Abstract
Antibiotics are the fundamental treatment for bacterial infections. However, they are associated with numerous side effects. Their adverse effects on the immune system are increasingly recognised, with several mechanisms identified. In this review, we focus on their direct effects on cellular immunity. We review the effects of antibiotics on mitochondrial function and how they impair specific immune cell functions including chemotaxis, phagocytosis, cytokine production, antigen presentation, and lymphocyte proliferation. Findings are described in a multitude of in vivo and in vitro models. However, their impact on patient immunity and clinical outcomes requires further research. Awareness of the potential adverse effects of antibiotics may improve antimicrobial stewardship. The use of therapeutic drug monitoring may help to reduce dose-dependent effects, which warrants further research.
1. Introduction
Antibiotics are the fundamental treatment for bacterial infections. However, given the lack of suitable rapid diagnostic tests, most patients with sepsis are commenced on broad-spectrum antibiotics and transitioned to narrow-spectrum if cultures identify a causative organism. Given the poor sensitivity of traditional cultures, many patients are not de-escalated to narrow-spectrum antibiotics. Additionally, the length of antibiotic course is highly variable, often between 3 and 14 days [1].
Many patients with sepsis develop immunosuppression, increasing their risk of secondary infection [2]. Mechanisms underpinning sepsis-induced immunosuppression are multifactorial but likely to include off-target effects of medications, including antibiotics. Although adverse effects of antibiotics on immune cell function are well described [3,4], their specific effects on immunosuppression after sepsis and critical illness are unknown. Prolonged use of antibiotics may exacerbate this immunosuppression, leaving septic patients more vulnerable to subsequent infections [5].
Data on antibiotic modulation of immunity have been mainly characterised in cell lines and animal models [4,6]; clinical data are limited [7]. Most antibiotic classes suppress both innate and adaptive immune responses [6]. It is imperative to understand off-target immune effects of specific antibiotic classes and to determine underlying mechanisms.
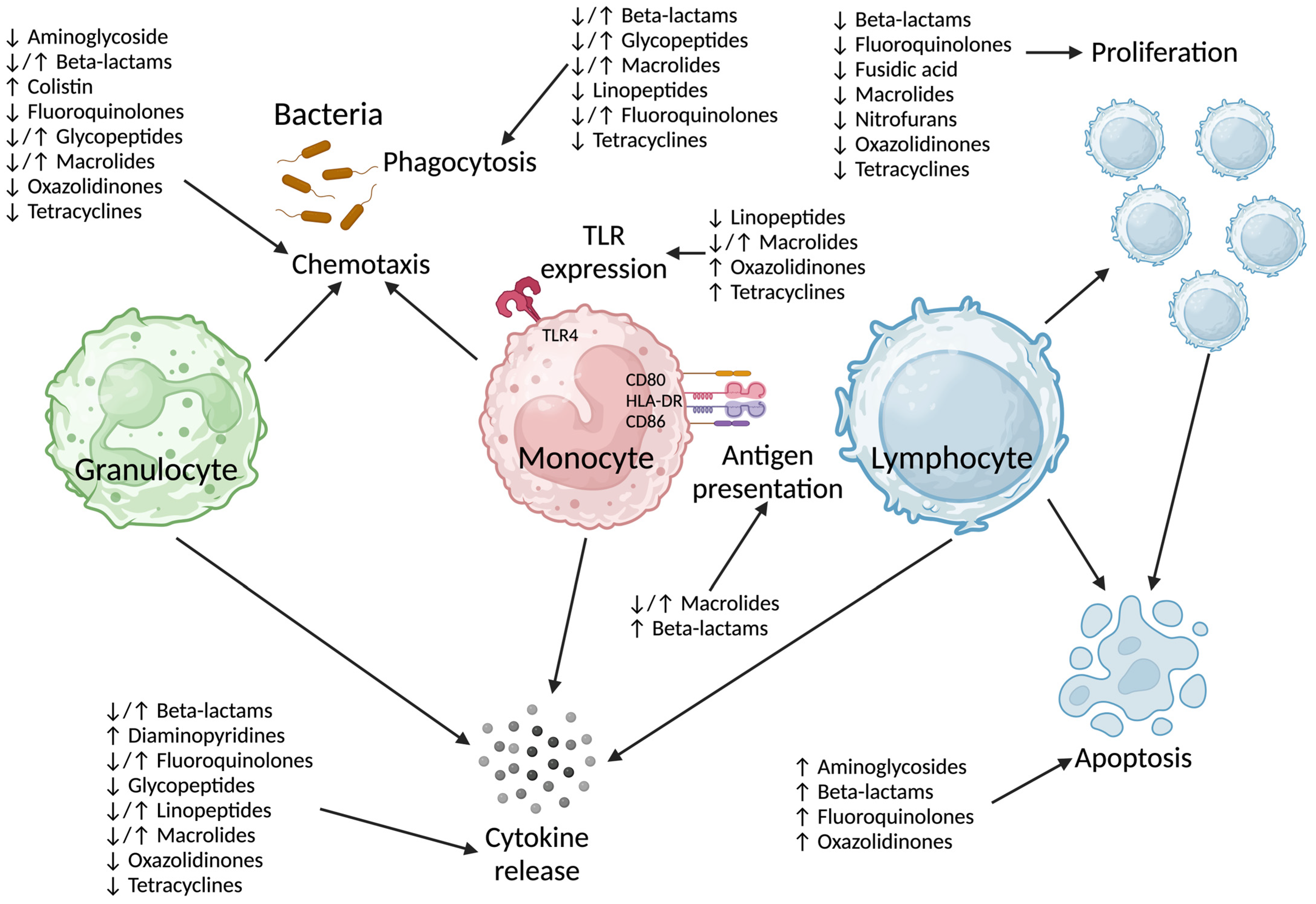
Antibiotics target (prokaryotic) bacterial cellular processes, although the antibiotic-related side effects experienced by patients clearly indicate off-target effects [8]. It is unclear if the mechanism(s) by which antibiotics impact on human immune cells are directly related to their antibacterial effects on DNA (deoxyribonucleic acid) transcription (ciprofloxacin) or protein translation (clarithromycin, gentamicin). The effect of beta-lactams on human immune cells is clearly unrelated to their mechanism of action on bacteria. Several pathways have been implicated in antibiotic-induced immunosuppression (Figure 1 and Supplementary Table S1).
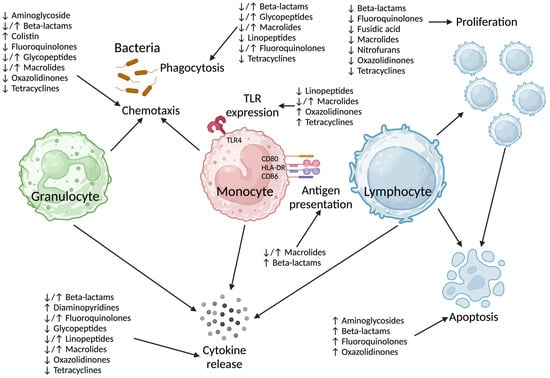
Figure 1.
Summary of immunosuppressive effects of antibiotics by class on immune cell function. TLR: Toll-like receptor, HLA-DR: Human Leukocyte Antigen—DR isotype, CD: cluster of differentiation. Created in www.BioRender.com.
In this review, we describe the effects of antibiotics on effects on mitochondrial function and specific immune cell functions including chemotaxis, phagocytosis, cytokine production by granulocytes and monocytes, antigen presentation (monocytes), and lymphocyte apoptosis and proliferation. Findings are described in a multitude of in vivo and in vitro models which highlight the potential relevance of the findings to clinical practise.
2. Cellular Dysfunction
2.1. Mitochondrial Dysfunction
Mitochondria are integral to regulating immune function; defects in leukocyte energy metabolism in septic patients are associated with immunosuppression [9]. The direct roles of mitochondria in innate and adaptive immune cells are wide-ranging, suggesting that mitochondrial dysfunction may play a significant causative role [10]. Given the current understanding of the prokaryotic origins of mitochondria, it is plausible that antibiotics targeting bacteria has detrimental effects on mitochondrial functionality.
For example, electron transport chain (ETC) adaptations serve as an early immunological–metabolic checkpoint for innate immune responses to bacterial infection [11]. Synthesis of mitochondrial DNA induced after the engagement of Toll-like receptors (TLRs) mediates NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome signalling in macrophages [12]. Antibiotics including lincosamides, macrolides, and fluoroquinolones accumulate in phagocytes and may interfere with the above processes [13]. The highly energy-dependent respiratory burst required for bacterial killing by macrophages is impaired by a dose-dependent inhibition of mitochondrial respiratory activity by ciprofloxacin [14].
The effects of antibiotics on immune system function are complex; observations from in vitro experiments may not necessarily translate to the in vivo situation. For instance, ciprofloxacin decreases the release of IL-1ß from human volunteer monocytes stimulated for 24 h in vitro with lipopolysaccharide (LPS) [15]; however, after administration of oral ciprofloxacin to healthy volunteers for 7 days, in vitro LPS stimulation enhanced IL-1ß production [16]. Previous work by our group has also demonstrated that ciprofloxacin and LPS in vitro stimulation suppresses pro-inflammatory cytokine release from volunteer and septic patient PBMCs after 24 h but not via mitochondrial pathways [5]. Whether these conflicting findings are due to differences between in vivo or in vitro models or due to the differences in duration of antibiotic administration remains to be elucidated. Animal models or cells isolated from septic patients administered ciprofloxacin could be used to confirm these findings.
Aminoglycoside antibiotics are a family of amino-modified sugars containing hydrophilic portions and cationic amine moieties that preferentially bind nucleic acids due to their negative charge. They can cause translational errors and the assembly of incorrect amino acid products or premature termination of protein synthesis [17,18]. While their effects on immune cell mitochondria are yet to be delineated, they do impact upon renal tubular epithelial mitochondria in animal models [19].
Aminoglycosides bind to human mitochondrial ribosomes [20]. In isolated mitochondria from rat renal tubular cells, aminoglycosides induced ETC uncoupling, increased mitochondrial membrane cation permeability [19], and collapse of the mitochondrial membrane potential [21]. This reduced oxidative phosphorylation [22] and the production of mitochondrial reactive oxygen species (ROS) [23]. However, there may be differing effects on different aspects of mitochondrial respiration [24], which may be why some studies demonstrated an increase in ROS [25]. Oxazolidinone antibiotics bind to mitochondrial ribosomes, reducing mitochondrial protein in non-immune cells [26] and the K562 lymphoblastoid and THP-1 monocyte cell lines [27,28].
In a rat model of gentamicin-induced renal toxicity, respiratory components including cytochrome C and NADH (nicotinamide adenine dinucleotide (NAD) + hydrogen (H)) were depleted. This was associated with an opening of the mitochondrial transition pore and an increase in ROS production [29]. The potency of the aminoglycosides in producing these effects correlates with the number of ionizable amino groups present on the aminoglycoside molecule, suggesting that cationic charge is an important molecular determinant of toxic effect [24]. Similar effects have been demonstrated in other cell types including mouse cochlear cells [30] but not liver cells [22], suggesting certain cell types are at increased risk. Mitochondria in peripheral blood monocular cells (PBMCs) may not be affected [5]; however, further research is required to explore the effects of aminoglycosides on mitochondrial function in other immune cell types.
2.2. Chemotaxis and Migration
Immune cells migrate from the blood to the source of infection via a chemokine gradient.
Mouse macrophage chemotaxis was increased by carbapenems [31] and by teicoplanin and vancomycin [32] but decreased by amoxicillin beta-lactams, clindamycin, and tetracycline [33]. Mouse neutrophil migration was decreased by linezolid [34], and rat neutrophil migration was increased by colistin [35].
In volunteer immune cells and PBMCs, erythromycin and roxithromycin increased migration or chemotaxis [36], while aminoglycosides and tetracyclines were inhibitory [37,38]. Penicillins [38,39], carbapenems [39,40], and linezolid had no effect [41,42]. Cephalosporins [37,39,43,44,45,46,47], teicoplanin [48,49], and vancomycin had differing model-dependent effects [49,50]. In in vivo healthy volunteer models, erythromycin impaired neutrophil migration via reduced IL-8 [51], and ceftriaxone impaired chemotaxis [52].
In patients, macrolides inhibited neutrophil chemotaxis and migration predominantly through reduced IL-8 in patients with COPD [53,54,55,56,57], bronchial hyperreactivity [58], chronic sinusitis [59,60,61,62], and allergy [63]. This effect was, however, not seen consistently with clarithromycin, although this be related to the different diseases studied [64].
2.3. Toll-Like Receptor Expression
Toll-like receptors (TLRs) are pattern recognition receptors which are expressed on a variety of immune cells including neutrophils and monocytes. TLRs are activated by numerous bacterial products including peptidoglycans and LPS. Activation is important in the initiation of the inflammatory cascade in response to infection [65].
In a mouse model, folimycin decreased surface expression of TLR mediated by inhibition of V-ATPases (vacuolar adenosine triphosphate-ases) [66]. In THP-1 cell lines, linezolid increased TLR expression (-1, -2, and -6), while daptomycin decreased it [67]. Erythromycin, moxifloxacin, and doxycycline increased TLR expression (-1, -2, -4, and -6) both in the THP-1 cell line and in patients following cardiac bypass [68]. These findings need to be confirmed in other diseases.
2.4. Cytokine Release
Cytokines are inflammatory mediators released by several cell types in response to infection which regulate the inflammatory and immune response. They are broadly characterised into pro-inflammatory (e.g., IL-1β and TNF-α), anti-inflammatory (e.g., IL-10), or mixed (IL-6).
Most antibiotics inhibit cytokine production and release. In ex vivo mouse models on antigen-presenting cells, roxifloxacin [69], erythromycin [70], azithromycin [70], and doxycycline inhibited the release of multiple pro-inflammatory cytokines [71]. One postulated mechanism was through the inhibition of mitochondrial protein translation and NLRP3 inflammasome assembly in bone marrow-derived macrophages [71].
Using in vivo and in vitro animal models, fluroquinolones inhibited some pro-inflammatory cytokines, although there were in-class differences related to antibiotic structure [72,73]. Macrolides were anti-inflammatory [74], while roxifloxacin had time-dependent effects, increasing pro-inflammatory release initially but causing inhibition after over 2 weeks’ treatment [75,76]. Linezolid and vancomycin also reduced cytokine release in pneumonia models [34,77,78,79,80]. In large animal pneumonia models, azithromycin inhibited IL-6 release [81], linezolid had no effect [82], and danofloxacin was predominately anti-inflammatory, reducing pro-inflammatory cytokine release yet increasing IL-10 [83].
In J774 macrophage cell lines, macrolides inhibited pro-inflammatory cytokine release through reduced COX-2 (cyclooxygenase-2) and nitric oxide synthase (NOS) expression [74]. In THP-1 monocyte cell lines, linezolid and vancomycin increased both pro- and anti-inflammatory cytokine release [67]; erythromycin, doxycycline, and moxifloxacin increased pro-inflammatory cytokine release [68]; grepafloxacin inhibited pro-inflammatory release [84]; while daptomycin had mixed effects on pro-inflammatory cytokine release [67].
In volunteer whole blood and PBMC models, cytokine release was reduced by linezolid [85,86], clindamycin [87], teicoplanin [88], erythromycin [86,89], ceftazidime [90], and tigecycline [91]. Meropenem had mixed effects, reducing the release of some pro-inflammatory cytokines [40]. Amoxicillin and trimethoprim, however, were pro-inflammatory [91,92], while penicillin and metronidazole had no effect [87,89,93]. Several studies yielded conflicting results. Vancomycin either decreased release or had no effect [85,93], while fluroquinolones either reduced [15,40,90,94,95,96,97,98,99,100], had no effect [101], or increased release [102].
In patient studies, clarithromycin given to COPD (chronic obstructive pulmonary disease) and asthmatic patients either had no effect [57,103] or reduced both pro- and anti-inflammatory cytokine release [104,105]. Erythromycin given to wheezy children reduced cytokine levels [106], while amoxicillin and penicillin given to allergy patients increased pro-inflammatory cytokine levels [107]. Norfloxacin in cirrhotic patients induced an immunosuppressive phenotype with an increased proportion of Tregs and IL-10 release [108]. Suggested mechanisms include a direct fluroquinolone effect on protein synthesis [15,94,99], mitochondrial ETC inhibition [100], inhibition of COX-2 [98], and upregulation of the rag1 (recombination activating gene 1) gene (responsible for T-cell receptor formation) [108].
Clarithromycin has different effects dependant on the patient population studied. In those with ventilator-associated or community-acquired pneumonia, it causes an increase in LPS-induced TNF-α release [109,110]. However, in patients with septic shock, it reduced release, but IL-6 release was maintained [110]. This suggests there may be a disease- or illness-severity-specific effect, although whilst the stimulation studies were performed at day 4 of enrolment, it was unclear whether there were differences in duration of admission prior to enrolment, which could also explain the findings. However, the different effects of clarithromycin on cytokine release highlights potential mechanisms to be explored.
2.5. Phagocytosis
Phagocytosis is an important early antibacterial mechanism of professional phagocytotic cells, including neutrophils and monocytes, for the elimination of pathogens. Antigen-presenting cells, including monocytes, present antigens processed from phagocytosed bacteria to trigger the adaptive immune system [111].
In mouse and rat macrophages, carbapenems increased phagocytosis [31], while amoxicillin, clindamycin, azithromycin, and erythromycin impaired it [33,70]. Vancomycin and teicoplanin had differing effects with both enhancement and impairment [32,112]. Daptomycin and lomefloxacin had no effect [112,113]. In the THP-1 cell line, antifungal agents suppressed phagocytosis [114].
In volunteer immune cells and PBMCs, meropenem and macrolides reduced neutrophil phagocytosis [40,115]. Cephalosporins, co-amoxiclav, and imipenem increased neutrophil phagocytosis [44,45,46,115,116,117], while macrolides increased monocyte phagocytosis [118]. Rokitamycin and linezolid had no effect [41,42,119]. Teicoplanin and vancomycin had differing dose-dependent effects [48,49]. Fluroquinolones including ciprofloxacin also had differing effects, with low doses enhancing phagocytosis [120,121] or having no effect [113,122], while inhibition could occur at supra-pharmacological doses [123].
In volunteer in vivo models, carbapenems increased phagocytosis [117], while ceftriaxone had no effect [52]. In patients, piperacillin, doxycycline, and moxifloxacin inhibited monocyte phagocytosis after cardiac surgery [68]. Azithromycin increased macrophage phagocytosis [124], and clarithromycin increased neutrophil phagocytosis in COPD patients [64]. Roxifloxacin also increased neutrophil phagocytosis [125].
Given the myriad of conflicting results with individual antibiotics or classes, likely related to different experimental conditions and the cell types studied, in vivo antibiotic dosing models would be required to answer definitively even if most phagocytosis assays are in vitro.
2.6. Antigen Presentation
Antigen presenting cells are key mediators between the innate and adaptive immune systems. Phagocytosed bacterial products are broken down into antigens and presented on the cell surface by major histocompatibility complex class II (MHC class II) molecules including Human Leukocyte Antigen—DR isotype (HLA-DR) to the T-cell receptor on lymphocytes. The response of the lymphocyte to the selected antigen is regulated by co-stimulatory molecules, of which monocyte CD80 and CD86 are well characterised [126]. Impaired antigen presentation is a common feature of sepsis-induced immunosuppression and is associated with the development of secondary infections and mortality [127,128].
In mice, roxithromycin impairs antigen-presenting cell MHC class II presentation [69] and CD80 and CD86 on B-cells [129,130], although this effect was only seen with longer courses [131].
In volunteer PBMCs, pefloxacin and ciprofloxacin had no effect on antigen presentation [132]. In PBMCs isolated from patients with allergies, there was an upregulation of HLA-DR, CD80, and CD86 with amoxicillin [133], while in PBMCs from cirrhotic patients, norfloxacin impaired CD80/86 expression [108]. It is unclear whether the different effect of norfloxacin compared to ciprofloxacin is related to the drug itself or the effect of cirrhosis in this population. Macrolides increased CD80 but not HLA-DR in patients with chronic sinusitis [134], while clarithromycin increased HLA-DR in patients with pneumonia and sepsis [135] and increased CD86 in patients with ventilator-associated pneumonia and sepsis [109]. The differences in these findings appear to be disease-related, with greater effect seen in acute illness with short duration of antibiotics compared to chronic illness with prolonged antibiotic course. A prolonged time course of changes in antigen presentation with clarithromycin would be required to confirm this, especially given changes between the clarithromycin and placebo groups were only seen 10 days after treatment [135].
2.7. Lymphocyte Proliferation
In response to infection, lymphocytes undergo clonal proliferation and differentiation into the various subclasses to facilitate bacterial clearance. Imbalances in proliferation have been associated with mortality in sepsis with reduced proliferation of Thelper cells but increased proliferation of the immunosuppressive Treg type [136].
Antibiotics have been demonstrated to inhibit cell proliferation. In volunteer PBMCs, fluroquinolones impair proliferation through alterations in IL-2 release and increased monocyte prostaglandin E2 release [95,98,102]. Additional mechanistic work in breast and lung cancer cell lines suggests the effect could also be caused by damaging mitochondria and deactivating the PI3K/Akt/mTOR (phosphatidylinositol 3-kinase/Ak strain transforming/mechanistic target of rapamycin) and MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinases) pathways [137,138]. Another potential mechanism could be through direct binding and inhibition of the T-cell receptor. In patients with allergies to fluroquinolones and amoxicillin, these antibiotics directly bind to the T-cell receptor, albeit stimulating proliferation in these cases [133,139].
Erythromycin, clindamycin, rifampicin, fusidic acid, nitrofurantoin, and doxycycline all inhibited proliferation of healthy volunteer lymphocytes, whereas penicillin, cephalosporins, aminoglycosides, chloramphenicol, sulfamethoxazole, trimethoprim [140], and macrolides did not [141,142]. However, in a mouse model, cefotaxime did inhibit lymphocyte proliferation [143]. Other cephalosporins and penicillins (including piperacillin) impaired proliferation; however, the effect of each antibiotic was not consistent amongst different cell types when comparing their effect on proliferation of chick embryos, lymphocyte cell lines, and mouse lymphocytes. This cell-specific effect may in part explain the conflicting results described; further research is required to identify the causative mechanism and whether the in vitro effect is seen in vivo [144].
2.8. Lymphocyte Apoptosis
Following clonal lymphocyte proliferation after infection, apoptosis occurs, leaving the memory cells quiescent, primed for subsequent re-infections. Sepsis is associated with increased apoptosis, causing lymphopenia and increased risk of subsequent infection [128].
Lymphocyte apoptosis is mediated by two main pathways, mitochondrial (which includes caspases-3 and -8 and Bcl-2 (B-cell lymphoma) proteins) and non-mitochondrial pathways [145].
Linezolid induced lymphocyte apoptosis through mitochondrial pathways by inhibiting mitochondrial protein synthesis and complex IV activity in volunteer PBMCs and skin nerve fibres [146] and in patient and rat skeletal muscle and liver [147]. Protein levels were reduced, while mitochondrial DNA levels remained similar, suggesting a direct action on the mitochondrial ribosome; certain polymorphisms appear to be at increased risk. Moxifloxacin increased murine macrophage cell death, although this could be ameliorated by the use of immunomodulatory compounds tinrostim and licopid [148].
The experimental beta-lactam, lactam 1, induced T-cell apoptosis in a Jurkat cell line [149] and a mouse breast cancer model [150] through direct damage to, and inhibition of, DNA replication. This led to p38 MAPK activation, S phase arrest, and apoptotic cell death mediated by caspase-3, -8, and -9 activation, cleavage of the pro-apoptotic Bcl-2 family protein Bid (BH3-interacting domain death agonist), and release of mitochondrial cytochrome c.
The fluroquinolone ciprofloxacin also induced Jurkat cell apoptosis through mitochondrial pathways by causing direct damage to mitochondrial DNA, inhibiting the respiratory chain and decreasing membrane potential [151]. Similar effects of mitochondrial-induced apoptosis have been demonstrated by ciprofloxacin on other cell lines, including colon and bladder tumour cells [152,153], and by levofloxacin in breast and lung cancer cell lines [137,138]. Whilst this suggests a consistent mechanism, the effect on primary lymphocytes and other immune cell types remains to be delineated.
Gentamicin-induced ETC inhibition activated caspases-3 and -9, leading to mitochondrial-induced cellular apoptosis in renal cell lines [154,155].
3. Clinical Consequences
Antibiotics remain the key management strategy for the treatment of bacterial infection. In light of the adverse effects on immune cell function described in this review, and the multitude of other deleterious effects on antimicrobial resistance and on the gut microbiota [6], we advocate strong antimicrobial stewardship [156], key features of which include the following:
Judicious initiation of antibiotics—The diagnosis of infection is not always straightforward. Recognising this, recent guidance now recommends consideration of illness severity as a guide to the urgency of antibiotic administration [157]. This allows time for the collection of appropriate cultures and consideration to many of the infection mimics prior to commencing antibiotics, especially as early antibiotic discontinuation even if infection is disproved is not always performed [158].
Reduced inappropriate usage—When antibiotics are required, broad-spectrum antibiotics are sometimes initiated, or the courses of antibiotics are sometimes unduly prolonged as a precaution. This is especially pertinent for community-acquired infections and surgical prophylaxis [159]. However, (appropriately) shorter duration of antibiotic courses are non-inferior to longer courses and may be associated with lower risk of development of subsequent infections [160,161].
Selecting narrow- over broad-spectrum antibiotics—Overuse of broad-spectrum antibiotics when not required is associated with increased risk of antimicrobial resistance but may also be associated with an increased risk of mortality [162]. Our review suggests carbapenems and piperacillin may have greater immunosuppressive effects over cephalosporins and amoxicillin—however, this needs to be explored further.
Therapeutic drug monitoring (TDM)—Many of the immunosuppressive effects demonstrated in this review were identified at higher concentrations. Whilst most antibiotic dosing is based on pharmacokinetic/pharmacodynamic studies in healthy volunteers, measured serum concentrations in septic patients vary significantly with both relative under- and overdosing demonstrated [163]. With the increasing interest in TDM, antibiotic dosing could be optimised to minimise the risk of supra-clinical concentrations which are associated with increased 28-day mortality potentially mediated through their deleterious immunosuppressive and other adverse effects [164].
4. Summary
Antibiotics are associated with multiple deleterious effects beyond their immediate side-effect profile. These include patient-specific effects of idiosyncratic drug reactions, disruption of microbiome and mitochondrial toxicity, and population-level effects including antimicrobial resistance. A growing body of evidence shows antibiotics can directly impact immune cell function, although their extent and the mechanisms by which these occur remains relatively unexplored.
Critical illness is associated with multiple immune defects which are associated with an increased risk of subsequent infections. While reduced monocyte HLA-DR expression and lymphopenia are well described, it is unclear whether these are isolated defects or symptomatic of wider immune cell dysfunction. The lack of benefit demonstrated by immunomodulatory treatments targeting these pathways suggests the latter; however, more research is required to explore this further.
Given the significant use of antibiotics in the critically ill, it is plausible that antibiotics may directly affect immune cell function, exacerbating the immunosuppressive state seen in critical illness. Confirmation of this would add support to rigorous antimicrobial stewardship goals aiming to reduce undue antimicrobial use, especially if the deleterious effects are related to duration of course or use of broad-spectrum agents or if there is evidence of a dose-dependent effect.
Given beta-lactam antibiotics are the most widely used class of antibiotics in the critically ill, they represent the best target for identification of immunosuppressive effects. The growing use of therapeutic drug monitoring for them also presents an opportunity to incorporate dosing regimens which ensure appropriate serum concentrations for bacterial killing whilst preventing supra-clinical concentrations which could have deleterious effects on immune cell function.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13111034/s1, Table S1: Summary of antibiotic effects on immune cell function.
Author Contributions
Writing—original draft preparation, T.A.C.S.; writing—review and editing, M.S. and N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Furukawa, Y.; Luo, Y.; Funada, S.; Onishi, A.; Ostinelli, E.; Hamza, T.; Furukawa, T.A.; Kataoka, Y. Optimal duration of antibiotic treatment for community-acquired pneumonia in adults: A systematic review and duration-effect meta-analysis. BMJ Open 2023, 13, e061023. [Google Scholar] [CrossRef] [PubMed]
- Conway Morris, A.; Datta, D.; Shankar-Hari, M.; Stephen, J.; Weir, C.J.; Rennie, J.; Antonelli, J.; Bateman, A.; Warner, N.; Judge, K.; et al. Cell-surface signatures of immune dysfunction risk-stratify critically ill patients: INFECT study. Intensive Care Med. 2018, 44, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.E., Jr.; Remington, J.S. Effect of antibiotics on the immune response. Am. J. Med. 1982, 72, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Anuforom, O.; Wallace, G.R.; Piddock, L.V. The immune response and antibacterial therapy. Med. Microbiol. Immunol. 2015, 204, 151–159. [Google Scholar] [CrossRef]
- Miller, M.; Melis, M.J.; Miller, J.R.C.; Kleyman, A.; Shankar-Hari, M.; Singer, M. Antibiotics, Sedatives, and Catecholamines Further Compromise Sepsis-Induced Immune Suppression in Peripheral Blood Mononuclear Cells. Crit. Care Med. 2024, 52, 596–606. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Routledge, M.; Schlebusch, S.; Lipman, J.; Conway Morris, A. Antimicrobial-associated harm in critical care: A narrative review. Intensive Care Med. 2020, 46, 225–235. [Google Scholar] [CrossRef]
- Snow, T.A.C.; Longobardo, A.; Brealey, D.; Down, J.; Satta, G.; Singer, M.; Arulkumaran, N. Beneficial ex vivo immunomodulatory and clinical effects of clarithromycin in COVID-19. J. Infect. Chemother. 2022, 28, 948–954. [Google Scholar] [CrossRef]
- Tosi, M.; Coloretti, I.; Meschiari, M.; De Biasi, S.; Girardis, M.; Busani, S. The Interplay between Antibiotics and the Host Immune Response in Sepsis: From Basic Mechanisms to Clinical Considerations: A Comprehensive Narrative Review. Antibiotics 2024, 13, 406. [Google Scholar] [CrossRef]
- Cheng, S.C.; Scicluna, B.P.; Arts, R.J.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.; Cremer, O.L.; et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef]
- Park, D.W.; Zmijewski, J.W. Mitochondrial Dysfunction and Immune Cell Metabolism in Sepsis. Infect. Chemother. 2017, 49, 10–21. [Google Scholar] [CrossRef]
- Garaude, J.; Acin-Perez, R.; Martinez-Cano, S.; Enamorado, M.; Ugolini, M.; Nistal-Villan, E.; Hervas-Stubbs, S.; Pelegrin, P.; Sander, L.E.; Enriquez, J.A.; et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016, 17, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, P.M. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Bhargava, P.; McCloskey, D.; Mao, N.; Palsson, B.O.; Collins, J.J. Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 2017, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Bailly, S.; Mahe, Y.; Ferrua, B.; Fay, M.; Tursz, T.; Wakasugi, H.; Gougerot-Pocidalo, M.A. Quinolone-induced differential modification of IL-1 alpha and IL-1 beta production by LPS-stimulated human monocytes. Cell. Immunol. 1990, 128, 277–288. [Google Scholar] [CrossRef]
- Bailly, S.; Fay, M.; Ferrua, B.; Gougerot-Pocidalo, M.A. Ciprofloxacin treatment in vivo increases the ex vivo capacity of lipopolysaccharide-stimulated human monocytes to produce IL-1, IL-6 and tumour necrosis factor-alpha. Clin. Exp. Immunol. 1991, 85, 331–334. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.; Guan, M.X. Mitochondrial DNA mutations associated with aminoglycoside induced ototoxicity. J. Otol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Fourmy, D.; Recht, M.I.; Blanchard, S.C.; Puglisi, J.D. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science 1996, 274, 1367–1371. [Google Scholar] [CrossRef]
- Weinberg, J.M.; Harding, P.G.; Humes, H.D. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. II. Effects on mitochondrial monovalent cation transport. Arch. Biochem. Biophys. 1980, 205, 232–239. [Google Scholar] [CrossRef]
- Hong, S.; Harris, K.A.; Fanning, K.D.; Sarachan, K.L.; Frohlich, K.M.; Agris, P.F. Evidence That Antibiotics Bind to Human Mitochondrial Ribosomal RNA Has Implications for Aminoglycoside Toxicity. J. Biol. Chem. 2015, 290, 19273–19286. [Google Scholar] [CrossRef]
- O’Reilly, M.; Young, L.; Kirkwood, N.K.; Richardson, G.P.; Kros, C.J.; Moore, A.L. Gentamicin Affects the Bioenergetics of Isolated Mitochondria and Collapses the Mitochondrial Membrane Potential in Cochlear Sensory Hair Cells. Front. Cell. Neurosci. 2019, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.F., Jr.; Bogusky, R.T.; Humes, H.D. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J. Pharmacol. Exp. Ther. 1980, 214, 709–715. [Google Scholar] [PubMed]
- Ueda, N.; Guidet, B.; Shah, S.V. Gentamicin-induced mobilization of iron from renal cortical mitochondria. Am. J. Physiol. 1993, 265 Pt 2, F435–F439. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M.; Simmons, F., Jr.; Humes, H.D. Alterations of mitochondrial respiration induced by aminoglycoside antibiotics. Res. Commun. Chem. Pathol. Pharmacol. 1980, 27, 521–531. [Google Scholar] [PubMed]
- Yang, C.L.; Du, X.H.; Han, Y.X. Renal cortical mitochondria are the source of oxygen free radicals enhanced by gentamicin. Ren. Fail. 1995, 17, 21–26. [Google Scholar] [CrossRef]
- McKee, E.E.; Ferguson, M.; Bentley, A.T.; Marks, T.A. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 2006, 50, 2042–2049. [Google Scholar] [CrossRef]
- Leach, K.L.; Swaney, S.M.; Colca, J.R.; McDonald, W.G.; Blinn, J.R.; Thomasco, L.M.; Gadwood, R.C.; Shinabarger, D.; Xiong, L.; Mankin, A.S. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 2007, 26, 393–402. [Google Scholar] [CrossRef]
- Milosevic, T.V.; Payen, V.L.; Sonveaux, P.; Muccioli, G.G.; Tulkens, P.M.; Van Bambeke, F. Mitochondrial Alterations (Inhibition of Mitochondrial Protein Expression, Oxidative Metabolism, and Ultrastructure) Induced by Linezolid and Tedizolid at Clinically Relevant Concentrations in Cultured Human HL-60 Promyelocytes and THP-1 Monocytes. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Morales, A.I.; Detaille, D.; Prieto, M.; Puente, A.; Briones, E.; Arevalo, M.; Leverve, X.; Lopez-Novoa, J.M.; El-Mir, M.Y. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010, 77, 861–869. [Google Scholar] [CrossRef]
- Desa, D.E.; Nichols, M.G.; Smith, H.J. Aminoglycosides rapidly inhibit NAD(P)H metabolism increasing reactive oxygen species and cochlear cell demise. J. Biomed. Opt. 2018, 24, 051403. [Google Scholar] [CrossRef]
- Nunez, R.M.; Rodriguez, A.B.; Barriga, C.; De la Fuente, M. In vitro and in vivo effects of Imipenem on phagocytic activity of murine peritoneal macrophages. APMIS 1989, 97, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Barriga, C.; Pedrera, I.; Rodriguez, A.B. Comparative study of the effect of teicoplanin and vancomycin upon the phagocytic process of peritoneal macrophages. Rev. Esp. Fisiol. 1996, 52, 215–222. [Google Scholar] [PubMed]
- Miyata, T.; Shinohara, M. Effect of antibiotics on rat leukocyte function. J. Osaka Dent. Univ. 1998, 32, 9–15. [Google Scholar] [PubMed]
- Jacqueline, C.; Broquet, A.; Roquilly, A.; Davieau, M.; Caillon, J.; Altare, F.; Potel, G.; Asehnoune, K. Linezolid dampens neutrophil-mediated inflammation in methicillin-resistant Staphylococcus aureus-induced pneumonia and protects the lung of associated damages. J. Infect. Dis. 2014, 210, 814–823. [Google Scholar] [CrossRef][Green Version]
- Stamatiou, R.; Vasilaki, A.; Tzini, D.; Deskata, K.; Zacharouli, K.; Ioannou, M.; Sgantzos, M.; Zakynthinos, E.; Makris, D. Colistin Effects on Emphysematous Lung in an LPS-Sepsis Model. Antibiotics 2023, 12, 1731. [Google Scholar] [CrossRef]
- Anderson, R. Erythromycin and roxithromycin potentiate human neutrophil locomotion in vitro by inhibition of leukoattractant-activated superoxide generation and autooxidation. J. Infect. Dis. 1989, 159, 966–973. [Google Scholar] [CrossRef]
- Sugita, K.; Nishimura, T. Effect of antimicrobial agents on chemotaxis of polymorphonuclear leukocytes. J. Chemother. 1995, 7, 118–125. [Google Scholar] [CrossRef]
- Belsheim, J.A.; Gnarpe, G.H. Antibiotics and granulocytes. Direct and indirect effects on granulocyte chemotaxis. Acta Pathol. Microbiol. Scand. C 1981, 89, 217–221. [Google Scholar]
- Fietta, A.; Sacchi, F.; Bersani, C.; Grassi, F.; Mangiarotti, P.; Grassi, G.G. Effect of beta-lactam antibiotics on migration and bactericidal activity of human phagocytes. Antimicrob. Agents Chemother. 1983, 23, 930–931. [Google Scholar] [CrossRef]
- Matera, G.; Berlinghieri, M.C.; Foca, A. Meropenem: Effects on human leukocyte functions and interleukin release. Int. J. Antimicrob. Agents 1995, 5, 129–133. [Google Scholar] [CrossRef]
- Naess, A.; Stenhaug Kilhus, K.; Nystad, T.W.; Sornes, S. Linezolid and human polymorphonuclear leukocyte function. Chemotherapy 2006, 52, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, S.; Pascual, A.; Garcia, I.; Perea, E.J. Effect of linezolid on the phagocytic functions of human polymorphonuclear leukocytes. Chemotherapy 2003, 49, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Labro, M.T.; Babin-Chevaye, C.; Hakim, J. Effects of cefotaxime and cefodizime on human granulocyte functions in vitro. J. Antimicrob. Chemother. 1986, 18, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fietta, A.; Merlini, C.; Gialdroni Grassi, G. In vitro activity of two new oral cephalosporins, cefixime and cefdinir (CI 983), on human peripheral mononuclear and polymorphonuclear leukocyte functions. Chemotherapy 1994, 40, 317–323. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Barriga, C.; De la Fuente, M. In vitro effect of cefoxitin on phagocytic function and antibody-dependent cellular cytotoxicity in human neutrophils. Comp. Immunol. Microbiol. Infect. Dis. 1993, 16, 37–50. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Barriga, C.; de la Fuente, M. Stimulation of phagocytic processes and antibody-dependent cellular cytotoxicity of human neutrophils by cefmetazole. Microbiol. Immunol. 1991, 35, 545–556. [Google Scholar] [CrossRef]
- Burgaleta, C.; Moreno, T. Effect of beta-lactams and aminoglycosides on human polymorphonuclear leucocytes. J. Antimicrob. Chemother. 1987, 20, 529–535. [Google Scholar] [CrossRef]
- Capodicasa, E.; Scaringi, L.; Rosati, E.; De Bellis, F.; Sbaraglia, G.; Marconi, P.; Del Favero, A. In-vitro effects of teicoplanin, teicoplanin derivative MDL 62211 and vancomycin on human polymorphonuclear cell function. J Antimicrob Chemother 1991, 27, 619–626. [Google Scholar] [CrossRef]
- Fietta, A.; Bersani, C.; De Rose, V.; Grassi, F.M.; Gialdroni Grassi, G. The effect of teicoplanin on leukocytic activity and intraleukocytic micro-organisms. J. Hosp. Infect. 1986, 7 (Suppl. A), 57–63. [Google Scholar] [CrossRef]
- Moran, F.J.; Puente, L.F.; Perez-Giraldo, C.; Blanco, M.T.; Hurtado, C.; Gomez-Garcia, A.C. Activity of vancomycin and teicoplanin against human polymorphonuclear leucocytes: A comparative study. J. Antimicrob. Chemother. 1991, 28, 415–418. [Google Scholar] [CrossRef]
- Schultz, M.J.; Speelman, P.; Hack, C.E.; Buurman, W.A.; van Deventer, S.J.; van Der Poll, T. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J. Antimicrob. Chemother. 2000, 46, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gialdroni Grassi, G.; Fietta, A.; Sacchi, F.; Derose, V. Influence of ceftriaxone on natural defense systems. Am. J. Med. 1984, 77, 37–41. [Google Scholar] [PubMed]
- Oda, H.; Kadota, J.; Kohno, S.; Hara, K. Erythromycin inhibits neutrophil chemotaxis in bronchoalveoli of diffuse panbronchiolitis. Chest 1994, 106, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Kadota, J.; Kohno, S.; Hara, K. Leukotriene B4 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Chest 1995, 108, 116–122. [Google Scholar] [CrossRef]
- Kadota, J.; Sakito, O.; Kohno, S.; Sawa, H.; Mukae, H.; Oda, H.; Kawakami, K.; Fukushima, K.; Hiratani, K.; Hara, K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 1993, 147, 153–159. [Google Scholar] [CrossRef]
- Sakito, O.; Kadota, J.; Kohno, S.; Abe, K.; Shirai, R.; Hara, K. Interleukin 1 beta, tumor necrosis factor alpha, and interleukin 8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis: A potential mechanism of macrolide therapy. Respiration 1996, 63, 42–48. [Google Scholar] [CrossRef]
- Banerjee, D.; Honeybourne, D.; Khair, O.A. The effect of oral clarithromycin on bronchial airway inflammation in moderate-to-severe stable COPD: A randomized controlled trial. Treat. Respir. Med. 2004, 3, 59–65. [Google Scholar] [CrossRef]
- Piacentini, G.L.; Peroni, D.G.; Bodini, A.; Pigozzi, R.; Costella, S.; Loiacono, A.; Boner, A.L. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: A preliminary report. Allergy Asthma Proc. 2007, 28, 194–198. [Google Scholar] [CrossRef]
- Suzuki, H.; Shimomura, A.; Ikeda, K.; Oshima, T.; Takasaka, T. Effects of long-term low-dose macrolide administration on neutrophil recruitment and IL-8 in the nasal discharge of chronic sinusitis patients. Tohoku J. Exp. Med. 1997, 182, 115–124. [Google Scholar] [CrossRef][Green Version]
- Cervin, A.; Wallwork, B.; Mackay-Sim, A.; Coman, W.B.; Greiff, L. Effects of long-term clarithromycin treatment on lavage-fluid markers of inflammation in chronic rhinosinusitis. Clin. Physiol. Funct. Imaging 2009, 29, 136–142. [Google Scholar] [CrossRef]
- Wallwork, B.; Coman, W.; Mackay-Sim, A.; Greiff, L.; Cervin, A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope 2006, 116, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Fujieda, S.; Mori, S.; Yamamoto, H.; Saito, H. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am. J. Rhinol. 2000, 14, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, T.; Takizawa, H.; Kawasaki, S.; Akiyama, N.; Sato, M.; Ito, K. Fourteen-member macrolides inhibit interleukin-8 release by human eosinophils from atopic donors. Antimicrob. Agents Chemother. 1999, 43, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Ferrara, F.; Dugnani, S.; Demartini, G.; Triscari, F.; Fraschini, F. Immunostimulation by clarithromycin in healthy volunteers and chronic bronchitis patients. J. Chemother. 1993, 5, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Kulatheepan, Y.; Jeyaseelan, S. Role of toll-like receptors and nod-like receptors in acute lung infection. Front. Immunol. 2023, 14, 1249098. [Google Scholar] [CrossRef] [PubMed]
- Eswarappa, S.M.; Basu, N.; Joy, O.; Chakravortty, D. Folimycin (concanamycin A) inhibits LPS-induced nitric oxide production and reduces surface localization of TLR4 in murine macrophages. Innate Immun. 2008, 14, 13–24. [Google Scholar] [CrossRef]
- Bode, C.; Muenster, S.; Diedrich, B.; Jahnert, S.; Weisheit, C.; Steinhagen, F.; Boehm, O.; Hoeft, A.; Meyer, R.; Baumgarten, G. Linezolid, vancomycin and daptomycin modulate cytokine production, Toll-like receptors and phagocytosis in a human in vitro model of sepsis. J. Antibiot. 2015, 68, 485–490. [Google Scholar] [CrossRef]
- Bode, C.; Diedrich, B.; Muenster, S.; Hentschel, V.; Weisheit, C.; Rommelsheim, K.; Hoeft, A.; Meyer, R.; Boehm, O.; Knuefermann, P.; et al. Antibiotics regulate the immune response in both presence and absence of lipopolysaccharide through modulation of Toll-like receptors, cytokine production and phagocytosis in vitro. Int. Immunopharmacol. 2014, 18, 27–34. [Google Scholar] [CrossRef]
- Ohshima, A.; Tokura, Y.; Wakita, H.; Furukawa, F.; Takigawa, M. Roxithromycin down-modulates antigen-presenting and interleukin-1 beta-producing abilities of murine Langerhans cells. J. Dermatol. Sci. 1998, 17, 214–222. [Google Scholar] [CrossRef]
- Ortega, E.; Escobar, M.A.; Gaforio, J.J.; Algarra, I.; Alvarez De Cienfuegos, G. Modification of phagocytosis and cytokine production in peritoneal and splenic murine cells by erythromycin A, azithromycin and josamycin. J. Antimicrob. Chemother. 2004, 53, 367–370. [Google Scholar] [CrossRef][Green Version]
- Liu, S.; Tan, M.; Cai, J.; Li, C.; Yang, M.; Sun, X.; He, B. Ribosome-targeting antibiotic control NLRP3-mediated inflammation by inhibiting mitochondrial DNA synthesis. Free Radic. Biol. Med. 2024, 210, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Strzepa, A.; Majewska-Szczepanik, M.; Kowalczyk, P.; Wozniak, D.; Motyl, S.; Szczepanik, M. Oral treatment with enrofloxacin early in life promotes Th2-mediated immune response in mice. Pharmacol. Rep. 2016, 68, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Fujii, M.; Ono, M.; Maezawa, K.; Hori, S.; Kizu, J. In vivo and in vitro effects of fluoroquinolones on lipopolysaccharide-induced pro-inflammatory cytokine production. J. Infect. Chemother. 2009, 15, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ianaro, A.; Ialenti, A.; Maffia, P.; Sautebin, L.; Rombola, L.; Carnuccio, R.; Iuvone, T.; D’Acquisto, F.; Di Rosa, M. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 2000, 292, 156–163. [Google Scholar] [PubMed]
- Konno, S.; Adachi, M.; Asano, K.; Kawazoe, T.; Okamoto, K.; Takahashi, T. Influences of roxithromycin on cell-mediated immune responses. Life Sci. 1992, 51, PL107–PL112. [Google Scholar] [CrossRef]
- Konno, S.; Adachi, M.; Asano, K.; Okamoto, K.; Takahashi, T. Anti-allergic activity of roxithromycin: Inhibition of interleukin-5 production from mouse T lymphocytes. Life Sci. 1993, 52, PL25–PL30. [Google Scholar] [CrossRef]
- Breslow-Deckman, J.M.; Mattingly, C.M.; Birket, S.E.; Hoskins, S.N.; Ho, T.N.; Garvy, B.A.; Feola, D.J. Linezolid decreases susceptibility to secondary bacterial pneumonia postinfluenza infection in mice through its effects on IFN-gamma. J. Immunol. 2013, 191, 1792–1799. [Google Scholar] [CrossRef]
- Kaku, N.; Morinaga, Y.; Takeda, K.; Kosai, K.; Uno, N.; Hasegawa, H.; Miyazaki, T.; Izumikawa, K.; Mukae, H.; Yanagihara, K. Antimicrobial and immunomodulatory effects of tedizolid against methicillin-resistant Staphylococcus aureus in a murine model of hematogenous pulmonary infection. Int. J. Med. Microbiol. 2016, 306, 421–428. [Google Scholar] [CrossRef]
- Yanagihara, K.; Kihara, R.; Araki, N.; Morinaga, Y.; Seki, M.; Izumikawa, K.; Kakeya, H.; Yamamoto, Y.; Yamada, Y.; Kohno, S.; et al. Efficacy of linezolid against Panton-Valentine leukocidin (PVL)-positive meticillin-resistant Staphylococcus aureus (MRSA) in a mouse model of haematogenous pulmonary infection. Int. J. Antimicrob. Agents 2009, 34, 477–481. [Google Scholar] [CrossRef]
- Verma, A.K.; Bauer, C.; Yajjala, V.K.; Bansal, S.; Sun, K. Linezolid Attenuates Lethal Lung Damage during Postinfluenza Methicillin-Resistant Staphylococcus aureus Pneumonia. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Mike, J.K.; White, Y.; Hutchings, R.S.; Vento, C.; Ha, J.; Manzoor, H.; Lee, D.; Losser, C.; Arellano, K.; Vanhatalo, O.; et al. Perinatal Azithromycin Provides Limited Neuroprotection in an Ovine Model of Neonatal Hypoxic-Ischemic Encephalopathy. Stroke 2023, 54, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.M.; Bruno, D.A.; Garcia-Morato, J.; Mann, K.C.; Risso Patron, J.; Sagardia, J.; Absi, R.; Garcia Bottino, M.; Marchetti, D.; Famiglietti, A.; et al. Effect of linezolid compared with glycopeptides in methicillin-resistant Staphylococcus aureus severe pneumonia in piglets. Chest 2009, 135, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Gao, W.; Tao, H.; Yang, J.; Huang, T. The regulation effects of danofloxacin on pig immune stress induced by LPS. Res. Vet. Sci. 2017, 110, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ives, T.J.; Schwab, U.E.; Ward, E.S.; Hall, I.H. In-vitro anti-inflammatory and immunomodulatory effects of grepafloxacin in zymogen A- or Staphylococcus aureus-stimulated human THP-1 monocytes. J. Infect. Chemother. 2003, 9, 134–143. [Google Scholar] [CrossRef]
- Franks, Z.; Campbell, R.A.; Vieira de Abreu, A.; Holloway, J.T.; Marvin, J.E.; Kraemer, B.F.; Zimmerman, G.A.; Weyrich, A.S.; Rondina, M.T. Methicillin-resistant Staphylococcus aureus-induced thrombo-inflammatory response is reduced with timely antibiotic administration. Thromb. Haemost. 2013, 109, 684–695. [Google Scholar] [CrossRef]
- Garcia-Roca, P.; Mancilla-Ramirez, J.; Santos-Segura, A.; Fernandez-Aviles, M.; Calderon-Jaimes, E. Linezolid diminishes inflammatory cytokine production from human peripheral blood mononuclear cells. Arch. Med. Res. 2006, 37, 31–35. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bryant, A.E.; Hackett, S.P. Antibiotic effects on bacterial viability, toxin production, and host response. Clin. Infect. Dis. 1995, 20 (Suppl. 2), S154–S157. [Google Scholar] [CrossRef]
- Foca, A.; Matera, G.; Berlinghieri, M.C. Inhibition of endotoxin-induced interleukin 8 release by teicoplanin in human whole blood. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 940–944. [Google Scholar] [CrossRef]
- Schultz, M.J.; Speelman, P.; Zaat, S.; van Deventer, S.J.; van der Poll, T. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob. Agents Chemother. 1998, 42, 1605–1609. [Google Scholar] [CrossRef]
- Vickers, I.E.; Smikle, M.F. The immunomodulatory effect of antibiotics on the secretion of tumour necrosis factor alpha by peripheral blood mononuclear cells in response to Stenotrophomonas maltophilia stimulation. West. Indian. Med. J. 2006, 55, 138–141. [Google Scholar] [CrossRef]
- Pichereau, S.; Moran, J.J.; Hayney, M.S.; Shukla, S.K.; Sakoulas, G.; Rose, W.E. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 2012, 67, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Reato, G.; Cuffini, A.M.; Tullio, V.; Palarchio, A.I.; Bonino, A.; Foa, R.; Carlone, N.A. Co-amoxiclav affects cytokine production by human polymorphonuclear cells. J. Antimicrob. Chemother. 1999, 43, 715–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lankelma, J.M.; Cranendonk, D.R.; Belzer, C.; de Vos, A.F.; de Vos, W.M.; van der Poll, T.; Wiersinga, W.J. Antibiotic-induced gut microbiota disruption during human endotoxemia: A randomised controlled study. Gut 2017, 66, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Bailly, S.; Fay, M.; Roche, Y.; Gougerot-Pocidalo, M.A. Effects of quinolones on tumor necrosis factor production by human monocytes. Int. J. Immunopharmacol. 1990, 12, 31–36. [Google Scholar] [CrossRef]
- Roche, Y.; Gougerot-Pocidalo, M.A.; Fay, M.; Etienne, D.; Forest, N.; Pocidalo, J.J. Comparative effects of quinolones on human mononuclear leucocyte functions. J. Antimicrob. Chemother. 1987, 19, 781–790. [Google Scholar] [CrossRef]
- Riesbeck, K.; Forsgren, A. Selective enhancement of synthesis of interleukin-2 in lymphocytes in the presence of ciprofloxacin. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 409–413. [Google Scholar] [CrossRef]
- Khan, A.A.; Slifer, T.R.; Remington, J.S. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 1998, 42, 1713–1717. [Google Scholar] [CrossRef]
- Mori, S.; Takahashi, H.K.; Liu, K.; Wake, H.; Zhang, J.; Liu, R.; Yoshino, T.; Nishibori, M. Ciprofloxacin inhibits advanced glycation end products-induced adhesion molecule expression on human monocytes. Br. J. Pharmacol. 2010, 161, 229–240. [Google Scholar] [CrossRef]
- Ono, Y.; Ohmoto, Y.; Ono, K.; Sakata, Y.; Murata, K. Effect of grepafloxacin on cytokine production in vitro. J. Antimicrob. Chemother. 2000, 46, 91–94. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Sauer, S.W.; Klemke, C.D.; Suss, D.; Okun, J.G.; Krammer, P.H.; Gulow, K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: Mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 2010, 184, 4827–4841. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kurita, C.; Usami, E.; Nakao, T.; Watanabe, S.; Kobayashi, J.; Yamazaki, F.; Nagai, H. Immunomodulatory action of levofloxacin on cytokine production by human peripheral blood mononuclear cells. Chemotherapy 1996, 42, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Roche, Y.; Fay, M.; Gougerot-Pocidalo, M.A. Enhancement of interleukin 2 production by quinolone-treated human mononuclear leukocytes. Int. J. Immunopharmacol. 1988, 10, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.J.; Chaudhuri, R.; Mair, F.; McSharry, C.; Greenlaw, N.; Weir, C.J.; Jolly, L.; Donnelly, I.; Gallacher, K.; Morrison, D.; et al. Randomised controlled trial of azithromycin in smokers with asthma. Eur. Respir. J. 2013, 42, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Aten, M.; Okada, P.J.; Bowlware, K.L.; Chavez-Bueno, S.; Mejias, A.; Rios, A.M.; Katz, K.; Olsen, K.; Ng, S.; Jafri, H.S.; et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: A double-blind, randomized, placebo-controlled trial. Ann. Allergy Asthma Immunol. 2006, 97, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Powell, H.; Boyle, M.J.; Scott, R.J.; Gibson, P.G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 148–155. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, Y.C.; Rhee, Y.K.; Lee, H.B. The effect of long-term treatment with erythromycin on Th1 and Th2 cytokines in diffuse panbronchiolitis. Biochem. Biophys. Res. Commun. 2004, 324, 114–117. [Google Scholar] [CrossRef]
- Lima, C.M.; Schroeder, J.T.; Galvao, C.E.; Castro, F.M.; Kalil, J.; Adkinson, N.F., Jr. Functional changes of dendritic cells in hypersensivity reactions to amoxicillin. Braz. J. Med. Biol. Res. 2010, 43, 964–968. [Google Scholar] [CrossRef]
- Juanola, O.; Gomez-Hurtado, I.; Zapater, P.; Moratalla, A.; Caparros, E.; Pinero, P.; Gonzalez-Navajas, J.M.; Gimenez, P.; Such, J.; Frances, R. Selective intestinal decontamination with norfloxacin enhances a regulatory T cell-mediated inflammatory control mechanism in cirrhosis. Liver Int. 2016, 36, 1811–1820. [Google Scholar] [CrossRef]
- Spyridaki, A.; Raftogiannis, M.; Antonopoulou, A.; Tsaganos, T.; Routsi, C.; Baziaka, F.; Karagianni, V.; Mouktaroudi, M.; Koutoukas, P.; Pelekanou, A.; et al. Effect of clarithromycin in inflammatory markers of patients with ventilator-associated pneumonia and sepsis caused by Gram-negative bacteria: Results from a randomized clinical study. Antimicrob. Agents Chemother. 2012, 56, 3819–3825. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Siampanos, A.; Bolanou, A.; Doulou, S.; Kakavoulis, N.; Tsiakos, K.; Katopodis, S.; Schinas, G.; Skorda, L.; Alexiou, Z.; et al. Clarithromycin for early anti-inflammatory responses in community-acquired pneumonia in Greece (ACCESS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2024, 12, 294–304. [Google Scholar] [CrossRef]
- Lim, J.J.; Grinstein, S.; Roth, Z. Diversity and Versatility of Phagocytosis: Roles in Innate Immunity, Tissue Remodeling, and Homeostasis. Front. Cell Infect. Microbiol. 2017, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.F. Effects of vancomycin, teicoplanin, daptomycin and coumermycin on normal immune capabilities. J. Chemother. 1991, 3, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Mato, R.; Corrales, I.; Prieto, J. Influence of lomefloxacin on phagocytosis and killing activity of macrophages and neutrophils. J. Antimicrob. Chemother. 1992, 30, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Muenster, S.; Bode, C.; Diedrich, B.; Jahnert, S.; Weisheit, C.; Steinhagen, F.; Frede, S.; Hoeft, A.; Meyer, R.; Boehm, O.; et al. Antifungal antibiotics modulate the pro-inflammatory cytokine production and phagocytic activity of human monocytes in an in vitro sepsis model. Life Sci. 2015, 141, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, C.; Parschalk, B.; Zedtwitz-Liebenstein, K.; Weihs, A.; el Menyawi, I.; Graninger, W. Effect of single oral dose of azithromycin, clarithromycin, and roxithromycin on polymorphonuclear leukocyte function assessed ex vivo by flow cytometry. Antimicrob. Agents Chemother. 1996, 40, 2039–2042. [Google Scholar] [CrossRef]
- Scheffer, J.; Knoller, J.; Cullmann, W.; Konig, W. Effects of cefaclor, cefetamet and Ro 40-6890 on inflammatory responses of human granulocytes. J. Antimicrob. Chemother. 1992, 30, 57–66. [Google Scholar] [CrossRef]
- Pasqui, A.L.; Di Renzo, M.; Bruni, F.; Fanetti, G.; Campoccia, G.; Auteri, A. Imipenem and immune response: In vitro and in vivo studies. Drugs Exp. Clin. Res. 1995, 21, 17–22. [Google Scholar]
- Yamaryo, T.; Oishi, K.; Yoshimine, H.; Tsuchihashi, Y.; Matsushima, K.; Nagatake, T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob. Agents Chemother. 2003, 47, 48–53. [Google Scholar] [CrossRef]
- Braga, P.C.; Maci, S.; Dal Sasso, M.; Fonti, E.; Ghessi, A. Effects of rokitamycin on phagocytosis and release of oxidant radicals of human polymorphonuclear leukocytes. Chemotherapy 1997, 43, 190–197. [Google Scholar] [CrossRef]
- Lianou, P.E.; Votta, E.G.; Papavassiliou, J.T.; Bassaris, H.P. In vivo potentiation of polymorphonuclear leukocyte function by ciprofloxacin. J. Chemother. 1993, 5, 223–227. [Google Scholar] [CrossRef]
- Herrera-Insua, I.; Jacques-Palaz, K.; Murray, B.E.; Rakita, R.M. The effect of antibiotic exposure on adherence to neutrophils of Enterococcus faecium resistant to phagocytosis. J. Antimicrob. Chemother. 1997, 39 (Suppl. A), 109–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forsgren, A.; Bergkvist, P.I. Effect of ciprofloxacin on phagocytosis. Eur. J. Clin. Microbiol. 1985, 4, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Gruger, T.; Morler, C.; Schnitzler, N.; Brandenburg, K.; Nidermajer, S.; Horre, R.; Zundorf, J. Influence of fluoroquinolones on phagocytosis and killing of Candida albicans by human polymorphonuclear neutrophils. Med. Mycol. 2008, 46, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Brozyna, S.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006, 28, 486–495. [Google Scholar] [CrossRef]
- Noma, T.; Hayashi, M.; Yoshizawa, I.; Aoki, K.; Shikishima, Y.; Kawano, Y. A comparative investigation of the restorative effects of roxithromycin on neutrophil activities. Int. J. Immunopharmacol. 1998, 20, 615–624. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohe, J.; Venet, F.; Debard, A.L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D., II; Kreisel, D.; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Suzuki, M.; Asano, K.; Yu, M.; Hisamitsu, T.; Suzaki, H. Inhibitory action of a macrolide antibiotic, roxithromycin, on co-stimulatory molecule expressions in vitro and in vivo. Mediators Inflamm. 2002, 11, 235–244. [Google Scholar] [CrossRef]
- Asano, K.; Suzuki, M.; Shimane, T.; Suzaki, H. Suppression of co-stimulatory molecule expressions on splenic B lymphocytes by a macrolide antibiotic, roxithromycin in vitro. Int. Immunopharmacol. 2001, 1, 1385–1392. [Google Scholar] [CrossRef]
- Kawazu, K.; Kurokawa, M.; Asano, K.; Mita, A.; Adachi, M. Suppressive activity of a macrolide antibiotic, roxithromycin on co-stimulatory molecule expression on mouse splenocytes in vivo. Mediators Inflamm. 2000, 9, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Roche, Y.; Fay, M.; Gougerot-Pocidalo, M.A. Effects of quinolones on interleukin 1 production in vitro by human monocytes. Immunopharmacology 1987, 13, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pena, R.; Lopez, S.; Mayorga, C.; Antunez, C.; Fernandez, T.D.; Torres, M.J.; Blanca, M. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to beta-lactams. J. Allergy Clin. Immunol. 2006, 118, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Iino, Y.; Sasaki, Y.; Kojima, C.; Miyazawa, T. Effect of macrolides on the expression of HLA-DR and costimulatory molecules on antigen-presenting cells in nasal polyps. Ann. Otol. Rhinol. Laryngol. 2001, 110 Pt 1, 457–463. [Google Scholar] [CrossRef]
- Karakike, E.; Scicluna, B.P.; Roumpoutsou, M.; Mitrou, I.; Karampela, N.; Karageorgos, A.; Psaroulis, K.; Massa, E.; Pitsoulis, A.; Chaloulis, P.; et al. Effect of intravenous clarithromycin in patients with sepsis, respiratory and multiple organ dysfunction syndrome: A randomized clinical trial. Crit. Care 2022, 26, 183. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Zhang, J. Repositioning of antibiotic levofloxacin as a mitochondrial biogenesis inhibitor to target breast cancer. Biochem. Biophys. Res. Commun. 2016, 471, 639–645. [Google Scholar] [CrossRef]
- Song, M.; Wu, H.; Wu, S.; Ge, T.; Wang, G.; Zhou, Y.; Sheng, S.; Jiang, J. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed. Pharmacother. 2016, 84, 1137–1143. [Google Scholar] [CrossRef]
- Schmid, D.A.; Depta, J.P.; Pichler, W.J. T cell-mediated hypersensitivity to quinolones: Mechanisms and cross-reactivity. Clin. Exp. Allergy 2006, 36, 59–69. [Google Scholar] [CrossRef]
- Banck, G.; Forsgren, A. Antibiotics and suppression of lymphocyte function in vitro. Antimicrob. Agents Chemother. 1979, 16, 554–560. [Google Scholar] [CrossRef]
- Kushiya, K.; Nakagawa, S.; Taneike, I.; Iwakura, N.; Imanishi, K.; Uchiyama, T.; Tsukada, H.; Gejyo, F.; Yamamoto, T. Inhibitory effect of antimicrobial agents and anisodamine on the staphylococcal superantigenic toxin-induced overproduction of proinflammatory cytokines by human peripheral blood mononuclear cells. J. Infect. Chemother. 2005, 11, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Karrow, N.A.; McCay, J.A.; Brown, R.D.; Musgrove, D.L.; Germolec, D.R.; White, K.L., Jr. Evaluation of the immunomodulatory effects of the macrolide antibiotic, clarithromycin, in female B6C3F1 mice: A 28-day oral gavage study. Drug Chem. Toxicol. 2001, 24, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Pulverer, G. Effects of cefodizime and cefotaxime on cellular and humoral immune responses. Infection 1992, 20 (Suppl. 1), S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Neftel, K.A.; Muller, M.R.; Widmer, U.; Hugin, A.W. Beta-lactam antibiotics inhibit human in vitro granulopoiesis and proliferation of some other cell types. Cell Biol. Toxicol. 1986, 2, 513–521. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 2007, 17, 759–771. [Google Scholar] [CrossRef]
- Garrabou, G.; Soriano, A.; Pinos, T.; Casanova-Molla, J.; Pacheu-Grau, D.; Moren, C.; Garcia-Arumi, E.; Morales, M.; Ruiz-Pesini, E.; Catalan-Garcia, M.; et al. Influence of Mitochondrial Genetics on the Mitochondrial Toxicity of Linezolid in Blood Cells and Skin Nerve Fibers. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Coster, R.V.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.J.; Groote, C.C.; Vandecasteele, S.; et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- Plekhova, N.G.; Kondrashova, N.M.; Somova, L.M.; Drobot, E.I.; Lyapun, I.N. Effects of immunomodulators on functional activity of innate immunity cells infected with Streptococcus pneumoniae. Bull. Exp. Biol. Med. 2015, 158, 461–464. [Google Scholar] [CrossRef]
- Smith, D.M.; Kazi, A.; Smith, L.; Long, T.E.; Heldreth, B.; Turos, E.; Dou, Q.P. A novel beta-lactam antibiotic activates tumor cell apoptotic program by inducing DNA damage. Mol. Pharmacol. 2002, 61, 1348–1358. [Google Scholar] [CrossRef]
- Chen, D.; Falsetti, S.C.; Frezza, M.; Milacic, V.; Kazi, A.; Cui, Q.C.; Long, T.E.; Turos, E.; Dou, Q.P. Anti-tumor activity of N-thiolated beta-lactam antibiotics. Cancer Lett. 2008, 268, 63–69. [Google Scholar] [CrossRef]
- Koziel, R.; Zablocki, K.; Duszynski, J. Calcium signals are affected by ciprofloxacin as a consequence of reduction of mitochondrial DNA content in Jurkat cells. Antimicrob. Agents Chemother. 2006, 50, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Ocker, M.; Ganslmayer, M.; Gerauer, H.; Hahn, E.G.; Schuppan, D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 2002, 86, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Aranha, O.; Zhu, L.; Alhasan, S.; Wood, D.P., Jr.; Kuo, T.H.; Sarkar, F.H. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cells. J. Urol. 2002, 167, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Denamur, S.; Boland, L.; Beyaert, M.; Verstraeten, S.L.; Fillet, M.; Tulkens, P.M.; Bontemps, F.; Mingeot-Leclercq, M.P. Subcellular mechanisms involved in apoptosis induced by aminoglycoside antibiotics: Insights on p53, proteasome and endoplasmic reticulum. Toxicol. Appl. Pharmacol. 2016, 309, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Servais, H.; Van Der Smissen, P.; Thirion, G.; Van der Essen, G.; Van Bambeke, F.; Tulkens, P.M.; Mingeot-Leclercq, M.P. Gentamicin-induced apoptosis in LLC-PK1 cells: Involvement of lysosomes and mitochondria. Toxicol. Appl. Pharmacol. 2005, 206, 321–333. [Google Scholar] [CrossRef]
- Cusack, R.; Little, E.; Martin-Loeches, I. Practical Lessons on Antimicrobial Therapy for Critically Ill Patients. Antibiotics 2024, 13, 162. [Google Scholar] [CrossRef]
- Suspected Sepsis: Recognition, Diagnosis and Early Management (NG51); National Institute for Health and Care Excellence (NICE): Manchester, UK, 2024.
- Pandolfo, A.M.; Horne, R.; Jani, Y.; Reader, T.W.; Bidad, N.; Brealey, D.; Enne, V.I.; Livermore, D.M.; Gant, V.; Brett, S.J.; et al. Understanding decisions about antibiotic prescribing in ICU: An application of the Necessity Concerns Framework. BMJ Qual. Saf. 2022, 31, 199–210. [Google Scholar] [CrossRef]
- Higgins, H.; Freeman, R.; Doble, A.; Hood, G.; Islam, J.; Gerver, S.; Henderson, K.L.; Demirjian, A.; Hopkins, S.; Ashiru-Oredope, D. Appropriateness of acute-care antibiotic prescriptions for community-acquired infections and surgical antibiotic prophylaxis in England: Analysis of 2016 national point prevalence survey data. J. Hosp. Infect. 2023, 142, 115–129. [Google Scholar] [CrossRef]
- Royer, S.; DeMerle, K.M.; Dickson, R.P.; Prescott, H.C. Shorter Versus Longer Courses of Antibiotics for Infection in Hospitalized Patients: A Systematic Review and Meta-Analysis. J. Hosp. Med. 2018, 13, 336–342. [Google Scholar] [CrossRef]
- Palin, V.; Welfare, W.; Ashcroft, D.M.; van Staa, T.P. Shorter and Longer Courses of Antibiotics for Common Infections and the Association with Reductions of Infection-Related Complications Including Hospital Admissions. Clin. Infect. Dis. 2021, 73, 1805–1812. [Google Scholar] [CrossRef]
- Chanderraj, R.; Admon, A.J.; He, Y.; Nuppnau, M.; Albin, O.R.; Prescott, H.C.; Dickson, R.P.; Sjoding, M.W. Mortality of Patients With Sepsis Administered Piperacillin-Tazobactam vs Cefepime. JAMA Intern. Med. 2024, 184, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D.O.; Kipper, K.; Baker, E.H.; Barker, C.I.S.; Oldfield, I.; Philips, B.J.; Johnston, A.; Rhodes, A.; Sharland, M.; Standing, J.F. beta-Lactam antimicrobial pharmacokinetics and target attainment in critically ill patients aged 1 day to 90 years: The ABDose study. J. Antimicrob. Chemother. 2020, 75, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Drager, S.; Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Verkaik, N.J.; van Vliet, P.; Purmer, I.M.; Osthoff, M.; Koch, B.C.P.; Endeman, H.; et al. Target attainment of beta-lactam antibiotics and ciprofloxacin in critically ill patients and its association with 28-day mortality. J. Crit. Care 2024, 154904. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).