The Use of Cefiderocol in Gram-Negative Bacterial Infections at International Medical Center, Jeddah, Saudi Arabia
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Participants and Data Collection
4.3. Inclusion and Exclusion Criteria
- Inclusion criteria: males and females, ages 18–80, hospitalized as inpatients or in the ICU, and receiving CFDC for MDR organisms.
- Exclusion criteria: patients receiving antibiotics with similar microbial coverage as CFDC for GNBI to minimize interference with the results.
4.4. Collection of Specimens and Culture Media
4.4.1. Culture Media
4.4.2. Sample Inoculation Procedure
4.5. Identification of Isolated Bacteria
4.5.1. Gram Stain Procedure
4.5.2. Vitek-2 System
4.6. Materials
4.6.1. Procedure for Vitek-2
4.6.2. Weekly Vitek-2 Quality Control
4.6.3. Media Quality Control
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syed, Y.Y. Cefiderocol: A review in serious Gram-negative bacterial infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Palasik, B.N. Combating antimicrobial resistance with cefiderocol for complicated infections involving the urinary tract. Ther. Adv. Urol. 2022, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A siderophore cephalosporin with activity against carbapenem-resistant and multidrug-resistant gram-negative bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic review of mechanisms of resistance, heteroresistance and in vivo emergence of resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76, V23–V37. [Google Scholar] [CrossRef]
- Abdul-Mutakabbir, J.C.; Alosaimy, S.; Morrisette, T.; Kebriaei, R.; Rybak, M.J. Cefiderocol: A novel Siderophore cephalosporin against multidrug-resistant gram-negative pathogens. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 1228–1247. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Koh, Y.S. A novel antibiotic agent, cefiderocol, for multidrug-resistant Gram-negative bacteria. JBV 2020, 50, 218–226. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram-negative bacteria: The study, the patient, the bug or the drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef]
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America antimicrobial resistant treatment guidance: Gram-negative bacterial infections. Clin. Infect. Dis. 2021, 72, e169–e183. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33106864 (accessed on 6 February 2023). [CrossRef]
- Wright, H.; Bonomo, R.A.; Paterson, D.L. New agents for the treatment of infections with Gram-negative bacteria: Restoring the miracle or false dawn? Clin. Microbiol. Infect. 2017, 23, 704–712. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int (accessed on 11 May 2021).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov (accessed on 11 May 2021).
- International Medical Center. Available online: https://www.imc.med.sa/ar (accessed on 5 March 2023).
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomized, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Matsunaga, Y.; Ariyasu, M.; Clevenbergh, P.; Echols, R.; Kaye, K.S.; Kollef, M.; Menon, A.; Pogue, J.M.; Shorr, A.F.; et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomized, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis 2021, 21, 213–225. [Google Scholar] [CrossRef]
- Falagas, M.E.; Skalidis, T.; Vardakas, K.Z.; Legakis, N.J.; Hellenic Cefiderocol Study Group. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J. Antimicrob. Chemother. 2017, 72, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Echols, R.; Wajima, T. Pharmacokinetic and pharmacodynamic profiles of Cefiderocol, a novel siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S552–S558. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, K.M.; Tsuji, M.; Wise, M.G.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, D.F. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 2018, 53, 177–184. [Google Scholar]
- Meschiari, M.; Volpi, S.; Faltoni, M.; Dolci, G.; Orlando, G.; Franceschini, E.; Menozzi, M.; Sarti, M.; Del Fabro, G.; Fumarola, B.; et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC-Antimicrob. Resist. 2021, 3, dlab188. [Google Scholar] [CrossRef]
- Ni, W.; Wang, Y.; Ma, X.; He, Y.; Zhao, J.; Guan, J.; Li, Y.; Gao, Z. In vitro and in vivo efficacy of cefiderocol plus tigecycline, colistin, or meropenem against carbapenem-resistant Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1451–1457. [Google Scholar] [CrossRef]
- Yassin, A.; Huralska, M.; Pogue, J.M.; Dixit, D.; Sawyer, R.G.; Kaye, K.S. Executive summary: State-of-the-art review: State of the management of infections caused by multidrug-resistant Gram-negative organisms. Clin. Infect. Dis. 2023, 77, 1223–1225. [Google Scholar] [CrossRef]
- Huang, N.; Jia, H.; Zhou, B.; Zhou, C.; Cao, J.; Liao, W.; Ye, J. Hypervirulent carbapenem-resistant Klebsiella pneumoniae causing highly fatal meningitis in southeastern China. Front. Public Health 2022, 10, 991306. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Assiri, A.; Almasri, M.; Roshdy, H.; Hathout, H.; Kaase, M.; Gatermann, S.G.; Yezli, S. Molecular characterization of carbapenemase production among Gram-negative bacteria in Saudi Arabia. Microb. Drug Resist. 2015, 21, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Sartor, A.L.; Balkhy, H.H.; Walsh, T.R.; Al Johani, S.M.; AlJindan, R.Y.; Alfaresi, M.; Ibrahim, E.; Al-Jardani, A.; Al-Abri, S.; et al. Molecular characterization of carbapenemase-producing E. coli and K. pneumoniae in the countries of the Gulf cooperation council: Dominance of OXA-48 and NDM producers. AAC 2014, 58, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; De Benedetto, I.; Pinna, S.M.; Vita, D.; Lupia, T.; Montrucchio, G.; De Rosa, F.G. Cefiderocol use in Gram-negative infections with limited therapeutic options: Is combination therapy the key? J. Infect. Public Health 2022, 15, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Simner, P.J.; Slover, C.M.; Yamano, Y.; Nagata, T.D.; Portsmouth, S. Cefiderocol treatment for patients with multidrug-and carbapenem-resistant Pseudomonas aeruginosa infections in the compassionate use program. Antimicrob. Agents Chemother. 2023, 67, e00194-23. [Google Scholar] [CrossRef]
- Miller, A.C.; Arakkal, A.T.; Sewell, D.K.; Segre, A.M.; Tholany, J.; Polgreen, P.M.; CDC MInD-Healthcare Group. Comparison of different antibiotics and the risk for community-associated Clostridioides difficile infection: A case-control study. Open Forum Infect. Dis. 2023, 10, ofad413. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Sferra, T.J.; Hernandez, A.V.; Donskey, C.J. Community-associated Clostridium difficile infection and antibiotics: A meta-analysis. J. Antimicrob. Chemother. 2013, 68, 1951–1961. [Google Scholar] [CrossRef]
- Sajib, M.I.; Monteforte, M.; Go, R. Clinical outcome of cefiderocol for infections with carbapenem-resistant organisms. Antibiotics 2023, 12, 936. [Google Scholar] [CrossRef]
- Cheesbrough, J.S.; Green, J.; Gallimore, C.I.; Wright, P.A.; Brown, D.W.G. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol. Infect. 2000, 125, 93–98. [Google Scholar] [CrossRef]
- Nimer, N.A.; Al-Saa’da, R.J.; Abuelaish, O. Accuracy of the VITEK 2 system for a rapid and direct identification and susceptibility testing of gram-negative rods and gram-positive cocci in blood samples. East. Mediterr. Health J. 2016, 22, 193–200. [Google Scholar] [CrossRef]
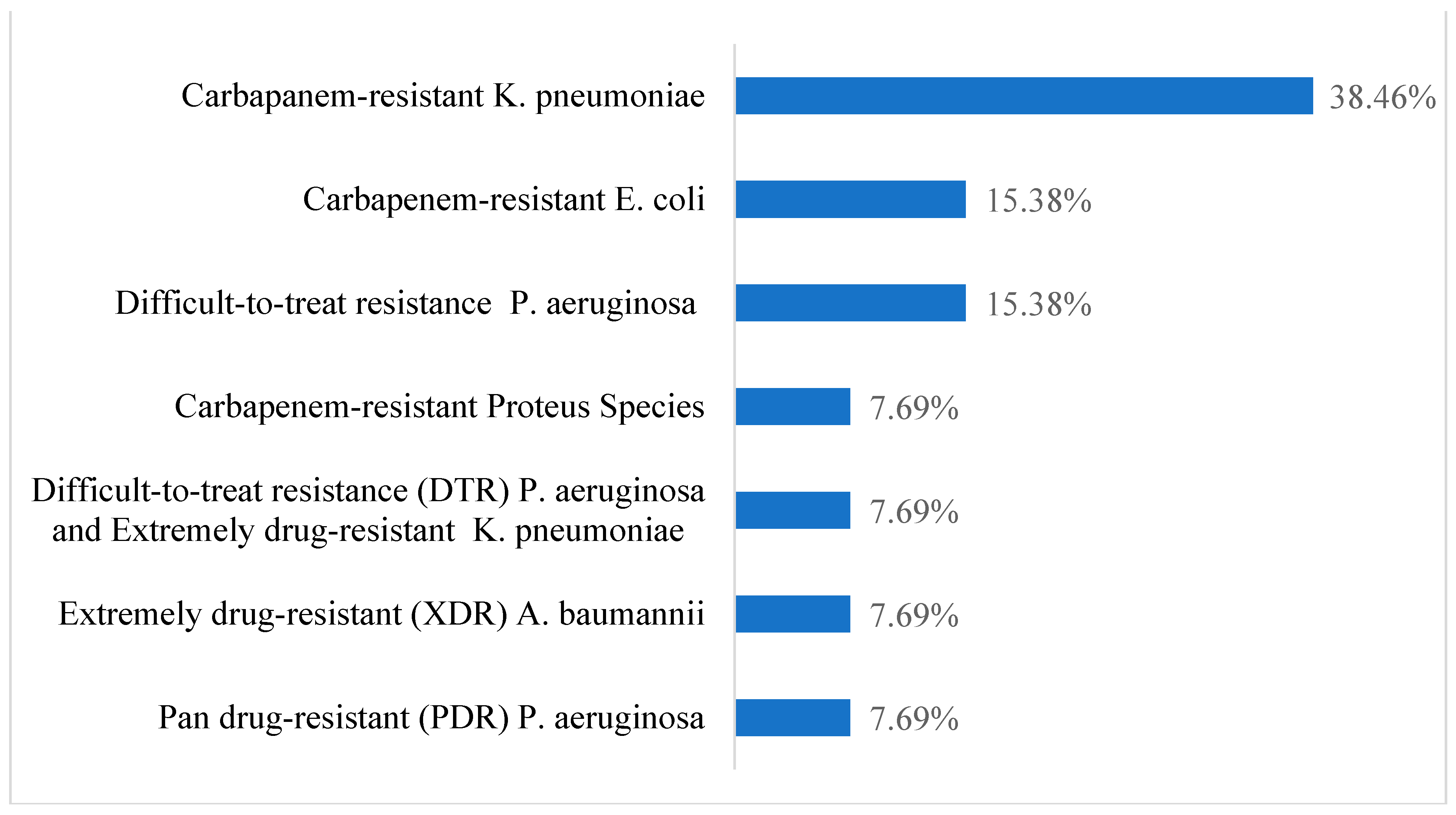

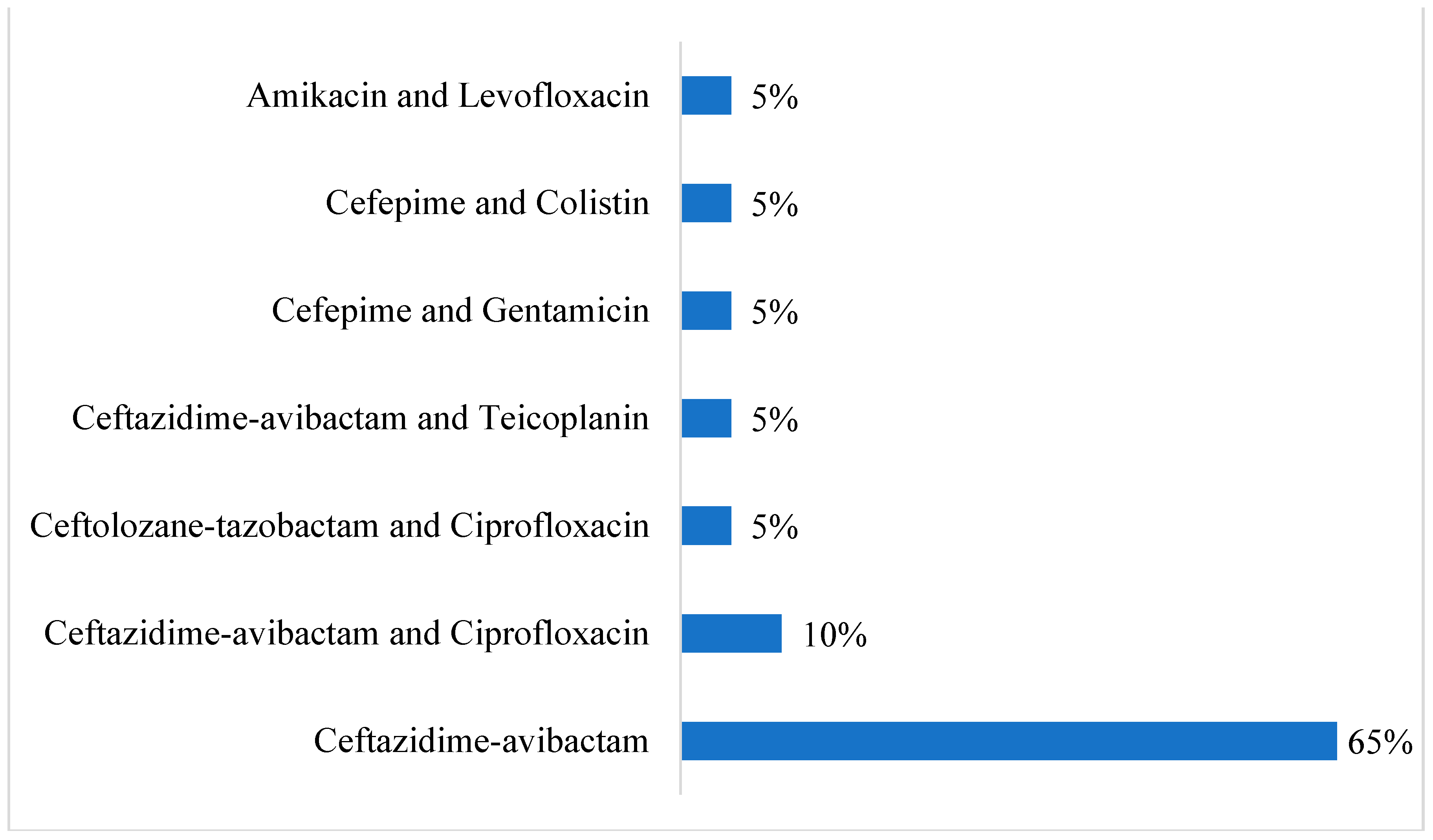
| Demographic Characteristics | Category | Case Group (n = 13) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| Gender | Male | 9 | 69.2 | 11 | 55.0 | 0.414 * |
| Female | 4 | 30.8 | 9 | 45.0 | ||
| ICU Stay | Yes | 5 | 38.5 | 7 | 35.0 | 0.840 * |
| No | 8 | 61.5 | 13 | 65.0 | ||
| Charlson Index | Mild | 0 | 0 | 2 | 10.0 | 0.306 * |
| Moderate | 5 | 38.5 | 4 | 20.0 | ||
| Severe | 8 | 61.5 | 14 | 70.0 | ||
| Age | Mean ± SD | 72.23 ± 10.450 | 72.40 ± 9.859 | 0.963 + | ||
| Length of Hospital Stay | Mean ± SD | 40.54 ± 55.667 | 42.25 ± 63.649 | 0.937 + | ||
| Category | Case Group (n = 13) | Control Group (n = 20) | p-Value | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| Indwelling Catheter | Yes | 12 | 92.3 | 15 | 75.0 | 0.208 * |
| No | 1 | 7.7 | 5 | 25.0 | ||
| Mechanical Ventilation | Yes | 5 | 38.5 | 10 | 50.0 | 0.515 * |
| No | 8 | 61.5 | 10 | 50.0 | ||
| Clinical Characteristics | Category | Case Group (n = 13) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| COVID-19 | Yes | 1 | 7.7 | 2 | 10.0 | 0.975 * |
| No | 10 | 76.9 | 15 | 75.0 | ||
| Not-tested | 2 | 15.4 | 3 | 15.0 | ||
| Diabetes miletus | Yes | 8 | 61.5 | 12 | 60.0 | 0.930 * |
| No | 5 | 38.5 | 8 | 40.0 | ||
| Hypertension | Yes | 10 | 76.9 | 16 | 80.0 | 0.833 * |
| No | 3 | 23.1 | 4 | 20.0 | ||
| Renal impairment | Yes | 8 | 61.5 | 10 | 50.0 | 0.515 * |
| No | 5 | 38.5 | 10 | 50.0 | ||
| Heart disease | Yes | 8 | 61.5 | 13 | 65.0 | 0.840 * |
| No | 5 | 38.5 | 7 | 35.0 | ||
| Chronic liver disease | Yes | 2 | 15.4 | 2 | 10.0 | 0.643 * |
| No | 11 | 84.6 | 18 | 90.0 | ||
| Solid organ malignancies | Brain Tumor | 1 | 7.7 | 0 | 0 | 0.282 * |
| Colon Adenocarcinoma | 0 | 1 | 5.0 | |||
| Prostate Cancer | 1 | 7.7 | 0 | 0 | ||
| None | 11 | 84.6 | 19 | 95.0 | ||
| Clostridioides difficile infection | Yes | 2 | 15.4 | 0 | 0 | 0.070 * |
| No | 11 | 84.6 | 20 | 100 | ||
| Category | Case Group (n = 13) | Control Group (n = 20) | p-Value | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| Type of Infection | Pneumonia | 7 | 53.8 | 13 | 65.0 | 0.284 * |
| UTI | 6 | 46.2 | 5 | 25.0 | ||
| Necrotizing fasciitis | 0 | 0 | 2 | 10.0 | ||
| Category | Case Group (n = 13) | Control Group (n = 20) | p-Value | |||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | |||
| Enzymes | NDM | 4 | 30.8 | 1 | 5.0 | 0.052 * |
| NDM and KPC | 1 | 7.7 | 1 | 5.0 | ||
| OXA-48 | 0 | 0 | 8 | 40.0 | ||
| OXA-48 and NDM | 1 | 7.7 | 0 | 0 | ||
| VIM | 1 | 7.7 | 0 | 0 | ||
| Phenotype | XDR | 0 | 0 | 1 | 5.0 | |
| Resistance Gene | No genes detected | 6 | 46.2 | 9 | 45.0 | |
| Mortality Within 30-Days | Case Group (n = 13) | Control Group (n = 20) | p-Value | ||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Alive | 10 | 76.9 | 15 | 75.0 | 0.975 * |
| Dead | 2 | 15.4 | 3 | 15.0 | |
| Missing information | 1 | 7.7 | 2 | 10.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaki, R.; Taj, A.; Bagaaifar, S. The Use of Cefiderocol in Gram-Negative Bacterial Infections at International Medical Center, Jeddah, Saudi Arabia. Antibiotics 2024, 13, 1043. https://doi.org/10.3390/antibiotics13111043
Kaki R, Taj A, Bagaaifar S. The Use of Cefiderocol in Gram-Negative Bacterial Infections at International Medical Center, Jeddah, Saudi Arabia. Antibiotics. 2024; 13(11):1043. https://doi.org/10.3390/antibiotics13111043
Chicago/Turabian StyleKaki, Reham, Amjad Taj, and Sultan Bagaaifar. 2024. "The Use of Cefiderocol in Gram-Negative Bacterial Infections at International Medical Center, Jeddah, Saudi Arabia" Antibiotics 13, no. 11: 1043. https://doi.org/10.3390/antibiotics13111043
APA StyleKaki, R., Taj, A., & Bagaaifar, S. (2024). The Use of Cefiderocol in Gram-Negative Bacterial Infections at International Medical Center, Jeddah, Saudi Arabia. Antibiotics, 13(11), 1043. https://doi.org/10.3390/antibiotics13111043






