Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities
Abstract
:1. Introduction
2. Results
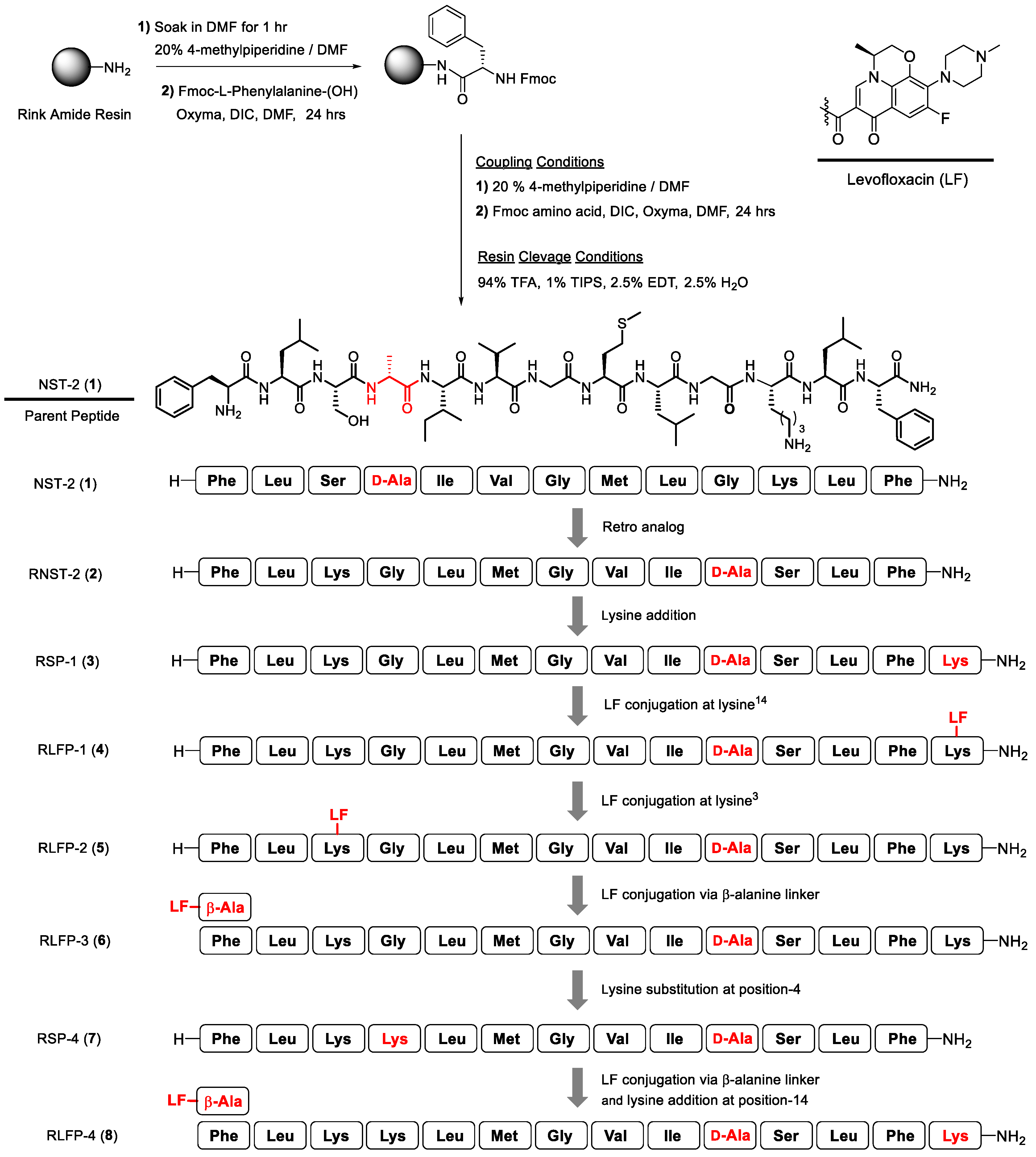
2.1. Circular Dichroism (CD) and Secondary Structure Analysis
| Peptide Name | Systematic Name | Sequence |
|---|---|---|
| NST-2 (1) | [G4a]-SHa | H-Phe1-Leu2-Ser3-D-Ala4-Ile5-Val6-Gly7-Met8-Leu9-Gly10-Lys11-Leu12-Phe13-NH2 |
| RNST-2 (2) | R[G4a]-SHa | H-Phe1-Leu2-Lys3-Gly4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-NH2 |
| RSP-1 (3) | RNST-2-14K | H-Phe1-Leu2-Lys3-Gly4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-Lys14-NH2 |
| RLFP-1 (4) | RRNST-2-14K-14LF | H-Phe1-Leu2-Lys3-Gly4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-Lys14(LF)-NH2 |
| RLFP-2 (5) | RNST-2-14K-3LF | H-Phe1-Leu2-Lys3(LF)-Gly4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-Lys14-NH2 |
| RLFP-3 (6) | RNST-2-14K-1LF | LF-β-Ala-Phe1-Leu2-Lys3-Gly4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-Lys14-NH2 |
| RSP-4 (7) | RNST-2-G4K | H-Phe1-Leu2-Lys3-Lys4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-NH2 |
| RLFP-4 (8) | RNST-2-G4K-1LF | LF-β-Ala-Phe1-Leu2-Lys3-Lys4-Leu5-Met6-Gly7-Val8-Ile9-D-Ala10-Ser11-Leu12-Phe13-NH2 |
| Peptide Name | Chemical Formula | Exact Mass | Observed Mass * | Time(R) ** | ‡ | Yield § |
|---|---|---|---|---|---|---|
| RNST-2 (2) | C68H111N15O14S | 1393.8 | 1395.8 [M + H]+ | 3.1 | −202 | 25 |
| RSP-1 (3) | C74H123N17O15S | 1521.9 | 1523.9 [M + H]+ | 2.9 | −15 | 8 |
| RLFP-1 (4) | C92H141FN20O18S | 1865.0 | 1863.5 [M + H]+ | 3.3 | +120 | 22 |
| RLFP-2 (5) | C92H141FN20O18S | 1865.0 | 1912.3 [M + 2Na]2+ | 3.7 | +5 | 17 |
| RLFP-3 (6) | C95H146FN21O19S | 1936.0 | 1936.9 [M + H]+ | 3.6 | −45 | 26 |
| RSP-4 (7) | C72H120N16O14S | 1464.9 | 1467.0 [M + H]+ | 3.1 | +88 | 28 |
| RLFP-4 (8) | C93H143FN20O18S | 1879.1 | 941.7 [M + 2H]2+ | 3.0 | +131 | 31 |
| Peptide | Helix (%) | Antiparallel (%) | Parallel (%) | Turn (%) | Others (%) |
|---|---|---|---|---|---|
| NST-2 (1) | 81.5 | 18.5 | 0.00 | 0.00 | 0.00 |
| RNST (2) | 62.0 | 17.9 | 20.1 | 0.00 | 0.00 |
| RSP-1 (3) | 81.8 | 18.2 | 0.00 | 0.00 | 0.00 |
| RLFP-1 (4) | 80.1 | 19.9 | 0.00 | 0.00 | 0.00 |
| RLFP-2 (5) | 80.4 | 19.6 | 0.00 | 0.00 | 0.00 |
| RLFP-3 (6) | 82.2 | 17.8 | 0.00 | 0.00 | 0.00 |
| RSP-4 (7) | 56.8 | 22.1 | 0.00 | 7.6 | 13.6 |
| RLFP-4 (8) | 75.0 | 0.00 | 14.1 | 0.00 | 11.0 |
2.2. Antimicrobial Assay
2.3. Anticancer Activity and Hemolytic Effect of the Analogs
2.4. Selectivity Indexes of the Analogs
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Peptides Synthesis and Characterization
4.2.1. Synthesis of Temporin-SHa Retro-Analogs and Their Levofloxacin Conjugates
4.2.2. Mass and NMR Spectroscopic Analysis of Peptides
Synthesis of NST-2 Peptide (1)
Synthesis of RNST-2 Peptide (2)
Synthesis of RSP-1 Peptide (3)
Synthesis of RLFP-1 (4)
Synthesis of RLFP-2 Peptide (5)
Synthesis of RLFP-3 (6)
Synthesis of RSP-4 Peptide (7)
Synthesis of RLFP-4 Peptide (8)
4.2.3. Circular Dichroism (CD) and Secondary Structure Analysis
4.3. Biological Studies
4.3.1. Antibacterial Assay
4.3.2. Antifungal Assay
4.3.3. Antiproliferative Assay
4.3.4. Hemolytic Assay
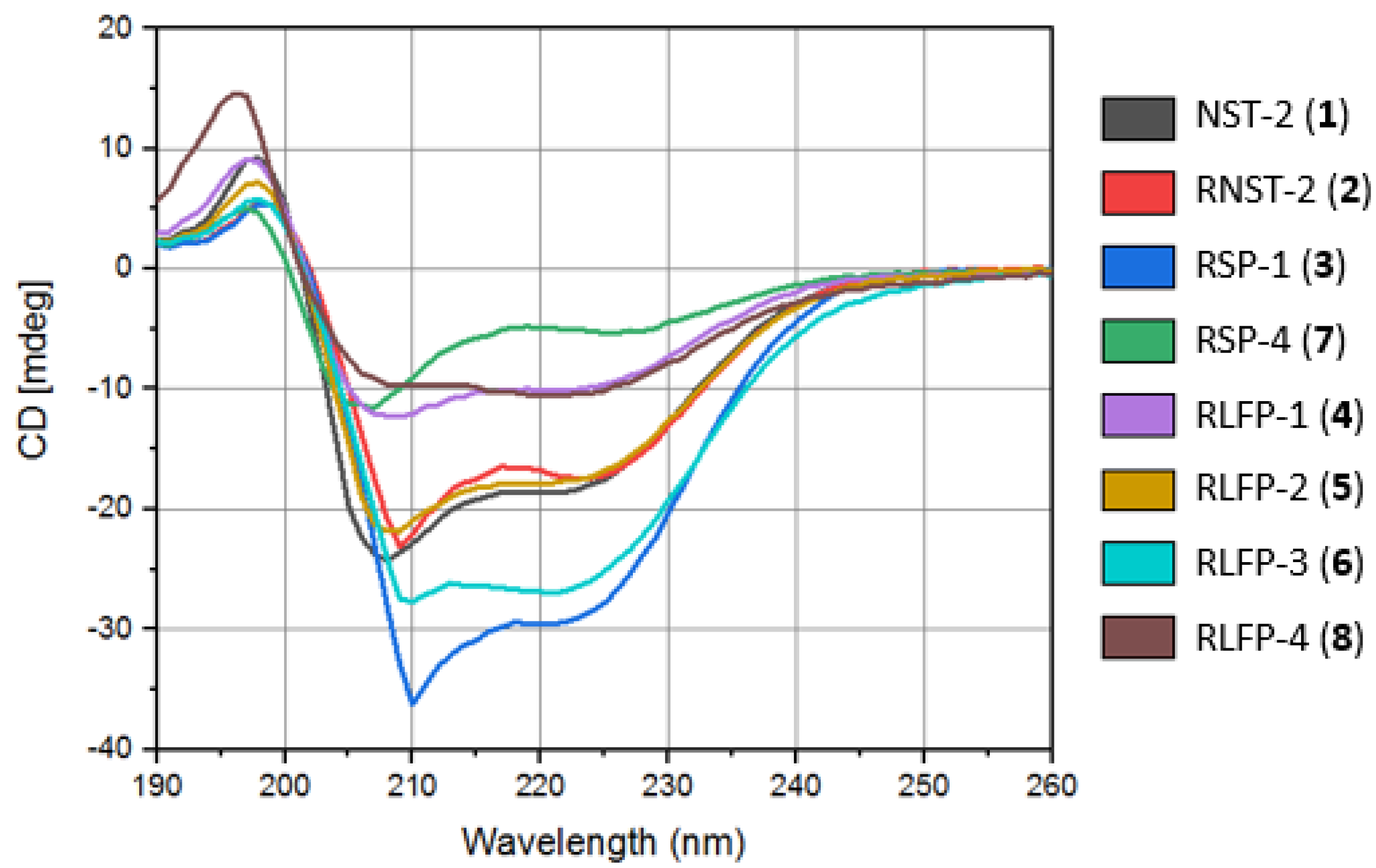
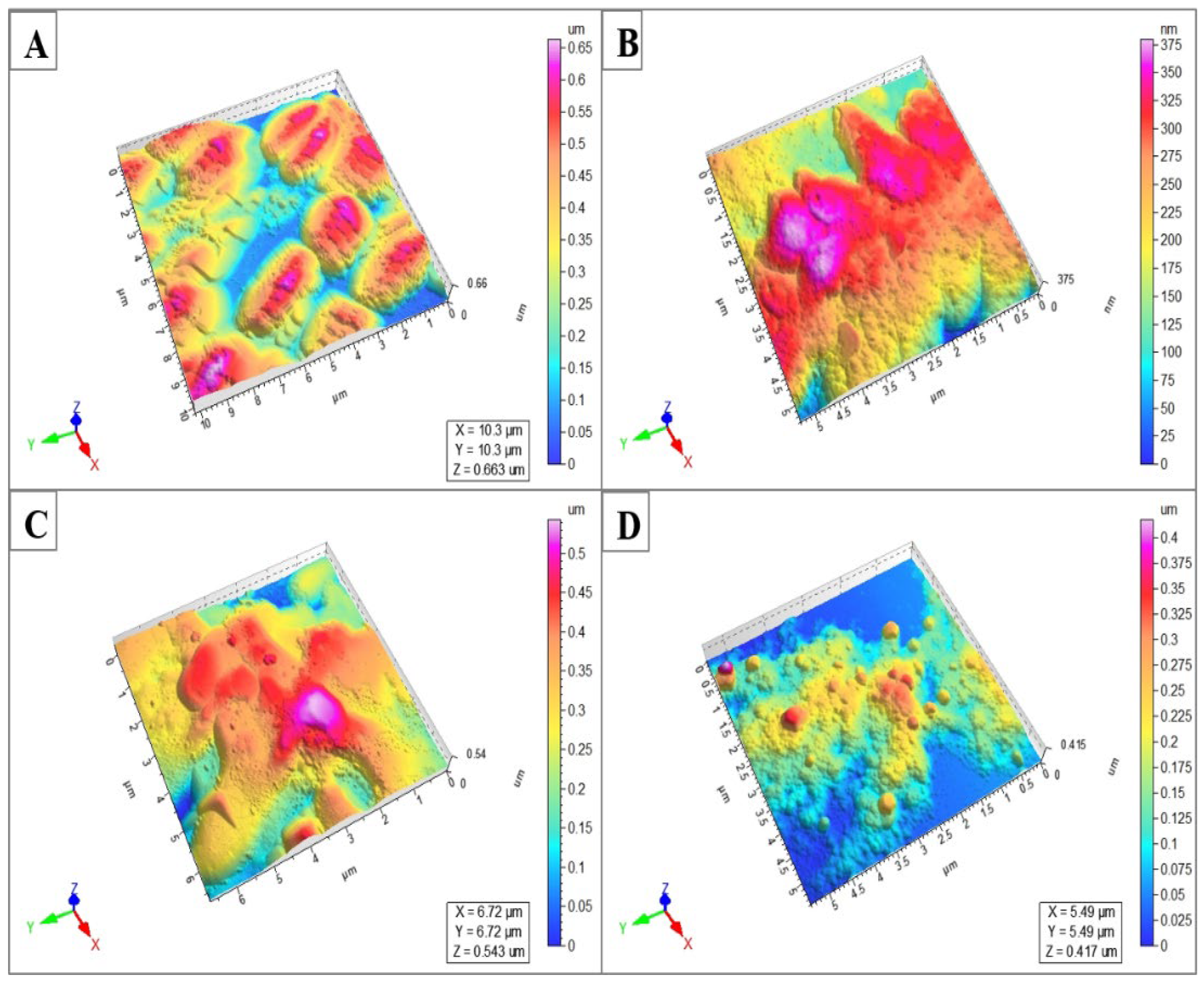
4.3.5. Atomic Force Microscopy (AFM) Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, X.; Lu, H.; Zhu, W.; Zhang, Y.; Huo, X.; Yang, C.; Xiao, S.; Zhang, Y.; Su, J. A novel antimicrobial peptide derived from bony fish IFN1 exerts potent antimicrobial and anti-inflammatory activity in mammals. Microbiol. Spectr. 2022, 10, e02013-21. [Google Scholar] [CrossRef]
- Buommino, E.; Carotenuto, A.; Antignano, I.; Bellavita, R.; Casciaro, B.; Loffredo, M.R.; Merlino, F.; Novellino, E.; Mangoni, M.L.; Nocera, F.P.; et al. The Outcomes of Decorated Prolines in the Discovery of Antimicrobial Peptides from Temporin-L. ChemMedChem 2019, 14, 1283–1290. [Google Scholar] [CrossRef]
- Bellavita, R.; Casciaro, B.; Di-Maro, S.; Brancaccio, D.; Carotenuto, A.; Falanga, A.; Cappiello, F.; Buommino, E.; Galdiero, S.; Novellino, E.; et al. First-in-class cyclic Temporin L analogue: Design, synthesis, and antimicrobial assessment. J. Med. Chem. 2021, 64, 11675–11694. [Google Scholar] [CrossRef]
- Manteghi, R.; Pallagi, E.; Olajos, G.; Csoka, I. Pegylation and formulation strategy of Anti-Microbial Peptide (AMP) according to the quality by design approach. Eur. J. Pharm. Sci. 2020, 144, 105197. [Google Scholar] [CrossRef] [PubMed]
- Moreira Brito, J.C.; Carvalho, L.R.; De-Souza, A.N.; Carneiro, G.; Magalhaes, P.P.; Farias, L.M.; Guimaraes, N.R.; Verly, R.M.; Resende Jm De-Lima, M.E. PEGylation of the antimicrobial peptide LyeTx Ib maintains structure-related biological properties and improves selectivity. Front. Mol. Biosci. 2022, 9, 1001508. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, X.; Chen, X.; Aramsantienchai, P.; Tong, Z.; Lin, H. Protein lipidation: Occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 2018, 118, 919–988. [Google Scholar] [CrossRef]
- Rai, J. Peptide and protein mimetics by retro and retroinverso analogs. Chem. Biol. Drug Des. 2019, 93, 724–736. [Google Scholar] [CrossRef]
- Nair, D.T.; Kaur, K.J.; Singh, K.; Mukherjee, P.; Rajagopal, D.; George, A.; Bal, V.; Rath, S.; Rao, K.V.S.; Salunke, D.M. Mimicry of native peptide antigens by the corresponding retro-inverso analogs is dependent on their intrinsic structure and interaction propensities. J. Immunol. 2003, 170, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Chorev, M. The partial retro–inverso modification: A road traveled together. Pept. Sci. 2005, 80, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, L.; Zheng, Y.; Liu, J.; Gong, J.; Qiu, Z.; Li, Y.; Qiao, J.; Huo, Y.X. Incorporation of non-canonical amino acids into antimicrobial peptides: Advances, Challenges, and Perspectives. Appl. Environ. Microbiol. 2022, 88, e01617-22. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Nickling, J.H.; Bartholomae, M.; Buivydas, A.; Kuipers, O.P.; Budisa, N. Prospects of in vivo incorporation of non-canonical amino acids for the chemical diversification of antimicrobial peptides. Front. Microbiol. 2017, 8, 124. [Google Scholar] [CrossRef]
- Castro, T.G.; Melle-Franco, M.; Sousa, C.E.; Cavaco-Paulo, A.; Marcos, J.C. Non-Canonical Amino Acids as Building Blocks for Peptidomimetics: Structure, Function, and Applications. Biomolecules 2023, 13, 981. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, L.; Zhang, T.; Shi, M.; Zhang, N.; Huang, J.; Xian, M. Biosynthesis and biotechnological application of non-canonical amino acids: Complex and Unclear. Biotechnol. Adv. 2018, 36, 1917–1927. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Gellman, S. Peptidomimetics. Acc. Chem. Res. 2008, 41, 1231–1232. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Vagner, J.; Qu, H.; Hruby, V.J. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 292–296. [Google Scholar] [CrossRef]
- Del Gatto, A.; Cobb, S.L.; Zhang, J.; Zaccaro, L. Peptidomimetics: Synthetic tools for drug discovery and development. Front. Chem. 2021, 9, 802120. [Google Scholar] [CrossRef] [PubMed]
- Güell, I.; Micaló, L.; Cano, L.; Badosa, E.; Ferre, R.; Montesinos, E.; Bardají, E.; Feliu, L.; Planas, M. Peptidotriazoles with antimicrobial activity against bacterial and fungal plant pathogens. Peptides 2012, 33, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.D.; Campbell, M.M. Partially modified retro-inverso peptides: Development, synthesis, and conformational behavior. Chem. Rev. 1998, 98, 763–796. [Google Scholar] [CrossRef] [PubMed]
- Staśkiewicz, A.; Ledwoń, P.; Rovero, P.; Papini, A.M.; Latajka, R. Triazole-modified peptidomimetics: An opportunity for drug discovery and development. Front. Chem. 2021, 9, 674705. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.Z.; Carneiro, H.C.; Lara, H.A.; Alves, R.B.; Resende, J.M.; Oliveira, H.M.; Silva, L.M.; Santos, D.A.; Freitas, P. Synthesis of a new peptide–coumarin conjugate: A potential agent against cryptococcosis. ACS Med. Chem. Lett. 2015, 6, 271–275. [Google Scholar] [CrossRef]
- Suhas, R.; Chandrashekar, S.; Gowda, D.C. Synthesis of uriedo and thiouriedo derivatives of peptide conjugated heterocycles—A new class of promising antimicrobials. Eur. J. Med. Chem. 2012, 48, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Yaqoob, S.; Qasim, M.W.; Ali, S.; Wang, Y.; Jiang, Z.H. A Robust, Gram-Scale and High-Yield Synthesis of MDP Congeners for Activation of the NOD2 Receptor and Vaccine Adjuvantation. Synthesis 2024, 56, 539–548. [Google Scholar] [CrossRef]
- Guzelj, S.; Weiss, M.; Slutter, B.; Frkanec, R.; Jakopin, Z. Covalently conjugated NOD2/TLR7 agonists are potent and versatile immune potentiators. J. Med. Chem. 2022, 65, 15085–15101. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Khanam, R.; Wasim Qasim, M.; Wang, Y.; Jiang, Z.H. Improved Synthesis of D-Isoglutamine: Rapid Access to Desmuramyl Analogues of Muramyl Dipeptide for the Activation of Intracellular NOD2 Receptor and Vaccine Adjuvant Applications. Eur. J. Org. Chem. 2021, 2021, 6688–6699. [Google Scholar] [CrossRef]
- Aamra, H.; Khan, F.A.; Jahan, H.; Zafar, M.; Ali, H.; Shaheen, F. Synthesis of novel benzimidazole containing antimicrobial peptides (AMPs) with significant inhibitory effect on multidrug resistant strain of Salmonella typhimurium. Synth. Commun. 2021, 51, 3620–3628. [Google Scholar] [CrossRef]
- Gattu, R.; Ramesh, S.S.; Nadigar, S.; Ramesh, S. Conjugation as a tool in therapeutics: Role of amino acids/peptides-bioactive (including Heterocycles) hybrid molecules in treating infectious diseases. Antibiotics 2023, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Molero, A.; Vendrell, M.; Bonaventura, J.; Zachmann, J.; Lopez, L.; Pardo, L.; Lluis, C.; Cortes, A.; Albericio, F.; Casado, V.; et al. A solid-phase combinatorial approach for indoloquinolizidine-peptides with high affinity at D1 and D2 dopamine receptors. Eur. J. Med. Chem. 2015, 97, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Z.; Cao, Y.; Gao, L.; Shao, J.; Zhao, Y.; Wei, G.; Li, J.; Zhu, H. Exploration of novel four-membered-heterocycle constructed peptidyl proteasome inhibitors with improved metabolic stability for cancer treatment. Bioorganic Chem. 2023, 138, 106626. [Google Scholar] [CrossRef]
- Khan, F.A.; Nasim, N.; Wang, Y.; Alhazmi, A.; Sanam, M.; Ul-Haq, Z.; Yalamti, D.; Ulanova, M.; Jiang, Z.H. Amphiphilic desmuramyl peptides for the rational design of new vaccine adjuvants: Synthesis, in vitro modulation of inflammatory response and molecular docking studies. Eur. J. Med. Chem. 2021, 209, 112863. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Ulanova, M.; Bai, B.; Yalamati, D.; Jiang, Z.H. Design, synthesis and immunological evaluation of novel amphiphilic desmuramyl peptides. Eur. J. Med. Chem. 2017, 141, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Gobec, M.; Tomašič, T.; Štimac, A.; Frkanec, R.; Trontelj, J.; Anderluh, M.; Mlinaric-Rascn, I.; Jakopin, Z. Discovery of nanomolar desmuramylpeptide agonists of the innate immune receptor nucleotide-binding oligomerization domain-containing protein 2 (NOD2) possessing immunostimulatory properties. J. Med. Chem. 2018, 61, 2707–2724. [Google Scholar] [CrossRef]
- Guzelj, S.; Nabergoj, S.; Gobec, M.; Pajk, S.; Klančič, V.; Slütter, B.; Frkanec, R.; Štimac, A.; Šket, P.; Plavec, J.; et al. Structural fine-tuning of desmuramylpeptide NOD2 agonists defines their in vivo adjuvant activity. J. Med. Chem. 2021, 64, 7809–7838. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Neundorf, I. Design and application of antimicrobial peptide conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef]
- Ceccherini, F.; Falciani, C.; Onori, M.; Scali, S.; Rossolini, G.M.; Bracci, L.; Pini, A. Antimicrobial activity of levofloxacin–M33 peptide conjugation or combination. MedChemComm 2016, 7, 258–262. [Google Scholar] [CrossRef]
- Riahifard, N.; Tavakoli, K.; Yamaki, J.; Parang, K.; Tiwari, R. Synthesis and evaluation of antimicrobial activity of [R4W4K]-Levofloxacin and [R4W4K]-Levofloxacin-Q conjugates. Molecules 2017, 22, 957. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Lohan, S.; Kato, S.; Tiwari, R.K. Combination of Amphiphilic Cyclic Peptide [R4W4] and Levofloxacin against Multidrug-Resistant Bacteria. Antibiotics 2022, 11, 416. [Google Scholar] [CrossRef]
- Atzori, A.; Baker, A.E.; Chiu, M.; Bryce, R.A.; Bonnet, P. Effect of sequence and stereochemistry reversal on p53 peptide mimicry. PLoS ONE 2013, 8, e68723. [Google Scholar] [CrossRef] [PubMed]
- Zerze, G.H.; Stillinger, F.H.; Debenedetti, P.G. Computational investigation of retro-isomer equilibrium structures: Intrinsically disordered, foldable, and cyclic peptides. FEBS Lett. 2020, 594, 104–113. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, L.D.; Romanelli, A. Temporins: Multifunctional peptides from frog skin. Int. J. Mol. Sci. 2023, 24, 5426. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Canofeni, S.; Miele, R.; Mangoni, M.L.; Barra, D. Temporins, antimicrobial peptides from the European red frog Rana temporaria. Eur. J. Biochem. 1996, 242, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. CMLS 2006, 63, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Mahalka, A.K.; Kinnunen, P.K. Binding of amphipathic α-helical antimicrobial peptides to lipid membranes: Lessons from temporins B and L. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1600–1609. [Google Scholar] [CrossRef]
- Romero, S.M.; Cardillo, A.B.; Martínez Ceron, M.C.; Camperi, S.A.; Giudicessi, S.L. Temporins: An approach of potential pharmaceutic candidates. Surg. Infect. 2020, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 283, 22907–22917. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Saravanan, R.; Mohanram, H.; Mangoni, M.L.; Bhattacharjya, S. NMR structures and interactions of temporin-1Tl and temporin-1Tb with lipopolysaccharide micelles: Mechanistic insights into outer membrane permeabilization and synergistic activity. J. Biol. Chem. 2011, 286, 24394–24406. [Google Scholar] [CrossRef]
- Bhattacharjya, S. NMR structures and interactions of antimicrobial peptides with lipopolysaccharide: Connecting structures to functions. Curr. Top. Med. Chem. 2016, 16, 4–15. [Google Scholar] [CrossRef]
- Pulido, D.; Nogués, M.; Boix, E.; Torrent, M. Lipopolysaccharide neutralization by antimicrobial peptides: A gambit in the innate host defense strategy. J. Innate Immun. 2012, 4, 327–336. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Malmsten, M. (Lipo) polysaccharide interactions of antimicrobial peptides. J. Colloid Interface Sci. 2015, 449, 136–142. [Google Scholar] [CrossRef]
- Khan, A.I.; Nazir, S.; Haque, M.N.U.; Maharjan, R.; Khan, F.A.; Olleik, H.; Courvoisier-Dezord, E.; Maresca, M.; Shaheen, F. Synthesis of Second-Generation Analogs of Temporin-SHa Peptide Having Broad-Spectrum Antibacterial and Anticancer Effects. Antibiotics 2024, 13, 758. [Google Scholar] [CrossRef]
- Khan, A.I.; Nazir, S.; Ullah, A.; Haque, M.N.U.; Maharjan, R.; Simjee, S.U.; Olleik, H.; Dezord, E.C.; Maresca, M.; Shaheen, F. Design, synthesis and characterization of [G10a]-Temporin SHa dendrimers as dual inhibitors of cancer and pathogenic microbes. Biomolecules 2022, 12, 770. [Google Scholar] [CrossRef]
- Raja, Z.; Andre, S.; Abbasi, F.; Humblot, V.; Lequin, O.; Bouceba, T.; Correia, I.; Casale, S.; Foulon, T.; Sereno, D.; et al. Insight into the mechanism of action of temporin-SHa, a new broad-spectrum antiparasitic and antibacterial agent. PLoS ONE 2017, 12, e0174024. [Google Scholar] [CrossRef]
- Olleik, H.; Baydoun, E.; Perrier, J.; Hijazi, A.; Raymond, J.; Manzoni, M.; Dupuis, L.; Pauleau, G.; Goudard, Y.; de La Villéon, B.; et al. Temporin-SHa and its analogs as potential candidates for the treatment of helicobacter pylori. Biomolecules 2019, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Khan, A.I.; Haque, M.N.U.; Maresca, M.; Choudhary, M.I.; Shaheen, F.; Simjee, S.U.A. Serum stable and low hemolytic temporin-SHa peptide analogs disrupt cell membrane of methicillin-resistant Staphylococcus aureus (MRSA). Probiotics Antimicrob. 2022, 14, 391–405. [Google Scholar] [CrossRef]
- Lucana, M.C.; Arruga, Y.; Petrachi, E.; Roig, A.; Lucchi, R.; Oller-Salvia, B. Protease-resistant peptides for targeting and intracellular delivery of Therapeutics. Pharmaceutics 2021, 13, 2065. [Google Scholar] [CrossRef]
- Amirkhanov, N.V.; Bardasheva, A.V.; Tikunova, N.V.; Pyshnyi, D.V. Synthetic antimicrobial peptides: III—Effect of cationic groups of lysine, arginine, and histidine on antimicrobial activity of peptides with a linear type of amphipathicity. Russ. J. Bioorganic Chem. 2021, 47, 681–690. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B. Lysine-enriched cecropin-mellitin antimicrobial peptides with enhanced selectivity. Antimicrob. Agents Chemother. 2008, 52, 4463–4465. [Google Scholar] [CrossRef] [PubMed]
- Taheri, B.; Mohammadi, M.; Momenzadeh, N.; Farshadzadeh, Z.; Roozbehani, M.; Dehghani, P.; Shamseddin, J. Substitution of lysine for isoleucine at the center of the nonpolar face of the antimicrobial peptide, piscidin-1, leads to an increase in the rapidity of bactericidal activity and a reduction in toxicity. Infect. Drug Resist. 2019, 12, 1629–1647. [Google Scholar] [CrossRef]
- Parker, W.; Song, P.S. Protein structures in SDS micelle-protein complexes. Biophys. J. 1992, 61, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E.W. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef]
- Micsonai, A.; Moussong, E.; Wien, F.; Boros, E.; Vadászi, H.; Murvai, N.; Lee, Y.H.; Molnar, T.; Refregiers, M.; Goto, Y.; et al. BeStSel: Webserver for secondary structure and fold prediction for protein CD spectroscopy. Nucleic Acids Res. 2022, 50, W90–W98. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Moussong, É.; Wien, F.; Boros, E.; Vadászi, H.; Murvai, N.; Lee, Y.H.; Molnar, T.; Refregiers, M.; Goto, Y.; et al. Bestsel: Updated webserver for secondary structure and fold prediction for protein CD spectroscopy. Biophys. J. 2023, 122, 179a. [Google Scholar] [CrossRef]
- Shaheen, F.; Nadeem-ul-Haque, M.; Ahmed, A.; Simjee, S.U.; Ganesan, A.; Jabeen, A.; Shah, Z.A.; Choudhary, M.I. Synthesis of breast cancer targeting conjugate of temporin-SHa analog and its effect on pro-and anti-apoptotic protein expression in MCF-7 cells. Peptides 2018, 106, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ghosh, J.K. Introduction of a lysine residue promotes aggregation of temporin L in lipopolysaccharides and augmentation of its antiendotoxin property. Antimicrob. Agents Chemother. 2013, 57, 2457–2466. [Google Scholar] [CrossRef]
- Mohanram, H.; Bhattacharjya, S. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob. Agents Chemother. 2014, 58, 1987–1996. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef] [PubMed]
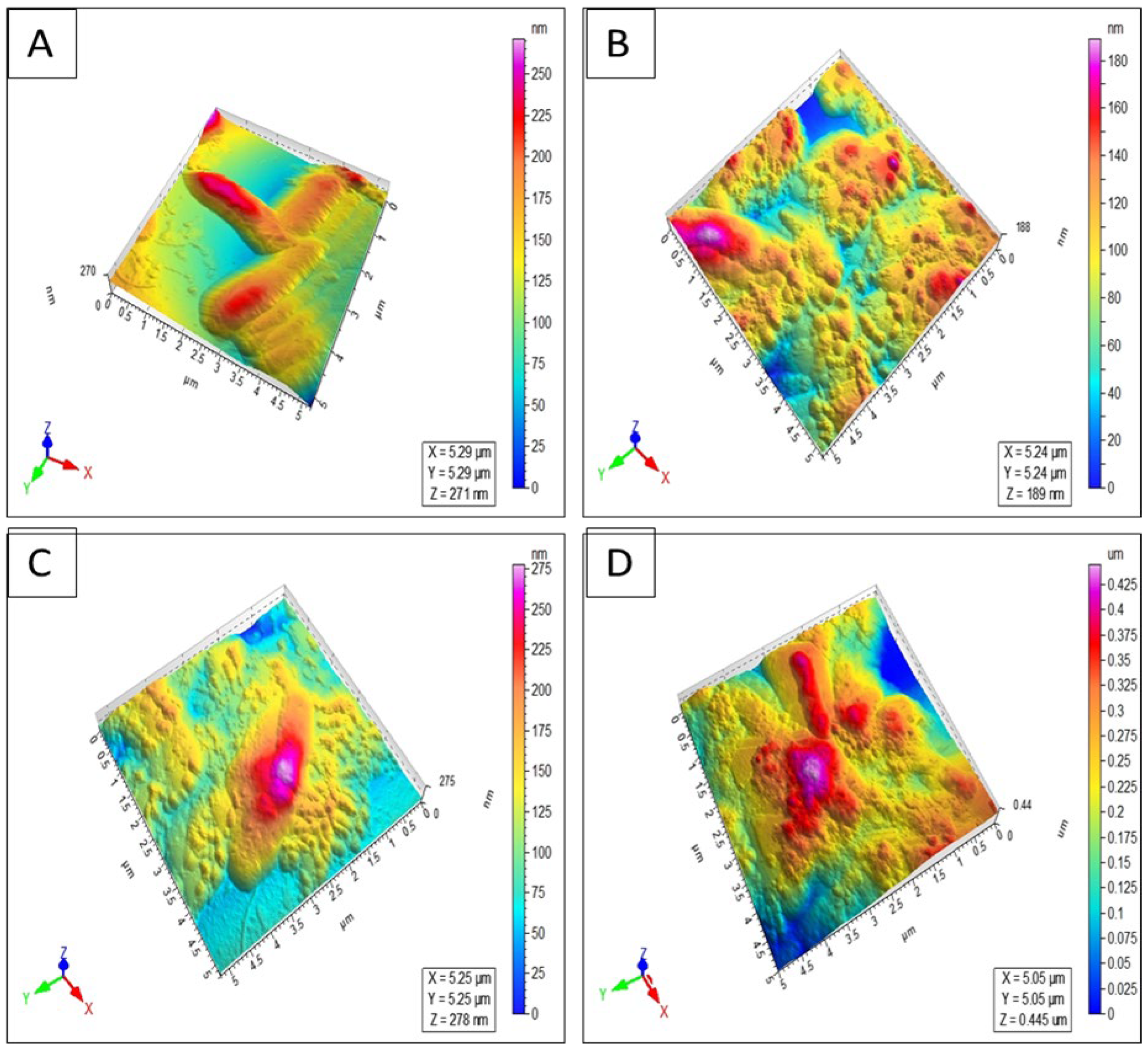

| Peptide | S. aureus (NCTC 13277) | B. subtilis (ATCC 23857) | S. typhi (ATCC 14028) | E. coli (ATCC 25922) | P. aeruginosa (ATCC 10145) | C. albicans (ATCC 36082) |
|---|---|---|---|---|---|---|
| NST-2 (1) | 14.34 | 6.25 | 250 | 125 | >250 | 20 |
| RNST-2 (2) | 3.12 | 1.56 | 50 | 50 | 200 | 15.6 |
| RSP-1 (3) | 6.25 | 1.56 | 50 | 25 | 50 | 15.6 |
| RLFP-1 (4) | 50 | 50 | 25 | >200 | >200 | >250 |
| RLFP-2 (5) | 100 | 50 | 200 | >200 | >200 | >250 |
| RLFP-3 (6) | 12.5 | 1.56 | 3.12 | 25 | 12.5 | 3.12 |
| RSP-4 (7) | 50 | 12.5 | 100 | 50 | 200 | 100 |
| RLFP-4 (8) | 6.25 | 1.56 | 50 | 25 | 12.5 | >200 |
| Levofloxacin | 25 | 0.39 | 0.39 | 0.78 | 1.56 | >250 |
| Peptide | IC50 on Breast Cancer (MCF-7) | IC50 on Cervical Cancer (HeLa) | HC50 on Human Red Blood Cells (RBCs) |
|---|---|---|---|
| NST-2 (1) | 17.9 | >100 | 90.0 |
| RNST-2 (2) | 53.0 | 60.0 | 13.8 |
| RSP-1 (3) | 42.7 | 33.5 | 51.0 |
| RLFP-1 (4) | >100 | >100 | 98.9 |
| RLFP-2 (5) | >100 | >100 | 25.0 |
| RLFP-3 (6) | 13.3 | 12.1 | 6.6 |
| RSP-4 (7) | 39.9 | 56.9 | >100 |
| RLFP-4 (8) | 23.8 | 15.5 | 4.5 |
| Levofloxacin | >100 | >100 | >100 |
| Peptide | Lower MIC | Lower IC50 | Hemolysis (HC50) | SI Based on MIC | SI Based on IC50 |
|---|---|---|---|---|---|
| NST-2 (1) | 6.25 | 17.9 | 90.0 | 14.4 | 5.0 |
| RNST-2 (2) | 1.56 | 53.0 | 13.8 | 8.8 | 0.2 |
| RSP-1 (3) | 1.56 | 33.5 | 51.0 | 32.6 | 1.5 |
| RLFP-1 (4) | 25 | >100 | 98.9 | 3.9 | <0.9 |
| RLFP-2 (5) | 50 | >100 | 25.0 | 0.5 | <0.2 |
| RLFP-3 (6) | 1.56 | 12.1 | 6.6 | 4.2 | 0.5 |
| RSP-4 (7) | 12.5 | 39.9 | >100 | >8.0 | >2.5 |
| RLFP-4 (8) | 1.56 | 15.5 | 4.5 | 2.8 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, S.; Khan, A.I.; Maharjan, R.; Khan, S.N.; Akram, M.A.; Maresca, M.; Khan, F.-A.; Shaheen, F. Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities. Antibiotics 2024, 13, 1213. https://doi.org/10.3390/antibiotics13121213
Nazir S, Khan AI, Maharjan R, Khan SN, Akram MA, Maresca M, Khan F-A, Shaheen F. Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities. Antibiotics. 2024; 13(12):1213. https://doi.org/10.3390/antibiotics13121213
Chicago/Turabian StyleNazir, Shahzad, Arif Iftikhar Khan, Rukesh Maharjan, Sadiq Noor Khan, Muhammad Adnan Akram, Marc Maresca, Farooq-Ahmad Khan, and Farzana Shaheen. 2024. "Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities" Antibiotics 13, no. 12: 1213. https://doi.org/10.3390/antibiotics13121213
APA StyleNazir, S., Khan, A. I., Maharjan, R., Khan, S. N., Akram, M. A., Maresca, M., Khan, F.-A., & Shaheen, F. (2024). Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities. Antibiotics, 13(12), 1213. https://doi.org/10.3390/antibiotics13121213








