Effect of Hydrogen Peroxide on Cyanobacterial Biofilms
Abstract
:1. Introduction
2. Results
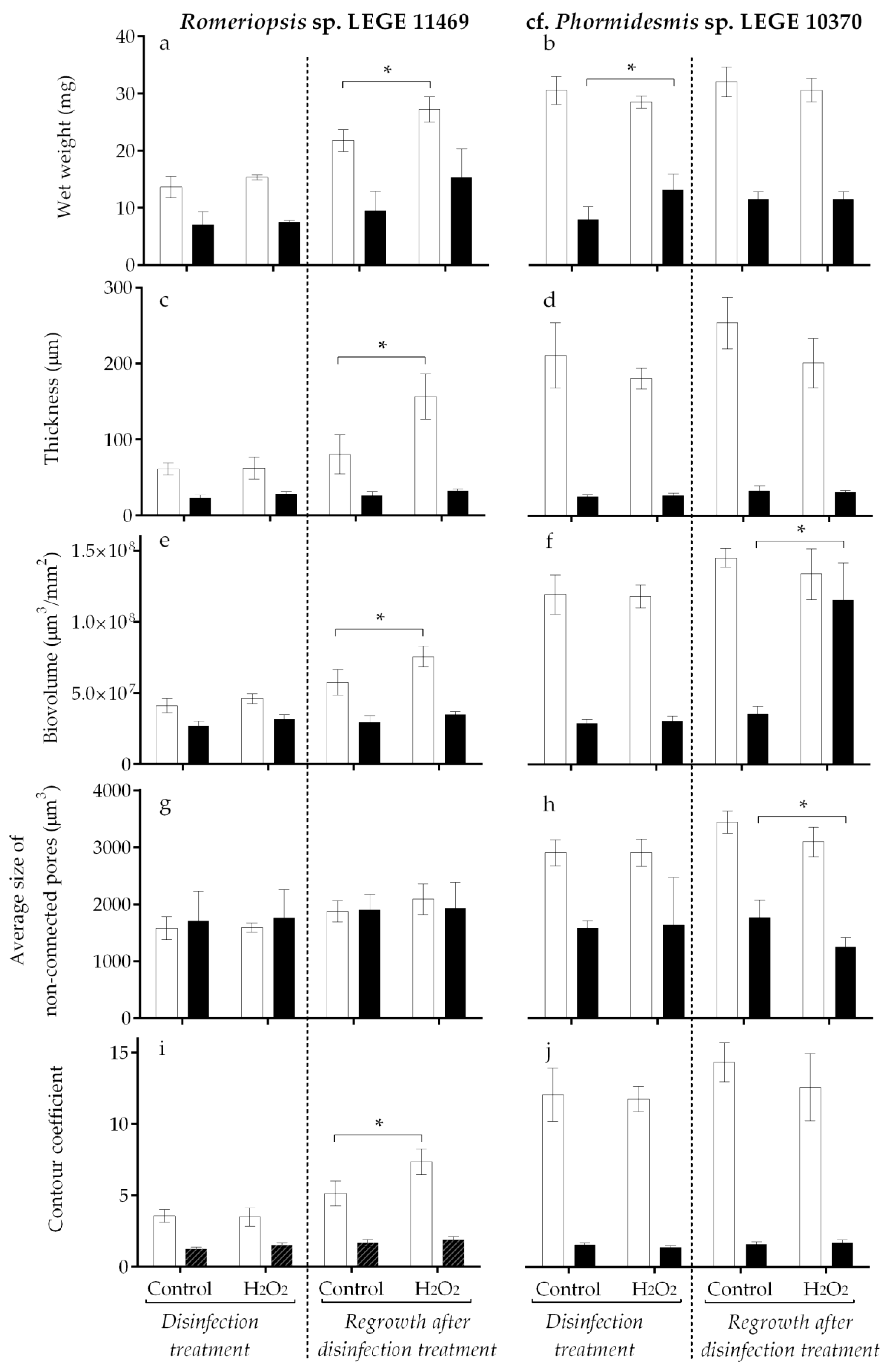
2.1. Biofilm Development
2.2. H2O2 Disinfection Treatment and Cyanobacterial Biofilm Regrowth
3. Discussion
4. Materials and Methods
4.1. Organism and Culture Conditions
4.2. Biofilm Formation
4.3. Biofilm Analysis
4.4. H2O2 Disinfection Treatment
4.5. Regrowth after the H2O2 Disinfection Treatment
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romeu, M.J.; Mergulhão, F. Development of Antifouling Strategies for Marine Applications. Microorganisms 2023, 11, 1568. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management Priorities for Marine Invasive Species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in Marine Aquaculture: A Review of Recent Research and Developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef]
- Demirel, Y.K.; Uzun, D.; Zhang, Y.; Fang, H.C.; Day, A.H.; Turan, O. Effect of Barnacle Fouling on Ship Resistance and Powering. Biofouling 2017, 33, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; Stepec, B.A.A.; Wade, S.A. Microbiologically Influenced Corrosion—More than Just Microorganisms. FEMS Microbiol. Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef] [PubMed]
- Bradbeer, S.J.; Coughlan, N.E.; Cuthbert, R.N.; Crane, K.; Dick, J.T.A.; Caffrey, J.M.; Lucy, F.E.; Renals, T.; Davis, E.; Warren, D.A.; et al. The Effectiveness of Disinfectant and Steam Exposure Treatments to Prevent the Spread of the Highly Invasive Killer Shrimp, Dikerogammarus villosus. Sci. Rep. 2020, 10, 1919. [Google Scholar] [CrossRef]
- Stout, J.B.; Avila, B.W.; Fetherman, E.R. Efficacy of Commercially Available Quaternary Ammonium Compounds for Controlling New Zealand Mudsnails Potamopyrgus antipodarum. N. Am. J. Fish. Manag. 2016, 36, 277–284. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, H.; Zheng, J.; Teng, F.; Wang, X.; Lou, K.; Zhang, X.; Tao, Y. Suppression of Water-Bloom Cyanobacterium Microcystis Aeruginosa by Algaecide Hydrogen Peroxide Maximized through Programmed Cell Death. J. Hazard. Mater. 2020, 393, 122394. [Google Scholar] [CrossRef]
- Cahill, P.L.; Lewis, P.N.; Solutions, B.; Tait, L.; Floerl, O. Treatment Agents for Biofouling in Internal Pipework of Recreational Vessels: A Review of Pipework Configurations, Biofouling Risk, and Operational Considerations; Publications Logistics Officer Ministry for Primary Industries: Wellington, New Zealand, 2019. [Google Scholar]
- Stockton-Fiti, K.A.; Moffitt, C.M. Safety and Efficacy of Virkon® Aquatic as a Control Tool for Invasive Molluscs in Aquaculture. Aquaculture 2017, 480, 71–76. [Google Scholar] [CrossRef]
- Mainous, M.E.; Kuhn, D.D.; Smith, S.A. Efficacy of Common Aquaculture Compounds for Disinfection of Flavobacterium Columnare and F. psychrophilum. J. Appl. Aquac. 2012, 24, 262–270. [Google Scholar] [CrossRef]
- Moffitt, C.M.; Barenberg, A.; Stockton, K.A.; Watten, B.J. Efficacy of Two Approaches for Disinfecting Surfaces and Water Infested with Quagga Mussel Veligers. In Biology and Management of Invasive Quagga and Zebra Mussels in the Western United States; Wong, W.H., Gerstenberger, S., Eds.; CRC PRESS: Boca Raton, FL, USA, 2015; pp. 467–477. [Google Scholar]
- Assaf, S.; Aaron, K. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, H.; Wen, Y. Effect of Hydrogen Peroxide on Microcystic Aeruginosa: Role of Cytochromes P450. Sci. Total Environ. 2018, 626, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Piel, T.; Sandrini, G.; Muyzer, G.; Brussaard, C.P.D.; Slot, P.C.; van Herk, M.J.; Huisman, J.; Visser, P.M. Resilience of Microbial Communities after Hydrogen Peroxide Treatment of a Eutrophic Lake to Suppress Harmful Cyanobacterial Blooms. Microorganisms 2021, 9, 1495. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shi, X.; Zhang, M.; Liu, C.; Chen, K. Comparison of Algal Harvest and Hydrogen Peroxide Treatment in Mitigating Cyanobacterial Blooms via an in Situ Mesocosm Experiment. Sci. Total Environ. 2019, 694, 133721. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, D.J. Chemicals Used as Disinfectants: Active Ingredients and Enhancing Additives. Rev. Sci. Tech. 1995, 14, 57–74. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of Hydrogen Peroxide as a Biocide: New Consideration of Its Mechanisms of Biocidal Action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Lin, L.; Shan, K.; Xiong, Q.; Zhou, Q.; Li, L.; Gan, N.; Song, L. The Ecological Risks of Hydrogen Peroxide as a Cyanocide: Its Effect on the Community Structure of Bacterioplankton. J. Oceanol. Limnol. 2018, 36, 2231–2242. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The Efficacy of Hydrogen Peroxide in Mitigating Communities across Four Lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting Cyanobacteria with Hydrogen Peroxide: A Laboratory Study on the Consequences for Phytoplankton Community and Diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.G.; Rajendran, S.; Wilson, A.E. Hydrogen Peroxide Treatment Promotes Chlorophytes over Toxic Cyanobacteria in a Hyper-Eutrophic Aquaculture Pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef]
- Sandrini, G.; Piel, T.; Xu, T.; White, E.; Qin, H.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to Hydrogen Peroxide of the Bloom-Forming Cyanobacterium Microcystis PCC 7806 Depends on Nutrient Availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Narváez-Zapata, J.; Tebbe, C.C.; Ortega-Morales, B.O. Molecular Diversity and Biomass of Epilithic Biofilms from Intertidal Rocky Shores in the Gulf of Mexico. Biofilms 2005, 2, 93–103. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.F. Marine Biofilms on Artificial Surfaces: Structure and Dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Velmourougane, K.; Prasanna, R. Phototrophic Biofilms: Diversity, Ecology and Applications. J. Appl. Phycol. 2017, 29, 2729–2744. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O. Marine and Estuarine Natural Microbial Biofilms: Ecological and Biogeochemical Dimensions. AIMS Microbiol. 2016, 2, 304–331. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Mitbavkar, S.; Raghu, C.; Rajaneesh, K.M.; Pavan, D. Picophytoplankton Community from Tropical Marine Biofilms. J. Exp. Mar. Biol. Ecol. 2012, 426–427, 88–96. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Foster, R.A.; Kuypers, M.M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen Fixation and Transfer in Open Ocean Diatom-Cyanobacterial Symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between Microbial Biofilms and Marine Fouling Algae: A Mini Review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef]
- Romeu, M.J.; Lima, M.; Gomes, L.C.; De Jong, E.D.; Morais, J.; Vasconcelos, V.; Pereira, M.F.R.; Soares, S.G.P.; Sjollema, J.; Mergulhão, F.J. The Use of 3D Optical Coherence Tomography to Analyze the Architecture of Cyanobacterial Biofilms Formed on a Carbon Nanotube Composite. Polymers 2022, 14, 4410. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental Assessment of the Performance of Two Marine Coatings to Curb Biofilm Formation of Microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Faria, S.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; de Jong, E.; Sjollema, J.; Vasconcelos, V.; Mergulhão, F.J. Unveiling the Antifouling Performance of Different Marine Surfaces and Their Effect on the Development and Structure of Cyanobacterial Biofilms. Microorganisms 2021, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Camps, M.; Barani, A.; Gregori, G.; Bouchez, A.; le Berre, B.; Bressy, C.; Blache, Y.; Briand, J.F. Antifouling Coatings Influence both Abundance and Community Structure of Colonizing Biofilms: A Case Study in the Northwestern Mediterranean Sea. Appl. Environ. Microbiol. 2014, 80, 4821–4831. [Google Scholar] [CrossRef]
- Hu, P.; Xie, Q.; Ma, C.; Zhang, G. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. [Google Scholar] [CrossRef]
- Romeu, M.J.; Alves, P.; Morais, J.; Miranda, J.M.; de Jong, E.D.; Sjollema, J.; Ramos, V.; Vasconcelos, V.; Mergulhão, F.J.M. Biofilm Formation Behaviour of Marine Filamentous Cyanobacterial Strains in Controlled Hydrodynamic Conditions. Environ. Microbiol. 2019, 21, 4411–4424. [Google Scholar] [CrossRef]
- Acosta, F.; Montero, D.; Izquierdo, M.; Galindo-Villegas, J. High-Level Biocidal Products Effectively Eradicate Pathogenic γ-Proteobacteria Biofilms from Aquaculture Facilities. Aquaculture 2021, 532, 736004. [Google Scholar] [CrossRef]
- Petrille, J.; Miller, S. Efficacy of Hydrogen Peroxide for Control of Adult Zebra Mussels, Dreissena Polymorpha, and Asian Clams, Corbicula Fluminea. In Proceedings of the 10th International Aquatic Nuisance Species and Zebra Mussel Conference, Toronto, ON, Canada, 13–17 February 2000; pp. 54–62. [Google Scholar]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen Peroxide Oxygenation and Disinfection Capacity in Recirculating Aquaculture Systems. Aquac. Eng. 2021, 92, 102140. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Piel, T.; Sandrini, G.; White, E.; Xu, T.; Schuurmans, J.M.; Huisman, J.; Visser, P.M. Suppressing Cyanobacteria with Hydrogen Peroxide Is More Effective at High Light Intensities. Toxins 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Weiss, G.; Murik, O.; Sukenik, A.; Lieman-Hurwitz, J.; Kaplan, A. The Response of Microcystis Aeruginosa Strain MGK to a Single or Two Consecutive H2O2 Applications. Environ. Microbiol. Rep. 2019, 11, 621–629. [Google Scholar] [CrossRef]
- Arndt, E.; Robinson, A.; Hester, S. Factors That Influence Vessel Biofouling and Its Prevention and Management; Cebra: Melbourne, Australia, 2021. [Google Scholar]
- Sharma, M.; Liu, H.; Tsesmetzis, N.; Handy, J.; Place, T.; Gieg, L.M. Diagnosing Microbiologically Influenced Corrosion at a Crude Oil Pipeline Facility Leak Site—A Multiple Lines of Evidence Approach. Int. Biodeterior. Biodegrad. 2022, 172, 105438. [Google Scholar] [CrossRef]
- Yazdi, M.; Khan, F.; Abbassi, R.; Quddus, N.; Castaneda-Lopez, H. A Review of Risk-Based Decision-Making Models for Microbiologically Influenced Corrosion (MIC) in Offshore Pipelines. Reliab. Eng. Syst. Saf. 2022, 223, 108474. [Google Scholar] [CrossRef]
- Sinha, A.K.; Eggleton, M.A.; Lochmann, R.T. An Environmentally Friendly Approach for Mitigating Cyanobacterial Bloom and Their Toxins in Hypereutrophic Ponds: Potentiality of a Newly Developed Granular Hydrogen Peroxide-Based Compound. Sci. Total Environ. 2018, 637–638, 524–537. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective Suppression of Harmful Cyanobacteria in an Entire Lake with Hydrogen Peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia Coli ATCC 12806 to Sublethal Concentrations of Food-Grade Biocides Influences Its Ability to Form Biofilm, Resistance to Antimicrobials, and Ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef]
- Romeu, M.J.; Rodrigues, D.; Azeredo, J. Effect of Sub-Lethal Chemical Disinfection on the Biofilm Forming Ability, Resistance to Antibiotics and Expression of Virulence Genes of Salmonella Enteritidis Biofilm-Surviving Cells. Biofouling 2020, 36, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial Diversity Held in Microbial Biological Resource Centers as a Biotechnological Asset: The Case Study of the Newly Established LEGE Culture Collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Hentschke, G.S.; Ramos, V.; Pinheiro, Â.; Barreiro, A.; Costa, M.S.; Rego, A.; Brule, S.; Vasconcelos, V.M.; Leão, P.N. Zarconia navalis gen. nov., sp. nov., Romeriopsis navalis gen. nov., sp. nov. and Romeriopsis marina sp. nov., Isolated from Inter-and Subtidal Environments from Northern Portugal. Int. J. Syst. Evol. Microbiol. 2022, 72, 5552. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for the Preparation of Modified Nutrient Solution Z8 for Algae. Nor. Inst. Water Res. Oslo 1972, 11, 5. [Google Scholar]
- Taylor, D.A. Introduction to Marine Engineering; Butterworth-Heinemann: Oxford, UK, 1996; ISBN 1110301197. [Google Scholar]
- Blain, S.; Guillou, J.; Tréguer, P.J.; Woerther, P.; Delauney, L.; Follenfant, E.; Gontier, O.; Hamon, M.; Leilde, B.; Mer, D.; et al. High Frequency Monitoring of the Coastal Marine Environment Using the MAREL Buoy. J. Environ. Monit. 2004, 6, 569–575. [Google Scholar] [CrossRef]
- King, R.K.; Flick, G.J.; Smith, S.A.; Pierson, M.D.; Boardman, G.D.; Coale, C.W. Comparison of Bacterial Presence in Biofilms on Different Materials Commonly Found in Recirculating Aquaculture Systems. J. Appl. Aquac. 2006, 18, 79–88. [Google Scholar] [CrossRef]
- Meireles, A.; Fulgêncio, R.; Machado, I.; Mergulhão, F.; Melo, L.; Simões, M. Characterization of the Heterotrophic Bacteria from a Minimally Processed Vegetables Plant. LWT—Food Sci. Technol. 2017, 85, 293–300. [Google Scholar] [CrossRef]
- Bakker, D.P.; Van Der Plaats, A.; Verkerke, G.J.; Busscher, H.J.; Mei, H.C. Van Der Comparison of Velocity Profiles for Different Flow Chamber Designs Used in Studies of Microbial Adhesion to Surfaces. Appl. Environ. Microbiol. 2003, 69, 6280–6287. [Google Scholar] [CrossRef]
- Silva, E.R.; Tulcidas, A.V.; Ferreira, O.; Bayón, R.; Igartua, A.; Mendoza, G.; Mergulhão, F.J.M.; Faria, S.I.; Gomes, L.C.; Carvalho, S.; et al. Assessment of the Environmental Compatibility and Antifouling Performance of an Innovative Biocidal and Foul-Release Multifunctional Marine Coating. Environ. Res. 2021, 198, 111219. [Google Scholar] [CrossRef]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton Bloom Status: Chlorophyll a Biomass as an Indicator of Water Quality Condition in the Southern Estuaries of Florida, USA. Ecol. Indic. 2009, 9, 56–67. [Google Scholar] [CrossRef]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric Determination of Hydrogen Peroxide Using Potassium Titanium(IV) Oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Russo, R.; Curtis, E.W.; Yanong, R.P.E. Preliminary Investigations of Hydrogen Peroxide Treatment of Selected Ornamental Fishes and Efficacy against External Bacteria and Parasites in Green Swordtails. J. Aquat. Anim. Health 2007, 19, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Magariños, B.; Irgang, R.; Toranzo, A.E. Use of Hydrogen Peroxide against the Fish Pathogen Tenacibaculum Maritimum and Its Effect on Infected Turbot (Scophthalmus Maximus). Aquaculture 2006, 257, 104–110. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Simões, M. Antimicrobial and Antibiofilm Potentiation by a Triple Combination of Dual Biocides and a Phytochemical with Complementary Activity. Food Res. Int. 2023, 167, 112680. [Google Scholar] [CrossRef]



| Symbol/Parameter | Description | Formula |
|---|---|---|
| x | Pixel position on the horizontal axis (width) | n.a. |
| y | Pixel position on the vertical axis (height) | |
| z | Pixel position on perpendicular axis (depth) | |
| i | Index of x,z position in the horizontal plane | |
| N | Number of voxels in the horizontal plane of the Region of Interest (ROI) | |
| Volume of a voxel (µm3) | ||
| Biofilm thickness at a given position i (µm) | ||
| Average biofilm thickness (µm) | ||
| Total area of the ROI (mm2) | ||
| Number of connected voxels identified as belonging to biofilm matrix/bacteria in the biofilm (biovolume) in a horizontal plane at position y | ||
| Number of voxels identified as belonging to biofilm matrix/bacteria in the biofilm (biovolume) in column i (vertical line of voxels at position i) and connected with the environment (including corner voxels) | ||
| Biovolume | Number of all connected voxels in all images of a horizontal plane multiplied by the voxel size, and it provides an estimate of the biomass in the biofilm (µm3) per area of the ROI | |
| Average size of non-connected pores | The determination of the average size of non-connected pores inside the biofilm structure was quantified, assuming the minimum size of non-connected pores equal to 1000 µm3 and defining 1 voxel size as corresponding to 117 µm3 | n.a. |
| Contour coefficient | Number of voxels connected to the background divided by the number of voxels of a horizontal plane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeu, M.J.; Morais, J.; Vasconcelos, V.; Mergulhão, F. Effect of Hydrogen Peroxide on Cyanobacterial Biofilms. Antibiotics 2023, 12, 1450. https://doi.org/10.3390/antibiotics12091450
Romeu MJ, Morais J, Vasconcelos V, Mergulhão F. Effect of Hydrogen Peroxide on Cyanobacterial Biofilms. Antibiotics. 2023; 12(9):1450. https://doi.org/10.3390/antibiotics12091450
Chicago/Turabian StyleRomeu, Maria João, João Morais, Vítor Vasconcelos, and Filipe Mergulhão. 2023. "Effect of Hydrogen Peroxide on Cyanobacterial Biofilms" Antibiotics 12, no. 9: 1450. https://doi.org/10.3390/antibiotics12091450
APA StyleRomeu, M. J., Morais, J., Vasconcelos, V., & Mergulhão, F. (2023). Effect of Hydrogen Peroxide on Cyanobacterial Biofilms. Antibiotics, 12(9), 1450. https://doi.org/10.3390/antibiotics12091450








