2011, 63, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yang, X.H.; Chen, D.; Peng, C.; Wang, G.S. A new flavonoid from the bract of Zea mays L. Chin. Chem. Lett. 2010, 21, 1350–1351. [Google Scholar] [CrossRef]
- Kaup, S.R.; Arunkumar, N.; Bernhardt, L.K.; Vasari, R.G.; Shetty, S.S.; Pi, S.R.; Arunkumar, B. Antihyperlipedemic activity of Cynodon dactylon extract in high-cholesterol diet fed Wistar rats. Genom. Med. Biomark. Health Sci. 2011, 3, 98–102. [Google Scholar] [CrossRef]
- Schreiner, G.E. Toxic nephropathy: Adverse renal effects caused by drugs and chemicals. JAMA Netw. Open 1965, 191, 849–850. [Google Scholar] [CrossRef]
- Maksimović, Z.; Malenčič, Đ.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, D.V.O.; Xavier, H.S.; Barista, J.E.M.; Castro-Chaves, C.D. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine 2005, 12, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.S.; Pais, A.A.; Tardivo, A.C.B.; Alves, M.J.Q.F. Effect of aqueous extract of corn silks (Zea mays L.) on the renal excretion of water and electrolytes and arterial pressure in anesthetized Wistar rats. Revi. Bras. Plantas Med. 2011, 13, 375–381. [Google Scholar] [CrossRef]
- Bai, H.; Hai, C.; Xi, M.; Liang, X.; Liu, R. Protective effect of maize silks (Maydis stigma) ethanol extracts on radiation-induced oxidative stress in mice. Plant Food Hum. Nutr. 2010, 65, 271–276. [Google Scholar] [CrossRef]
- Hu, Q.I.; Deng, Z.I. Protective effects of flavonoids from corn silk on oxidative stress induced by exhaustive exercise in mice. Afr. J. Biot. 2011, 10, 3163–3167. [Google Scholar]
- Guo, J.; Liu, T.; Han, L.; Liu, Y. The effects of corn silk on glycaemic metabolism. Nutr. Metab. 2009, 6, 47. [Google Scholar] [CrossRef]
- Sepehri, G.; Derakhshanfar, A.; Zade, F.Y. Protective effects of corn silk extract administration on gentamicin-induced nephrotoxicity in rat. Comp. Clin. Pathol. 2011, 20, 89–94. [Google Scholar] [CrossRef]
- Hongsuwan, N.; Sutthanut, K. Comparison of chemical compositions, nutritional values, antioxidant and tyrosinase inhibition activities in corn silk of three different corn varieties. J. Thai Trad. Alt. Med. 2018, 16, 362–371. [Google Scholar]
- Srisuksomwong, P.; Kaenhin, L.; Mungmai, L. Collagenase and tyrosinase inhibitory activities and stability of facial cream formulation containing cashew leaf extract. Cosmetics 2023, 10, 17. [Google Scholar] [CrossRef]
- Kishore, N.; Twilley, D.; Staden, A.B.; Verma, P.; Singh, B.; Cardinali, G.G.; Kovacs, D.; Picardo, M.; Kumar, V.; Lall, N. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their activities against mushroom tyrosinase. J. Nat. Prod. 2018, 81, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Söhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibitory by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Athipornchai, A.; Niyomtham, N.; Pabuprapap, W.; Ajavakorn, V.; Duca, M.; Azoulay, S.; Suksamrarn, A. Potent tyrosinase inhibitory activity of curcuminoid analoques and inhibition kinetics studied. Cosmetics 2021, 8, 35. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Xing, F.; Lei, W.; Tao, W.L.; Qing, Z. Studies on antimicrobial activity of aqueous extract of maize silk. Appl. Mech. Mater. 2012, 140, 426–430. [Google Scholar]
- Nessa, F.; Ismail, Z.; Mohamed, N. Antimicrobial activities of extracts and flavonoid glycosides of corn silk (Zea mays L.). Int. J. Biotechnol. Wellness Ind. 2012, 1, 115–121. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Ali, M.; Alhazmi, H.A.; Ansari, S.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and Bioactive Profile Compilations of Less Discussed but Effective Naturally Growing Saudi Plant. In Plant and Human Health; Ozturk, M., Hakeem, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 343–352. [Google Scholar]
- Abirami, S.; Priyalakshmi, M.; Soundariya, A.; Samrot, A.V.; Saigeetha, S.; Emilin, R.R.; Dhiva, S.; Inbathamizh, L. Antimicrobial activity, antiproliferative activity, amylase inhibitory activity and phytochemical analysis of ethanol extract of corn (Zea mays L.) silk. CRGSC 2021, 4, 100089. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Harida, M. Anti-inflammatory property of n-Hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Britto, D.; Leslee, C.; Kuppannan, S.B.; Seedevj, P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activity. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Patel, J.; Reddy, V.; Kumar, G.S.; Satyasai, D.; Bajari, B. Gas chromatography and mass spectroscopy analysis of bioactive components on the leaf extract of Terminalia coriacea: A potential folklore medicinal plant. Int. J. Green Pharm. 2017, 11, 140. [Google Scholar]
- Sheela, D.; Uthayakumari, F. GC-MS analysis of bioactive constituents from coastal sand Dune taxon-Sesuvium portulacastrum (L.). Biosci. Discov. 2013, 4, 47–53. [Google Scholar]
- Elezabeth, V.D.; Arumugam, S. GC-MS analysis of bioactive constituents of Indigofera suffruticosa leaves. J. Chem. Pharmaceut. Res. 2014, 6, 294–300. [Google Scholar]
- Sermakkani, M.; Thangapandian, V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharmaceut. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Deori, M.; Devi, D.; Devi, R. Nutrient composition and antioxidant activities of muga and eri silkworm pupae. Int. J. Sci. Nat. 2014, 5, 636–640. [Google Scholar]
- Obadoni, B.O.; Ochuko, P.O. Phytochemical studies and comparative efficacy of the crude extracts of some homostatic plants in Edo and Delta states of Nigeria. Glob. J. Pure Appl. Sci. 2001, 8, 203–208. [Google Scholar]
- Hou, W.C.; Chen, Y.C.; Chen, H.J.; Lin, Y.H.; Yang, L.L.; Lee, M.H. Antioxidant activities of trypsin inhibitor, a33 KDa root storage protein of sweet potato. J. Agric. Food Chem. 2001, 49, 2978–2981. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Jantakee, K.; Tragoolpua, Y. Activities of different types of Thai honey on pathogenic bacteria causing skin diseases, tyrosinase enzyme and generating free radical. Biol. Res. 2015, 48, 4. [Google Scholar] [CrossRef]
- Leelapornpisid, P.; Mungmai, L.; Sirithunyalug, B.; Jiranusornkul, S.; Peerapornpisal, Y. A novel moisturizer extracted from freshwater macroalga [Rhizoclonium hieroglyphicum (C. Agardh) Kützing] for skin care cosmetic. Chiang Mai J. Sci. 2014, 41, 1195–1207. [Google Scholar]
- Eiamthaworn, K.; Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Cordyceps militaris extracts against some skin pathogenic bacteria and antioxidant activity. J. Fungi 2022, 8, 327. [Google Scholar] [CrossRef] [PubMed]
| Biological Activity | Ethanolic Extract | Ethyl Acetate Extract |
|---|---|---|
| Total phenolic content (mg gallic acid equivalent/g extract) | 28.27 ± 0.86 * | 12.81 ± 0.17 |
| Flavonoid content (mg quercetin equivalent/g extract) | 4.71 ± 0.79 * | 2.23 ± 0.57 |
| Antioxidant Activity | Ethanolic Extract | Ethyl Acetate Extract |
|---|---|---|
| DPPH radical scavenging (mg gallic acid equivalent/g extract) | 5.22 ± 0.87 | 5.19 ± 0.37 |
| Reducing power assay (mg gallic acid equivalent/g extract) | 13.20 ± 0.42 * | 1.09 ± 0.17 |
| Treatment | Diameter of Inhibition Zone on Bacteria (mm) | |
|---|---|---|
| C. acnes | S. epidermidis | |
| Ethanolic extract of CS, 500 mg/mL | 11.7 ± 1.2 | 9.30 ± 0.6 |
| Gentamycin, 1 mg/mL | 23.3 ± 0.6 | 23.7 ± 0.6 |
| Treatment | C. acnes | S. epidermidis | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| CSA | 15.625 | 15.625 | 125 | 125 |
| Gentamycin | 0.0625 | 0.0625 | 0.0156 | 0.0156 |
| Conditions | Color | pH | Viscosity (Pa·s/cP) | Centrifugation Test | Phase Separation | Homogeneity |
|---|---|---|---|---|---|---|
| RT | Pale yellow | 7.60 | 4395.5 | Stable | No | Good |
| 4 °C | Pale yellow | 7.41 | 4551.0 | Stable | No | Good |
| 45 °C | Pale yellow | 7.13 | 6181.5 | Stable | No | Good |
| H/C | Pale yellow | 7.57 | 5500.0 | Stable | No | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yucharoen, R.; Srisuksomwong, P.; Julsrigival, J.; Mungmai, L.; Kaewkod, T.; Tragoolpua, Y. Antioxidant, Anti-Tyrosinase, and Anti-Skin Pathogenic Bacterial Activities and Phytochemical Compositions of Corn Silk Extracts, and Stability of Corn Silk Facial Cream Product. Antibiotics 2023, 12, 1443. https://doi.org/10.3390/antibiotics12091443
Yucharoen R, Srisuksomwong P, Julsrigival J, Mungmai L, Kaewkod T, Tragoolpua Y. Antioxidant, Anti-Tyrosinase, and Anti-Skin Pathogenic Bacterial Activities and Phytochemical Compositions of Corn Silk Extracts, and Stability of Corn Silk Facial Cream Product. Antibiotics. 2023; 12(9):1443. https://doi.org/10.3390/antibiotics12091443
Chicago/Turabian StyleYucharoen, Raenu, Pawalee Srisuksomwong, Jakaphun Julsrigival, Lapatrada Mungmai, Thida Kaewkod, and Yingmanee Tragoolpua. 2023. "Antioxidant, Anti-Tyrosinase, and Anti-Skin Pathogenic Bacterial Activities and Phytochemical Compositions of Corn Silk Extracts, and Stability of Corn Silk Facial Cream Product" Antibiotics 12, no. 9: 1443. https://doi.org/10.3390/antibiotics12091443






