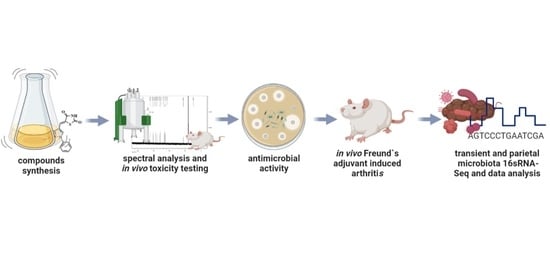
A New 4-Thiazolidinone Derivative (Les-6490) as a Gut Microbiota Modulator: Antimicrobial and Prebiotic Perspectives
Abstract
:1. Introduction
2. Results
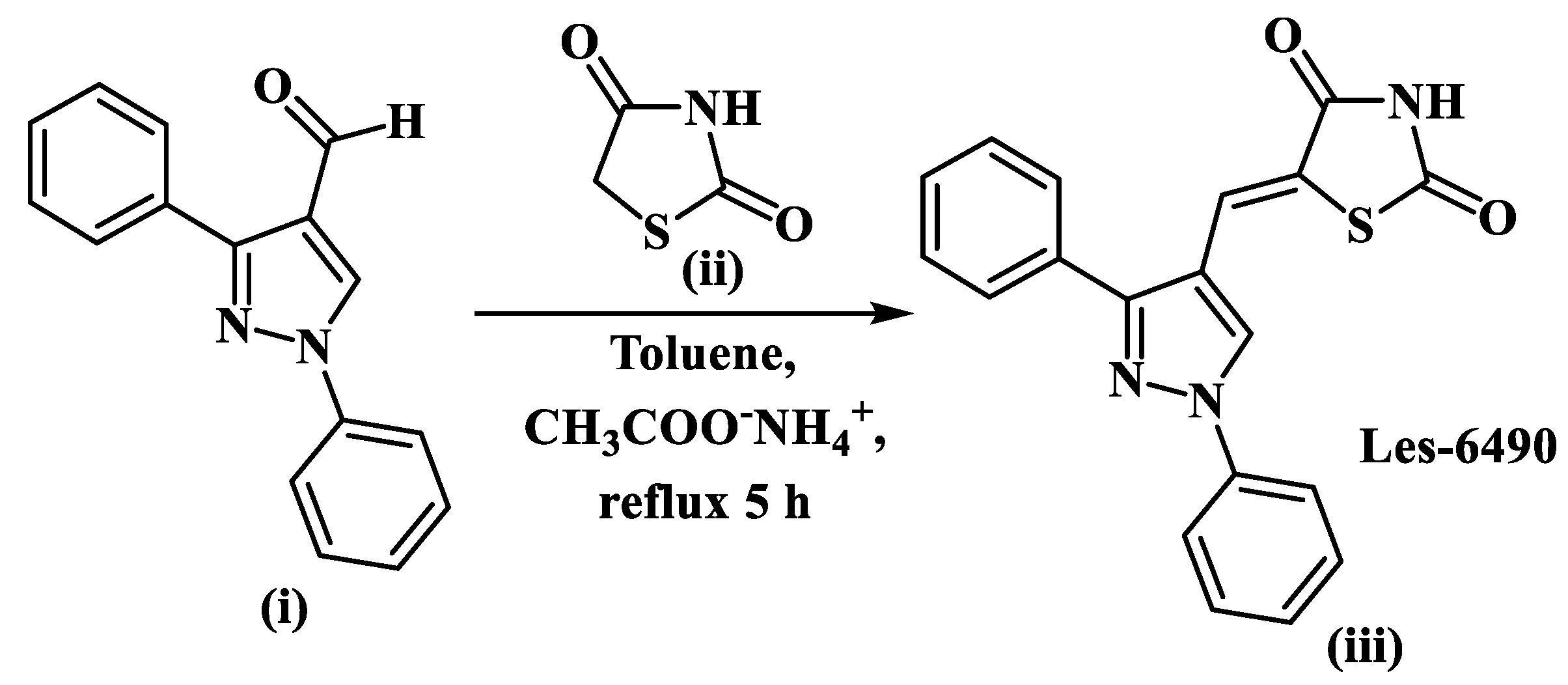
2.1. Chemistry
2.2. Antimicrobial Activity In Vitro and Clinical Indicators In Vivo
2.3. Cytotoxicity
2.3.1. Cell Lines
2.3.2. Acute Toxicity in Mice
2.4. 16S rRNA Sequence
2.4.1. Composition of Microbial Community Analysis
2.4.2. Alpha Diversity Indices
2.4.3. Beta Diversity Analysis
Principal Component Analysis (PCA) and Non-Metric Multidimensional Scaling (NMDS)
Multi-Response Permutation Procedure (MRPP) Analysis
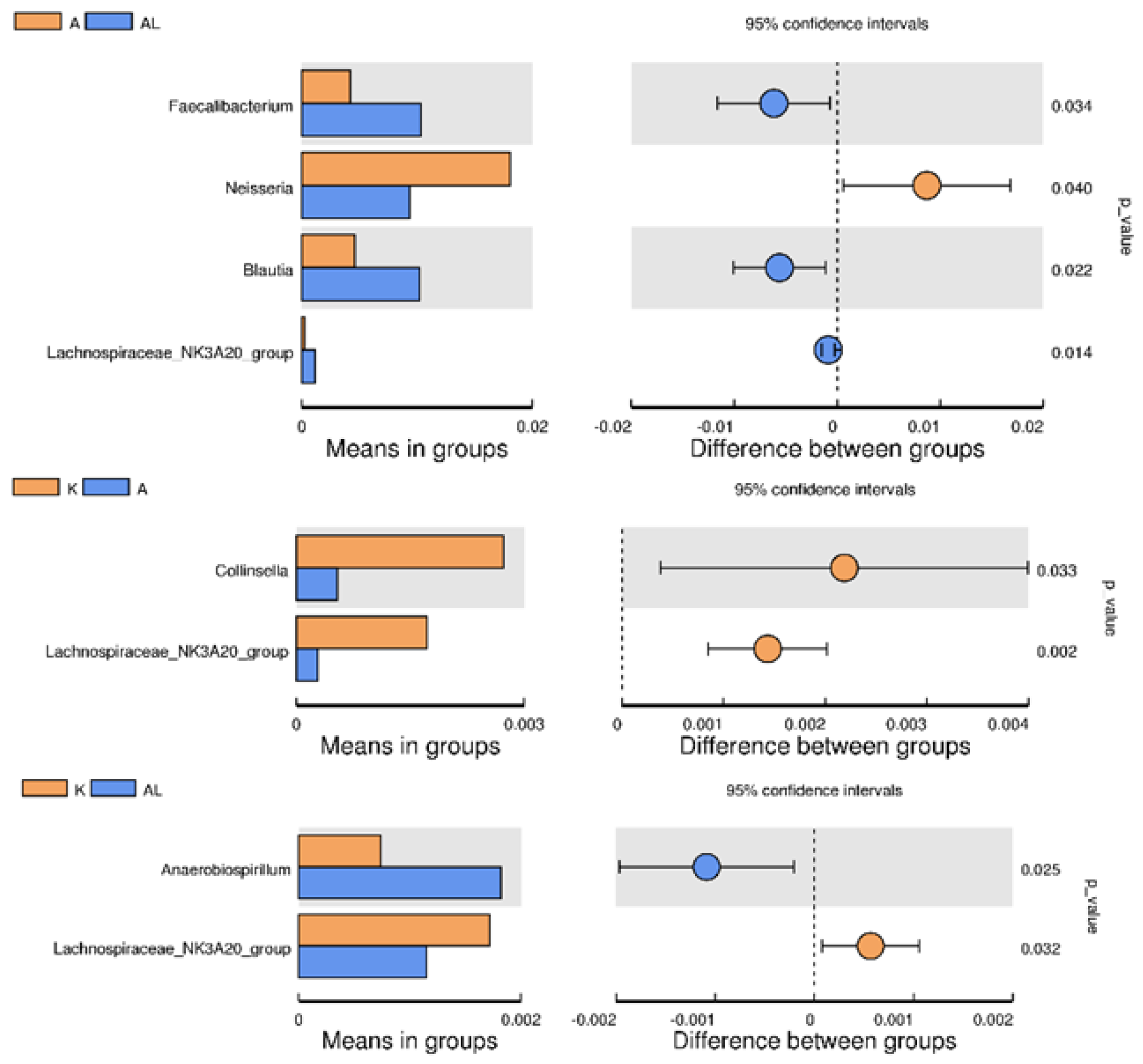
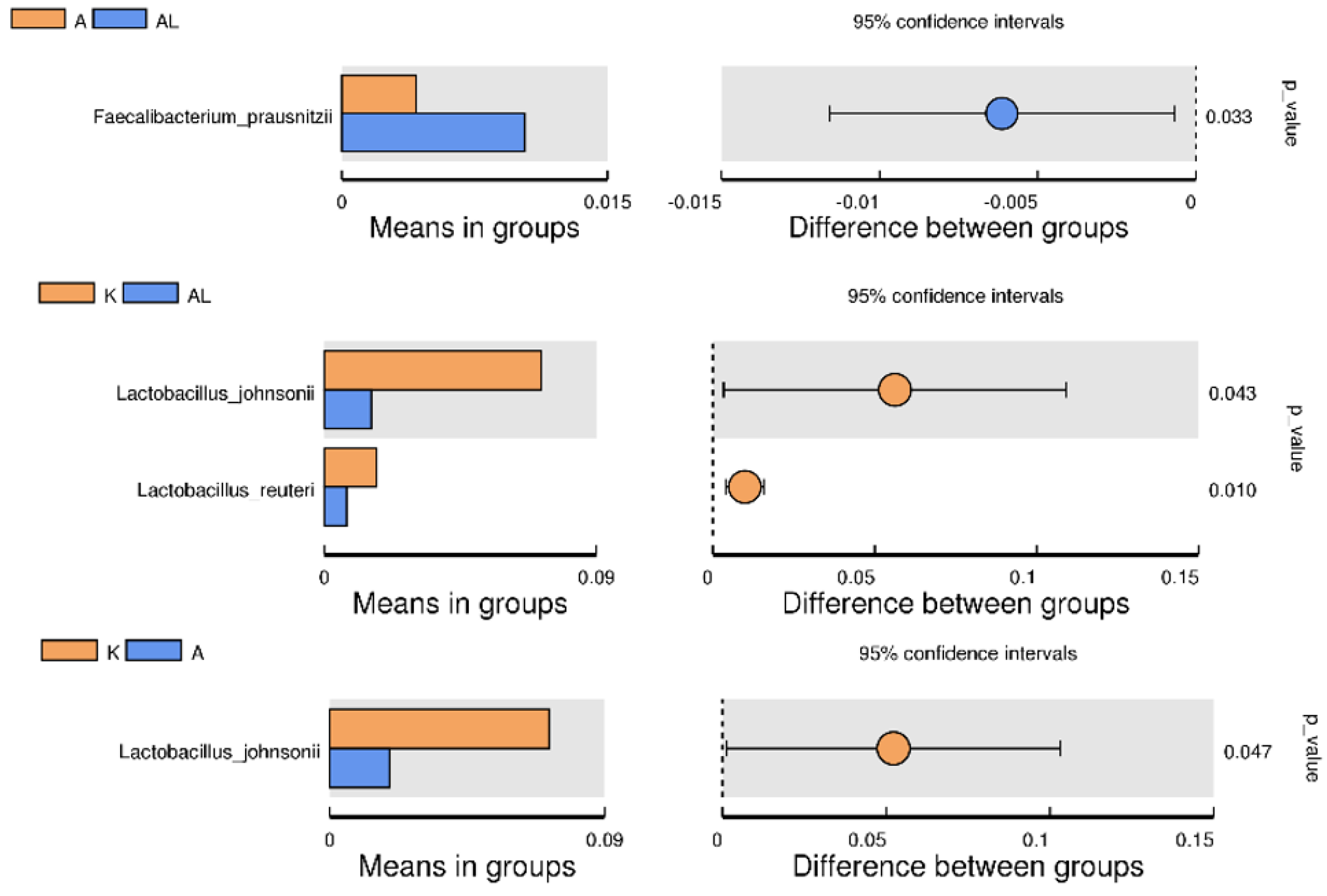
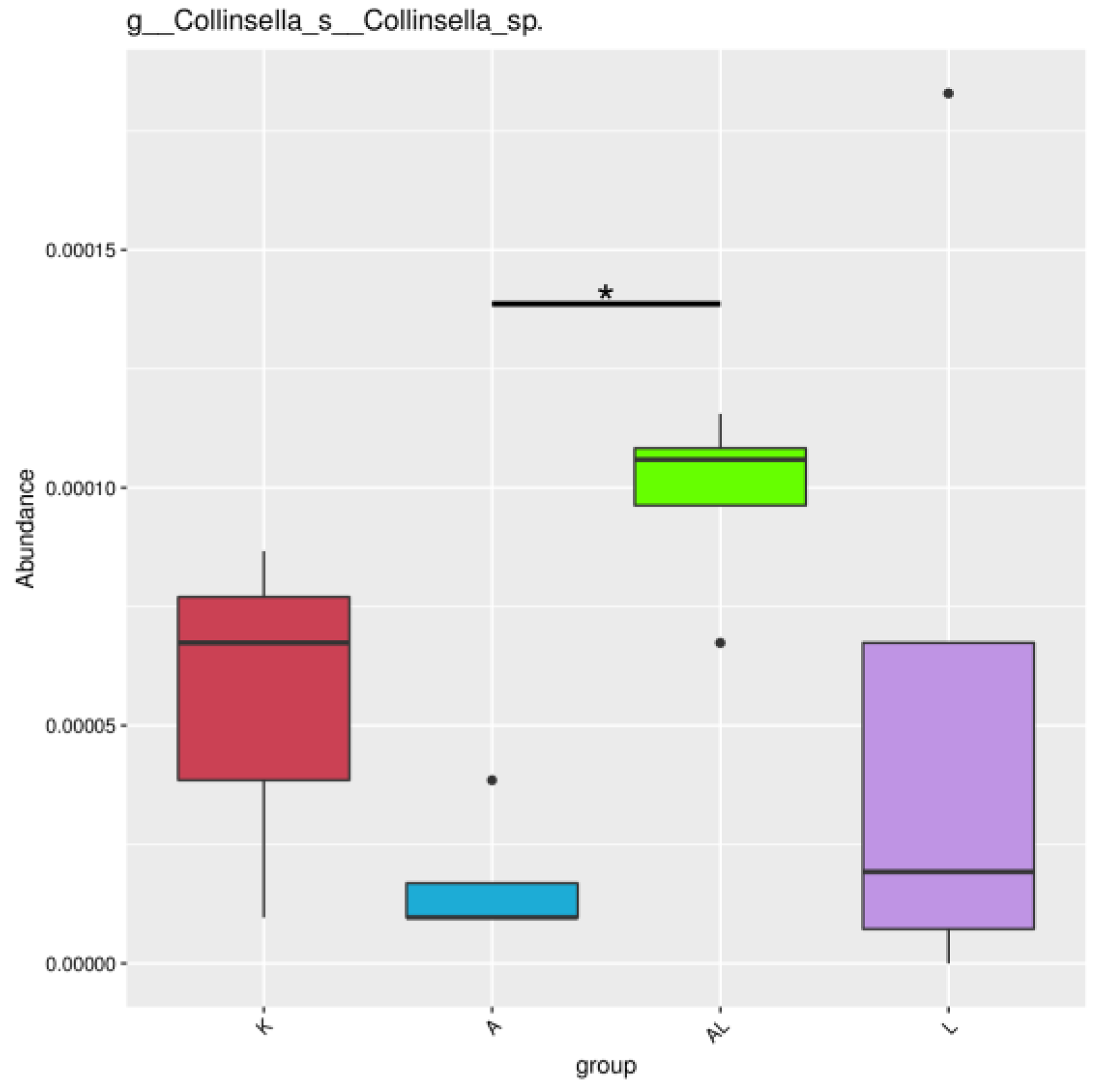
2.4.4. Between-Group Variation Analysis of Species
2.4.5. MetaStat
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Remarks
4.1.2. General Procedure for the Synthesis of 5-(1,3-diphenyl-1H-pyrazol-4-ylmethylene)-thiazolidine-2,4-dione Les-6490 (iii)
4.2. Antimicrobial Activity In Vitro
4.3. In Vivo Protocol
4.4. Cytotoxicity
4.4.1. Cell Lines
4.4.2. Acute Toxicity in Mice
4.5. 16S rRNA Sequencing
4.5.1. Extraction of Genome DNA
4.5.2. Amplicon Generation
4.5.3. PCR Products Quantification and Qualification, Mixing, and Purification
4.5.4. Library Preparation and Sequencing
4.5.5. Paired-End Read Assembly and Quality Control
4.5.6. OTU Clustering
4.5.7. Taxonomic Annotation
4.5.8. Phylogenetic Relationship Construction
4.5.9. Data Normalization
4.5.10. Alpha Diversity
4.5.11. Beta Diversity
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Lozynskyi, A.V.; Derkach, H.O.; Zasidko, V.V.; Konechnyi, Y.T.; Finiuk, N.S.; Len, Y.T.; Kutsyk, R.V.; Regeda, M.S.; Lesyk, R.B. Antimicrobial and Cytotoxic Activities of Thiazolo[4,5-b]Pyridine Derivatives. Biopolym. Cell 2021, 37, 153–164. [Google Scholar] [CrossRef]
- Konechnyi, Y.T.; Lozynskyi, A.V.; Horishny, V.Y.; Konechna, R.T.; Vynnytska, R.B.; Korniychuk, O.P.; Lesyk, R.B. Synthesis of Indoline-Thiazolidinone Hybrids with Antibacterial and Antifungal Activities. Biopolym. Cell 2020, 36, 381–391. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marsland, B.J.; Bunyavanich, S.; O’Mahony, L.; Leung, D.Y.M.; Muraro, A.; Fleisher, T.A. The Microbiome in Allergic Disease: Current Understanding and Future Opportunities—2017 PRACTALL Document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 2017, 139, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yao, J.; Deng, Q.; Li, X.; He, Y.; Ren, X.; Zheng, Y.; Song, R.; Zhong, X.; Ma, J.; et al. Relationship between Gut Microbiota and Rheumatoid Arthritis: A Bibliometric Analysis. Front. Immunol. 2023, 14, 1131933. [Google Scholar] [CrossRef]
- Attur, M.; Scher, J.U.; Abramson, S.B.; Attur, M. Role of Intestinal Dysbiosis and Nutrition in Rheumatoid Arthritis. Cells 2022, 11, 2436. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Panebianco, C.; Salemi, S.; Sorgi, M.; Di Rosa, R.; Tropea, A.; Sgrulletti, M.; Salerno, G.; Terracciano, F.; D’Amelio, R.; et al. Analysis of Gut Microbiota in Rheumatoid Arthritis Patients: Disease-Related Dysbiosis and Modifications Induced by Etanercept. Int. J. Mol. Sci. 2018, 19, 2938. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Guo, F.; Wu, W.; Zhang, T. Characterization of Tetracycline Resistant Bacterial Community in Saline Activated Sludge Using Batch Stress Incubation with High-Throughput Sequencing Analysis. Water Res. 2013, 47, 4207–4216. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Avershina, E.; Frisli, T.; Rudi, K. De Novo Semi-Alignment of 16S RRNA Gene Sequences for Deep Phylogenetic Characterization of Next Generation Sequencing Data. Microbes Environ. 2013, 28, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.-J.; Li, Y.; Xiong, W.-J. Shenqi Yanshen Formula (SQYSF) Protects against Chronic Kidney Disease by Modulating Gut Microbiota. Bioengineered 2022, 13, 5625–5637. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Nie, S. Deciphering Diet-Gut Microbiota-Host Interplay: Investigations of Pectin. Trends Food Sci. Technol. 2020, 106, 171–181. [Google Scholar] [CrossRef]
- Zaongo, S.D.; Chen, Y. Gut Microbiota: A Potential Key Player in Boosting Immune Reconstitution of Immunological Nonresponders. Future Microbiol. 2023, 18, 83–85. [Google Scholar] [CrossRef]
- Shelby, R.D.; Mar, P.; Janzow, G.E.; Mashburn-Warren, L.; Tengberg, N.; Navarro, J.B.; Allen, J.M.; Wickham, J.; Wang, Y.; Bailey, M.T.; et al. Antibacterial and Anti-Inflammatory Effects of Lactobacillus Reuteri in Its Biofilm State Contribute to Its Beneficial Effects in a Rat Model of Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 2021, 57, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; Miessi, D.M.J.; Esgalha da Rocha, T.; Gomes, N.A.; Nuernberg, M.A.A.; de Medeiros Cardoso, J.; Ervolino, E.; Theodoro, L.H. The Effects of Lactobacillus Reuteri on the Inflammation and Periodontal Tissue Repair in Rats: A Pilot Study. Saudi Dent. J. 2022, 34, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-Derived Probiotic Lactobacillus Reuteri Strains Differentially Reduce Intestinal Inflammation. Am. J. Physiol. Liver Physiol. 2010, 299, G1087–G1096. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Sugimoto, Y.; Aoyagi, Y.; Igarashi, Y.; Furumai, T.; Saito, N.; Yamada, Y.; Asao, T.; Oki, T. New Cdc25B Tyrrosine Phosphatase Inhibitors, Nocardiones A and B, Produced by Nocardia Sp. TP-A0248. Taxonomy, Fermentation, Isolation, Structural Elucidation and Biological Prbperties. J. Antibiot. 2000, 53, 337–344. [Google Scholar] [CrossRef]
- Liu, H.; Sun, D.; Du, H.; Zheng, C.; Li, J.; Piao, H.; Li, J.; Sun, L. Synthesis and Biological Evaluation of Tryptophan-Derived Rhodanine Derivatives as PTP1B Inhibitors and Anti-Bacterial Agents. Eur. J. Med. Chem. 2019, 172, 163–173. [Google Scholar] [CrossRef]
- Briedis, K.M. The Distribution and Evolution of Protein Kinase and Phosphatase Families in the Three Superkingdoms of Life; University of California: San Diego, CA, USA, 2008. [Google Scholar]
- Lacerda, D.C.; Trindade da Costa, P.C.; Pontes, P.B.; Carneiro dos Santos, L.A.; Cruz Neto, J.P.R.; Silva Luis, C.C.; de Sousa Brito, V.P.; de Brito Alves, J.L. Potential Role of Limosilactobacillus Fermentum as a Probiotic with Anti-Diabetic Properties: A Review. World J. Diabetes 2022, 13, 717–728. [Google Scholar] [CrossRef]
- Rumynska, T.M. The Effect of the Nimesulide and a New 4-Thiazolidinone Derivative on Hematological Parameters in the Conditions of an Experimental Inflammatory Process. Anim. Biol. 2023, 25, 33–36. [Google Scholar] [CrossRef]
- Belkacemi, S.; Tidjani Alou, M.; Million, M.; Levasseur, A.; Khelaifia, S.; Raoult, D. Prevalence of Treponema Species in the Gut Microbiome Is Linked to Bifidobacterium Sp. and Bacteroides Sp. Res. Sq. 2020, 1–28. [Google Scholar] [CrossRef]
- Beck, C.N.; Zhao, J.; Erf, G.F. Vaccine Immunogenicity versus Gastrointestinal Microbiome Status: Implications for Poultry Production. Appl. Sci. 2024, 14, 1240. [Google Scholar] [CrossRef]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of Reduced Microbial Diversity in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 6951091. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. 16S RRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Gorkiewicz, G.; Feierl, G.; Schober, C.; Dieber, F.; Köfer, J.; Zechner, R.; Zechner, E.L. Species-Specific Identification of Campylobacters by Partial 16S RRNA Gene Sequencing. J. Clin. Microbiol. 2003, 41, 2537–2546. [Google Scholar] [CrossRef]
- Peker, N.; Garcia-Croes, S.; Dijkhuizen, B.; Wiersma, H.H.; van Zanten, E.; Wisselink, G.; Friedrich, A.W.; Kooistra-Smid, M.; Sinha, B.; Rossen, J.W.A.; et al. A Comparison of Three Different Bioinformatics Analyses of the 16S–23S RRNA Encoding Region for Bacterial Identification. Front. Microbiol. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, X.; Zhang, H.; Yang, X.; Hong, N.; Yang, Y.; Chen, H.; Yu, C. Faecalibacterium Prausnitzii Inhibits Interleukin-17 to Ameliorate Colorectal Colitis in Rats. PLoS ONE 2014, 9, e109146. [Google Scholar] [CrossRef] [PubMed]
- Effendi, R.M.R.A.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia Muciniphila and Faecalibacterium Prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium Butyrate Attenuates High-Fat Diet-Induced Steatohepatitis in Mice by Improving Gut Microbiota and Gastrointestinal Barrier. World J. Gastroenterol. 2017, 23, 60. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Madrazo-Clemente, P.; Martínez-Cuesta, M.d.C.; Peláez, C.; Ortiz, A.; Sánchez-Niño, M.D.; Esteban, J.; Requena, T. Lyso-Gb3 Modulates the Gut Microbiota and Decreases Butyrate Production. Sci. Rep. 2019, 9, 12010. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial Effect of Butyrate-producing Lachnospiraceae on Stress-induced Visceral Hypersensitivity in Rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Zhang, B.; Yue, R.; Chen, Y.; Huang, X.; Yang, M.; Shui, J.; Peng, Y. The Herbal Medicine Scutellaria-Coptis Alleviates Intestinal Mucosal Barrier Damage in Diabetic Rats by Inhibiting Inflammation and Modulating the Gut Microbiota. Evid. -Based Complement. Altern. Med. 2020, 2020, 4568629. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green Tea Polyphenols Decrease Weight Gain, Ameliorate Alteration of Gut Microbiota, and Mitigate Intestinal Inflammation in Canines with High-Fat-Diet-Induced Obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Zhao, Y.; Yang, X. High l-Carnitine Ingestion Impairs Liver Function by Disordering Gut Bacteria Composition in Mice. J. Agric. Food Chem. 2020, 68, 5707–5714. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low Dietary Fiber Intake Increases Collinsella Abundance in the Gut Microbiota of Overweight and Obese Pregnant Women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Li, K.; Shi, Y.; Guo, X.; Wang, L.; Li, D. Sandalwood Seed Oil Improves Insulin Sensitivity in High-Fat/High-Sucrose Diet-Fed Rats Associated with Altered Intestinal Microbiota and Its Metabolites. Food Funct. 2021, 12, 9739–9749. [Google Scholar] [CrossRef]
- Yin, X.; Peng, J.; Zhao, L.; Yu, Y.; Zhang, X.; Liu, P.; Feng, Q.; Hu, Y.; Pang, X. Structural Changes of Gut Microbiota in a Rat Non-Alcoholic Fatty Liver Disease Model Treated with a Chinese Herbal Formula. Syst. Appl. Microbiol. 2013, 36, 188–196. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef]
- Ruiz-Limón, P.; Mena-Vázquez, N.; Moreno-Indias, I.; Manrique-Arija, S.; Lisbona-Montañez, J.M.; Cano-García, L.; Tinahones, F.J.; Fernández-Nebro, A. Collinsella Is Associated with Cumulative Inflammatory Burden in an Established Rheumatoid Arthritis Cohort. Biomed. Pharmacother. 2022, 153, 113518. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Zou, Y.; Dai, Y.; Luo, G.; Zhang, X.; Xiao, L. Characterization a Novel Butyric Acid-Producing Bacterium Collinsella Aerofaciens Subsp. Shenzhenensis Subsp. Nov. Microorganisms 2019, 7, 78. [Google Scholar] [CrossRef]
- Kwon, J.; Bae, M.; Szamosvári, D.; Cassilly, C.D.; Bolze, A.S.; Jackson, D.R.; Xavier, R.J.; Clardy, J. Collinsella Aerofaciens Produces a PH-Responsive Lipid Immunogen. J. Am. Chem. Soc. 2023, 145, 7071–7074. [Google Scholar] [CrossRef] [PubMed]
- Ather, A.Q.; Tahir, M.N.; Khan, M.A.; Mehmood, K.; Chaudhry, F. 1,3-Diphenyl-1 H-Pyrazole-4-Carbaldehyde. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o3170. [Google Scholar] [CrossRef] [PubMed]
- Turkevych, N.M.; Vvedenskij, V.M.; Petlichnaya, L.P. Method of Obtaining Pseudothiohydantoin and Thiazolidinedione-2,4. Ukr. Khim. Zh. 1961, 27, 680–681, Reprinted in Chem. Abstr. 1962, 56, 73455. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. EUCAST Reading Guide for Broth Microdilution. Available online: www.eucast.org (accessed on 1 January 2022).
- Noh, A.S.M.; Chuan, T.D.; Khir, N.A.M.; Zin, A.A.M.; Ghazali, A.K.; Long, I.; Ab Aziz, C.B.; Ismail, C.A.N. Effects of Different Doses of Complete Freund’s Adjuvant on Nociceptive Behaviour and Inflammatory Parameters in Polyarthritic Rat Model Mimicking Rheumatoid Arthritis. PLoS ONE 2021, 16, e0260423. [Google Scholar] [CrossRef]
- Ilkiv, I.; Lesyk, R.; Sklyarov, O. Evaluation of Novel 4-Thiazolidinone-Based Derivatives as Possible Cytoprotective Agents Against Stress Model In Rats. J. Appl. Pharm. Sci. 2017, 7, 199–203. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Saksena, A.; Pal, R.; Jaiswal, R.; Kumar, R. Effect of Boswellia Serrata Extract on Acute Inflammatory Parameters and Tumor Necrosis Factor-α in Complete Freund’s Adjuvant-Induced Animal Model of Rheumatoid Arthritis. Int. J. Appl. Basic Med. Res. 2019, 9, 100. [Google Scholar] [CrossRef]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Smith, W.G. 1 Pharmacological Screening Tests. Prog. Med. Chem. 1961, 1, 1–33. [Google Scholar]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S RRNA Sequence Formation and Detection in Sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]


| N | Type of Species | Species of Bacteria and Fungi | Zone of Growth Inhibition (mm ± SE) and Some MIC Value (μM) | |||||
|---|---|---|---|---|---|---|---|---|
| Les-6490 | DMSO | Vanco- Mycin | Cipro- Floxacin | Clotri- MAZOLE | ||||
| 1 | Gram-negative bacteria | Reference strains | Pseudomonas aeruginosa ATCC 10145 | 00 | 7.0 ± 0.3 | - | 35.0 ± 0.3 | - |
| 2 | Raoultella terrigena ATCC 33257 | 00 | 6.5 ± 0.25 | - | 42.0 ± 0.5 | - | ||
| 3 | Clinical strains | Klebsiella pneumoniae 189 | 00 | n/a | - | 20.0 ± 0.2 | - | |
| 4 | Aeromonas hydrophila N196 | 00 | n/a | - | 27.0 ± 0.4 | - | ||
| 5 | Wild non-pathogenic probiotic strain | Limosilactobacillus fermentum | 00 | n/a | - | 43.0 ± 0.5 | - | |
| 6 | Gram-positive bacteria | Reference strains | Streptococcus agalactiae ATCC 13813 | 00 | n/a | 32.0 ± 0.5 | - | - |
| 7 | Staphylococcus aureus subsp. aureus ATCC 25923 | 15.4 ± 0.4 MIC 2880 μM | n/a | 32.0 ± 0.5 | 35.0 ± 0.5 | - | ||
| 8 | Clinical strains | Staphylococcus aureus N 23 | 00 | n/a | 11.4 ± 0.3 | 9.0 ± 0.2 | - | |
| 9 | Fungi | Reference strains | Candida. albicans (ATCC 885-653) | 20.0 ± 0.4 | n/a | - | - | 18.0 ± 0.5 |
| 10 | Clinical strains | Candida albicans N67 | 22.0 ± 0.3 | n/a | - | - | 11.0 ± 0.3 MIC 2.9 µM | |
| 11 | Saccharomyces cerevisiae N62 | 25.0 ± 0.4 MIC 820 μM | n/a | - | - | 8.0 ± 0.2 | ||
| Comp./Cell Line | Isolated Lymphocytes | HaCaT |
|---|---|---|
| Les-6490 | >100 | >100 |
| Doxorubicin | 0.95 ± 0.20 | 5.21 ± 0.86 |
| Type of Microbiota | Group | A | Observed-Delta | Expected-Delta | Significance |
|---|---|---|---|---|---|
| Fecal–fecal | A-K | 0.06746 | 0.4858 | 0.521 | 0.085 |
| Fecal–fecal | A-AL | 0.05647 | 0.4808 | 0.5095 | 0.048 |
| Fecal–fecal | AL-K | 0.05719 | 0.4668 | 0.4951 | 0.118 |
| Fecal–parietal | AL-L | 0.2289 | 0.4949 | 0.6419 | 0.034 |
| Fecal–parietal | A-L | 0.2042 | 0.5116 | 0.6428 | 0.035 |
| Fecal–parietal | K-L | 0.1988 | 0.502 | 0.6266 | 0.03 |
| Group N | Group Name | Group Description | N of Samples (n) for 16S rRNA | Type of Studied Microbiota |
|---|---|---|---|---|
| 1 | A+L | Adjuvant (A) and compound (L) | 4 | F |
| 2 | A | Adjuvant (A) | 4 | F |
| 3 | K | Control without adjuvant and compound (K) | 3 | F |
| 4 | L | Compound without adjuvant (L) | 4 | P |
| Total | 11 | F | ||
| 4 | P | |||
| 15 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konechnyi, Y.; Rumynska, T.; Yushyn, I.; Holota, S.; Turkina, V.; Ryviuk Rydel, M.; Sękowska, A.; Salyha, Y.; Korniychuk, O.; Lesyk, R. A New 4-Thiazolidinone Derivative (Les-6490) as a Gut Microbiota Modulator: Antimicrobial and Prebiotic Perspectives. Antibiotics 2024, 13, 291. https://doi.org/10.3390/antibiotics13040291
Konechnyi Y, Rumynska T, Yushyn I, Holota S, Turkina V, Ryviuk Rydel M, Sękowska A, Salyha Y, Korniychuk O, Lesyk R. A New 4-Thiazolidinone Derivative (Les-6490) as a Gut Microbiota Modulator: Antimicrobial and Prebiotic Perspectives. Antibiotics. 2024; 13(4):291. https://doi.org/10.3390/antibiotics13040291
Chicago/Turabian StyleKonechnyi, Yulian, Tetyana Rumynska, Ihor Yushyn, Serhii Holota, Vira Turkina, Mariana Ryviuk Rydel, Alicja Sękowska, Yuriy Salyha, Olena Korniychuk, and Roman Lesyk. 2024. "A New 4-Thiazolidinone Derivative (Les-6490) as a Gut Microbiota Modulator: Antimicrobial and Prebiotic Perspectives" Antibiotics 13, no. 4: 291. https://doi.org/10.3390/antibiotics13040291
APA StyleKonechnyi, Y., Rumynska, T., Yushyn, I., Holota, S., Turkina, V., Ryviuk Rydel, M., Sękowska, A., Salyha, Y., Korniychuk, O., & Lesyk, R. (2024). A New 4-Thiazolidinone Derivative (Les-6490) as a Gut Microbiota Modulator: Antimicrobial and Prebiotic Perspectives. Antibiotics, 13(4), 291. https://doi.org/10.3390/antibiotics13040291