From Polymeric Nanoformulations to Polyphenols—Strategies for Enhancing the Efficacy and Drug Delivery of Gentamicin
Abstract
:1. Introduction
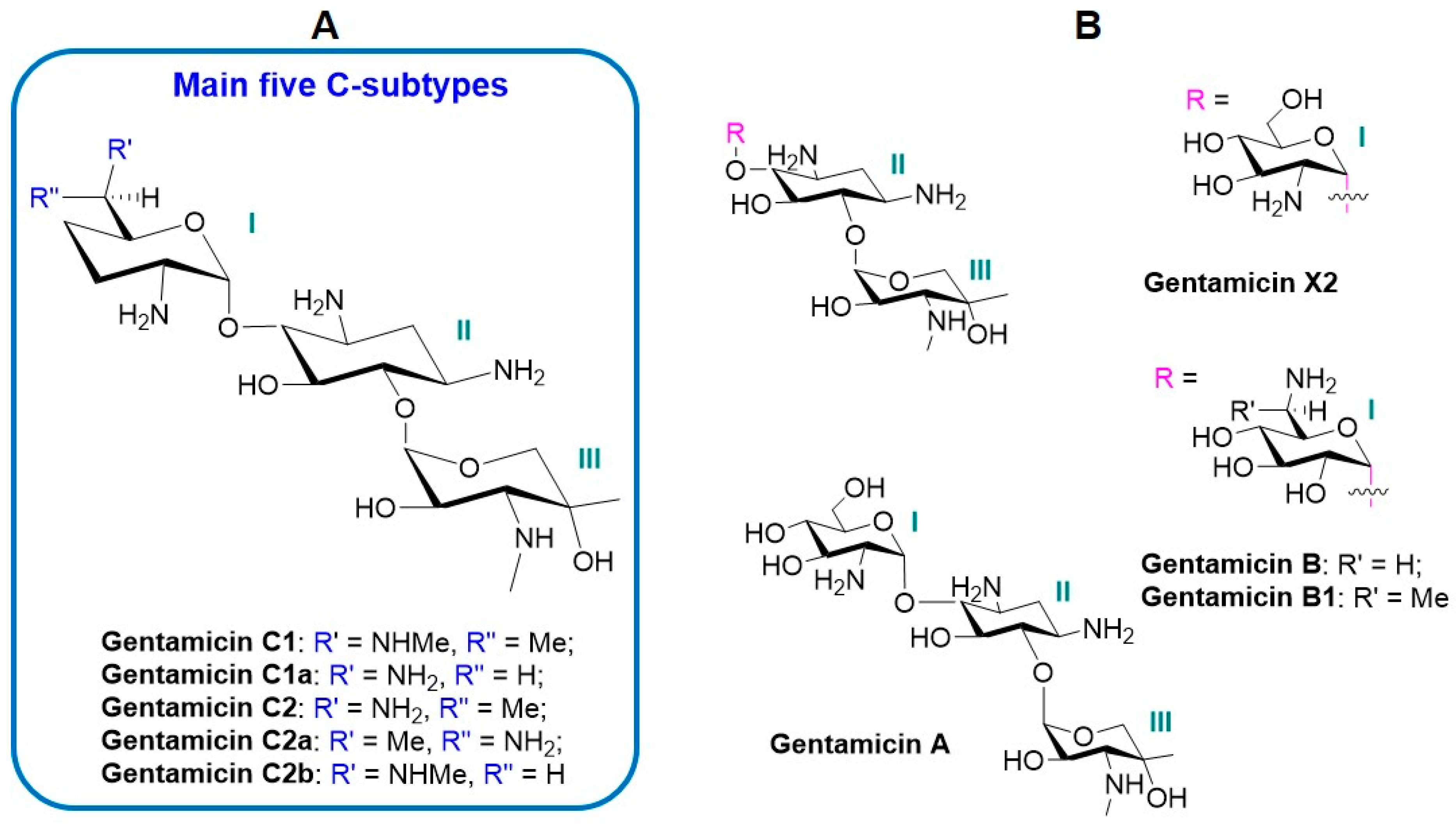
2. The Components and Properties of Gentamicin Complex
3. Pharmacology, Stability, and Administration of Gentamicin
4. Polymeric Nanoformulations of Gentamicin
4.1. Gentamicin-Loaded PLGA Nanoparticles
4.2. Gentamicin-Loaded Chitosan Nanoparticles
4.3. The Release of Gentamicin from Polymeric Nanoparticles
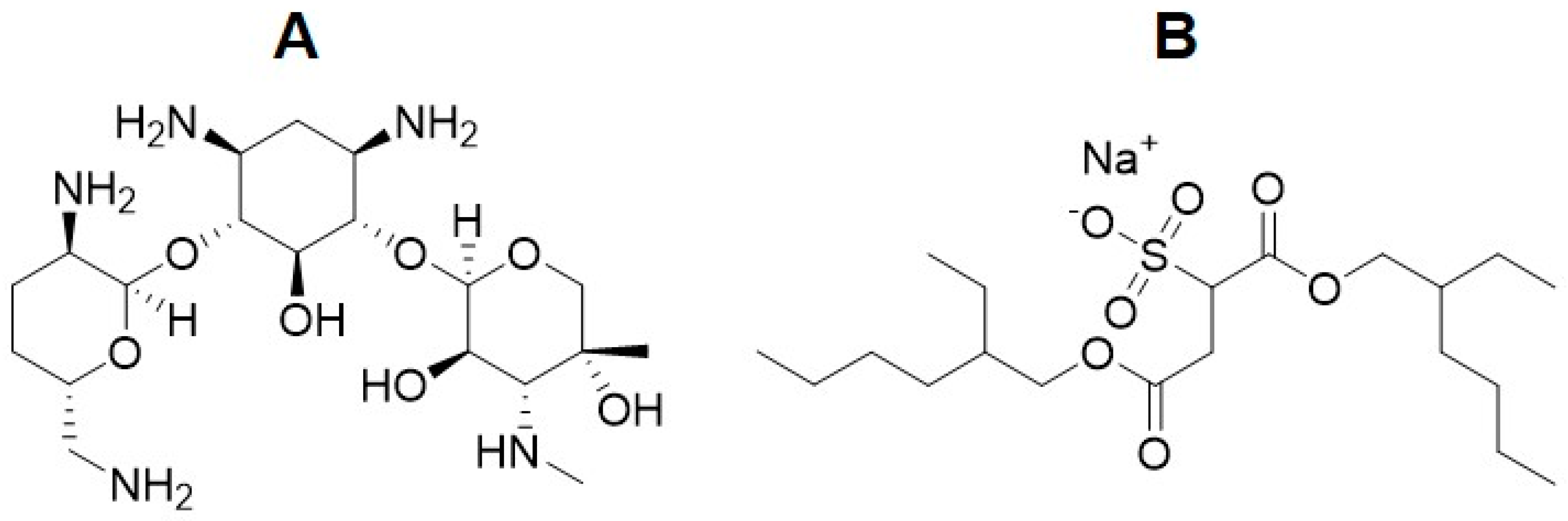
5. Hydrophobization of the Gentamicin Molecule
6. Gentamicin Combinations with Other Antibiotics
7. Gentamicin Combinations with Polyphenols
8. Gentamicin Combinations with Natural Products
| Natural Product | Bacteria | Bacterial Strain Type | Antibacterial Effect on Gentamicin | FICI | Synergy/Partial Synergy | Ref. |
|---|---|---|---|---|---|---|
| Aniba rosaeodora essential oil | B. cereus | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL | 0.30 | Synergy | [40] |
| B. subtilis | Reference | MIC reduction from 0.25 μg/mL to 0.06 μg/mL | 0.34 | Synergy | [40] | |
| S. aureus | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL and from 0.06 μg/mL to 0.01 μg/mL | 0.30 | Synergy | [40] | |
| E. coli | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL | 0.35 | Synergy | [40] | |
| A. baumannii | Reference | MIC reduction from 4.00 μg/mL to 0.24 μg/mL | 0.11 | Synergy | [40] | |
| S. marcescens | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL | 0.30 | Synergy | [40] | |
| Y. enterocolitica | Reference | MIC reduction from 0.25 μg/mL to 0.01 μg/mL | 0.11 | Synergy | [40] | |
| Clinopodium vulgare L. extracts | B. subtilis | Clinical isolates | Reduction in MIC | 0.395–0.44 | Synergy | [164] |
| Daphne genkwa extract | S. aureus (methicillin-resistant) | Reference | Reduction in MIC | 0.750 | Partial synergy | [165] |
| Magnolia officinalis extract | S. aureus (methicillin-resistant) | Reference | Reduction in MIC | 0.750 | Partial synergy | [165] |
| Kaempferia parviflora extracts | K. pneumoniae | Clinical isolates | Reduction in MIC | 0.141–0.625 | Synergy/Partial synergy | [162] |
| P. aeruginosa | Clinical isolates | Reduction in MIC | 0.133–0.625 | Synergy/Partial synergy | [162] | |
| A. baumannii | Clinical isolates | Reduction in MIC | 0.133–0.563 | Synergy/Partial synergy | [162] | |
| Mentha piperita L. essential oil | B. cereus | Reference | MIC reduction from 2.00 μg/mL to 0.06 μg/mL | 0.08 | Synergy | [163] |
| B. subtilis | Reference | MIC reduction from 0.50 μg/mL to 0.01 μg/mL | 0.07 | Synergy | [163] | |
| S. aureus | Reference | MIC reduction from 2.0 μg/mL to 0.06 μg/mL; from 0.5 μg/mL to 0.06 μg/mL; from 8.0 μg/mL to 2.0 μg/mL | 0.103–0.3 | Synergy | [163] | |
| E. faecalis | Reference | MIC reduction from 8.0 μg/mL to 1.0 μg/mL | 0.32 | Synergy | [163] | |
| E. coli | Reference | MIC reduction from 1.0 μg/mL to 0.03 μg/mL | 0.43 | Synergy | [163] | |
| K. pneumoniae | Reference | MIC reduction from 32.0 μg/mL to 1.0 μg/mL | 0.43 | Synergy | [163] | |
| A. baumannii | Reference | MIC reduction from 8.00 μg/mL to 0.5 μg/mL | 0.46 | Synergy | [163] | |
| P. aeruginosa | Reference | MIC reduction from 2.00 μg/mL to 0.06 μg/mL | 0.08 | Synergy | [163] | |
| Pelargonium graveolens essential oil | B. cereus | Reference | MIC reduction from 0.50 μg/mL to 0.125 μg/mL | 0.30 | Synergy | [40] |
| B. subtilis | Reference | MIC reduction from 0.25 μg/mL to 0.06 μg/mL | 0.34 | Synergy | [40] | |
| S. aureus | Reference | MIC reduction from 0.50 μg/mL to 0.01 μg/mL and from 0.12 to 0.04 μg/mL | 0.28–0.35 | Synergy | [40] | |
| E. coli | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL | 0.30 | Synergy | [40] | |
| A. baumannii | Reference | MIC reduction from 4.00 μg/mL to 0.24 μg/mL | 0.11 | Synergy | [40] | |
| S. marcescens | Reference | MIC reduction from 0.50 μg/mL to 0.12 μg/mL | 0.45 | Synergy | [40] | |
| Y. enterocolitica | Reference | MIC reduction from 0.25 μg/mL to 0.03 μg/mL | 0.22 | Synergy | [40] | |
| Propolis | B. subtilis | Reference | MIC reduction from 1.25 μg/mL to 0.05 μg/mL | NA | Synergy | [161] |
| B. cereus | Reference | MIC reduction from 1.25 μg/mL to 0.05 μg/mL | NA | Synergy | [161] | |
| B. megaterium | Reference | MIC reduction from 0.25 μg/mL to 0.01 μg/mL | NA | Synergy | [161] | |
| S. aureus (methicillin sensitive) | Reference | MIC reduction from 1.5 μg/mL to 0.5 μg/mL | NA | Synergy | [161] | |
| S. aureus (methicillin-resistant) | Reference | MIC reduction from >1.5 μg/mL to 0.5 μg/mL | NA | Synergy | [161] | |
| E. coli | Reference | MIC reduction from >1.5 μg/mL to 0.5 μg/mL | NA | Synergy | [161] |
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.W.; Lau, Y.Y.; Krishnan, T.; Chan, K.G.; Chang, C.Y. Recent Advances in Molecular Diagnosis of Pseudomonas aeruginosa Infection by State-of-the-Art Genotyping Techniques. Front. Microbiol. 2018, 9, 1104. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential synergistic activity of quercetin with antibiotics against multidrug-resistant clinical strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.G.; dos Santos, H.S.; Bandeira, P.N.; Rodrigues, T.H.S.; Matos, M.G.C.; Nascimento, M.F.; de Carvalho, G.G.C.; Braz-Filho, R.; Teixeira, A.M.R.; Tintino, S.R.; et al. Evaluation of antibacterial and enhancement of antibiotic action by the flavonoid kaempferol 7-O-β-D-(6″-O-cumaroyl)-glucopyranoside isolated from Croton piauhiensis müll. Microb. Pathog. 2020, 143, 104144. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Wu, P.; Chen, B. Update on new medicinal applications of gentamicin: Evidence-based review. J. Formos. Med. Assoc. 2014, 113, 72–82. [Google Scholar] [CrossRef]
- Alapi, M.E.; Fisher, J. Analogue-Based Drug Discovery; Fischer, J., Ganellin, C.R., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany; KgaA: Weinheim, Germany, 2006; p. 507. ISBN 9783527607495. [Google Scholar]
- WHO Electronic Essential Medicines List. Gentamicin. Model List of Essential Medicines. Available online: https://list.essentialmeds.org/medicines/229 (accessed on 19 February 2024).
- Gentamicin sulfate. In Merative Micromedex® DRUGDEX® (Electronic Version); Merative Healthcare Solutions/EBSCO Information Services: Greenwood Village, CO, USA; Cambridge, MA, USA; Available online: https://www.dynamed.com/drug-monograph/gentamicin-sulfate (accessed on 16 March 2024).
- Elsevier Drug Information. Drug Monograph. Gentamicin. In ClinicalKey; Elsevier: Amsterdam, The Netherlands, 2024; Available online: https://www.clinicalkey.com/#!/content/drug_monograph/6-s2.0-275 (accessed on 16 March 2024).
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gentamicin Sulfate PEG-PLGA/PLGA-H Nanoparticles: Screening Design and Antimicrobial Effect Evaluation toward Clinic Bacterial Isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin, a new antibiotic complex from micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Isoherranen, N.; Lavy, E.; Soback, S. Pharmacokinetics of gentamicin C1, C1a, and C2 in beagles after a single intravenous dose. Antimicrob. Agents Chemother. 2000, 44, 1443–1447. [Google Scholar] [CrossRef]
- Athauda, I.D.; Shetty, M.G.; Pai, P.; Hegde, M.; Gurumurthy, S.C.; Babitha, K.S. Enhanced Bactericidal Effects and Drug Delivery with Gentamicin-Conjugated Nanoparticles. J. Clust. Sci. 2023, 35, 371–390. [Google Scholar] [CrossRef]
- Deubner, R.; Schollmayer, C.; Wienen, F.; Holzgrabe, U. Assignment of the major and minor components of gentamicin for evaluation of batches. Magn. Reson. Chem. 2003, 41, 589–598. [Google Scholar] [CrossRef]
- Friesen, W.J.; Johnson, B.; Sierra, J.; Zhuo, J.; Vazirani, P.; Xue, X.; Tomizawa, Y.; Baiazitov, R.; Morrill, C.; Ren, H.; et al. The minor gentamicin complex component, X2, is a potent premature stop codon readthrough molecule with therapeutic potential. PLoS ONE 2018, 13, e0206158. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.E.; Song, Y.; Greenhouse, R.; Lin, R.; Perez, A.; Atkinson, P.J.; MacDonald, J.P.; Siddiqui, Z.; Lagasca, D.; Comstock, K.; et al. Dissociating antibacterial from ototoxic effects of gentamicin C-subtypes. Proc. Natl. Acad. Sci. USA 2020, 117, 32423–32432. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.; Parkin, J.; Adelanwa, A.; Cheneke, B.; Movileanu, L.; Khalid, S.; van den Berg, B. Toward understanding the outer membrane uptake of small molecules by Pseudomonas aeruginosa. J. Biol. Chem. 2013, 288, 12042–12053. [Google Scholar] [CrossRef] [PubMed]
- Claes, P.J.; Busson, R.; Vanderhaeghe, H. Determination of the component ratio of commercial gentamicins by high-performance liquid chromatography using pre-column derivatization. J. Chromatogr. A 1984, 298, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Kohlhepp, S.J.; Loveless, M.O.; Kohnen, P.W.; Houghton, D.C.; Bennett, W.M.; Gilbert, D.N. Nephrotoxicity of the Constituents of the Gentamicin Complex. J. Infect. Dis. 1984, 149, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Rajasekaran, P.; Haldimann, K.; Vasella, A.; Böttger, E.C.; Hobbie, S.N.; Crich, D. Synthesis of Gentamicins C1, C2, and C2a and Antiribosomal and Antibacterial Activity of Gentamicins B1, C1, C1a, C2, C2a, C2b, and X2. ACS Infect. Dis. 2023, 9, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ni, X.; Ren, J.; Gao, H.; Wang, D.; Xia, H. Biosynthesis of Epimers C2 and C2a in the Gentamicin C Complex. ChemBioChem 2015, 16, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, R.M.; Reilly, J.P.; Running, W.; Campos, S.B.; Santos, J.R.; Phillips, C.L.; Molitoris, B.A. A Non-Nephrotoxic Gentamicin Congener That Retains Antimicrobial Efficacy. J. Am. Soc. Nephrol. 2006, 17, 2697–2705. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sone, M.; Umemura, M.; Nabeshima, T.; Nakashima, T.; Hellström, S. Comparisons of cochleotoxicity among three gentamicin compounds following intratympanic application. Acta Otolaryngol. 2008, 128, 245–249. [Google Scholar] [CrossRef]
- Ishikawa, M.; García-Mateo, N.; Čusak, A.; López-Hernández, I.; Fernández-Martínez, M.; Müller, M.; Rüttiger, L.; Singer, W.; Löwenheim, H.; Kosec, G.; et al. Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Sci. Rep. 2019, 9, 2410. [Google Scholar] [CrossRef]
- Recht, M.I.; Puglisi, J.D. Aminoglycoside resistance with homogeneous and heterogeneous populations of antibiotic-resistant ribosomes. Antimicrob. Agents Chemother. 2001, 45, 2414–2419. [Google Scholar] [CrossRef]
- Rosenkrantz, B.E.; Greco, J.R.; Hoogerheide, J.G.; Oden, E.M. Gentamicin Sulfate. In Analytical Profiles of Drug Substances; Florey, K., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 9, pp. 295–340. [Google Scholar] [CrossRef]
- Tangy, F.; Moukkadem, M.; Vindimian, E.; Capmau, M.L.; Le Goffic, F. Mechanism of action of gentamicin components. Characteristics of their binding to Escherichia coli ribosomes. Eur. J. Biochem. 1985, 147, 381–386. [Google Scholar] [CrossRef]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream Infections and Infective Endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef]
- Prior, S.; Gamazo, C.; Irache, J.M.; Merkle, H.P.; Gander, B. Gentamicin encapsulation in PLA/PLGA microspheres in view of treating Brucella infections. Int. J. Pharm. 2000, 196, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Duran, S.; Anwar, J.; Moin, S.T. Interaction of gentamicin and gentamicin-AOT with poly-(lactide-co-glycolate) in a drug delivery system—Density functional theory calculations and molecular dynamics simulation. Biophys. Chem. 2023, 294, 106958. [Google Scholar] [CrossRef] [PubMed]
- Crcek, M.; Zdovc, J.; Kerec Kos, M. A review of population pharmacokinetic models of gentamicin in paediatric patients. J. Clin. Pharm. Ther. 2019, 44, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Optimizing aminoglycoside use. Crit. Care Clin. 2011, 27, 107–121. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Chiesa, E.; Genta, I.; Modena, T.; Bruni, G.; Grisoli, P.; Conti, B. Release Profile of Gentamicin Sulfate from Polylactide-co-Polycaprolactone Electrospun Nanofiber Matrices. Pharmaceutics 2019, 11, 161. [Google Scholar] [CrossRef]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; van den Broek, A.K.; de Vroom, S.L.; Prins, J.M.; Mathôt, R.A.A.; van Hest, R.M. Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Gentamicin. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–6. [Google Scholar] [CrossRef]
- Bailey, D.N.; Briggs, J.R. Gentamicin and Tobramycin Binding to Human Serum In Vitro. J. Anal. Toxicol. 2004, 28, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Drevets, D.A.; Canono, B.P.; Leenen, P.J.; Campbell, P.A. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 1994, 62, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Piarulli, M.; Corbo, F.; Muraglia, M.; Carone, A.; Vitali, M.E.; Vitali, C. In vitro synergistic antibacterial action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 2010, 17, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wan, P.; Li, P.; Wang, J.; Guo, S.; Zhang, Y.; An, Y.; Ye, C.; Liu, Z.; Gao, J.; et al. Mechanism and Prevention of Ototoxicity Induced by Aminoglycosides. Front. Cell Neurosci. 2021, 15, 692762. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Genta, I.; Grisoli, P.; Merelli, A.; Tomasi, C.; Conti, B. An experimental design approach to the preparation of pegylated polylactide-co-glicolide gentamicin loaded microparticles for local antibiotic delivery. Mater. Sci. Eng. C 2016, 58, 909–917. [Google Scholar] [CrossRef]
- Abbasi, M.Y.; Chaijamorn, W.; Wiwattanawongsa, K.; Charoensareerat, T.; Doungngern, T. Recommendations of Gentamicin Dose Based on Different Pharmacokinetic/Pharmacodynamic Targets for Intensive Care Adult Patients: A Redefining Approach. Clin. Pharmacol. 2023, 15, 67–76. [Google Scholar] [CrossRef]
- Cepec, E.; Trček, J. Antimicrobial Resistance of Acetobacter and Komagataeibacter Species Originating from Vinegars. Int. J. Environ. Res. Public. Health 2022, 19, 463. [Google Scholar] [CrossRef]
- Li, P.K.T.; Szeto, C.C.; Piraino, B.; de Arteaga, J.; Fan, S.; Figueiredo, A.E.; Fish, D.N.; Goffin, E.; Kim, Y.L.; Salzer, W.; et al. ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit. Dial. Int. 2016, 36, 481–508. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; de Waal, A.; Beumer, J.; Dutta, D.; Heo, I.; et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 2021, 16, 4633–4649. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef] [PubMed]
- Alhariri, M.; Majrashi, M.A.; Bahkali, A.H.; Almajed, F.S.; Azghani, A.O.; Khiyami, M.A.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M.A. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, 12, 6949–6961. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Joly, H.; Omri, A. Liposomes as a carrier for gentamicin delivery: Development and evaluation of the physicochemical properties. Int. J. Pharm. 2008, 359, 254–263. [Google Scholar] [CrossRef]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time–kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; Ge, Y.; Gasik, M.; Nordström, K.; Natri, O.; Hannula, S.-P. Silica-Gentamicin Nanohybrids: Synthesis and Antimicrobial Action. Materials 2016, 9, 170. [Google Scholar] [CrossRef]
- Perni, S.; Martini-Gilching, K.; Prokopovich, P. Controlling release kinetics of gentamicin from silica nano-carriers. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 541, 212–221. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Nichita, C.; Bălan, A.; Rădiţoiu, A.; Căprărescu, S.; Raduly, F.M.; Manea, R.; Şomoghi, R.; Nicolae, C.A.; et al. Preparation and Characterization of Silica Nanoparticles and of Silica-Gentamicin Nanostructured Solution Obtained by Microwave-Assisted Synthesis. Materials 2021, 14, 2086. [Google Scholar] [CrossRef]
- Katva, S.; Das, S.; Moti, H.S.; Jyoti, A.; Kaushik, S. Antibacterial Synergy of Silver Nanoparticles with Gentamicin and Chloramphenicol against Enterococcus faecalis. Pharmacogn. Mag. 2018, 13, S828–S833. [Google Scholar] [CrossRef] [PubMed]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef]
- Feizi, S.; Cooksley, C.M.; Nepal, R.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Silver nanoparticles as a bioadjuvant of antibiotics against biofilm-mediated infections with methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in chronic rhinosinusitis patients. Pathology 2022, 54, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Aguiar, S.; Bettencourt, A.; Gaspar, M.M. Lipid-based nanosystems for targeting bone implant-associated infections: Current approaches and future endeavors. Drug Deliv. Transl. Res. 2021, 11, 72–85. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Lecaroz, C.; Gamazo, C.; Renedo, M.J.; Blanco-Prieto, M.J. Biodegradable micro- and nanoparticles as long-term delivery vehicles for gentamicin. J. Microencapsul. 2006, 23, 782–792. [Google Scholar] [CrossRef]
- Lecaroz, M.C.; Blanco-Prieto, M.J.; Campanero, M.A.; Salman, H.; Gamazo, C. Poly(D,L-lactide-coglycolide) particles containing gentamicin: Pharmacokinetics and pharmacodynamics in Brucella melitensis-infected mice. Antimicrob. Agents Chemother. 2007, 51, 1185–1190. [Google Scholar] [CrossRef]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Włoch, M.; Pamuła, E. Gentamicin loaded PLGA nanoparticles as local drug delivery system for the osteomyelitis treatment. Acta Bioeng. Biomech. 2015, 17, 41–48. [Google Scholar] [CrossRef]
- Dhal, C.; Mishra, R. Formulation development and in vitro evaluation of gentamicin sulfate-loaded PLGA nanoparticles based film for the treatment of surgical site infection by Box–Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Greene, M.K.; Insua, J.L.; Pessoa, J.S.; Small, D.M.; Smyth, P.; McCann, A.P.; Cogo, F.; Bengoechea, J.A.; Taggart, C.C.; et al. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J. Control. Release 2018, 279, 316–325. [Google Scholar] [CrossRef]
- Sun, Y.; Bhattacharjee, A.; Reynolds, M.; Li, Y.V. Synthesis and characterizations of gentamicin-loaded poly-lactic-co-glycolic (PLGA) nanoparticles. J. Nanoparticle Res. 2021, 23, 155. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert. Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Cao, X.; Dai, L.; Sun, S.; Ma, R.; Liu, X. Preparation and performance of porous hydroxyapatite/poly(lactic-co-glycolic acid) drug-loaded microsphere scaffolds for gentamicin sulfate delivery. J. Mater. Sci. 2021, 56, 15278–15298. [Google Scholar] [CrossRef]
- Sivaraman, B.; Ramamurthi, A. Multifunctional nanoparticles for doxycycline delivery towards localized elastic matrix stabilization and regenerative repair. Acta Biomater. 2013, 9, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Razei, A.; Cheraghali, A.M.; Saadati, M.; Fasihi Ramandi, M.; Panahi, Y.; Hajizade, A.; Siadat, S.D.; Behrouzi, A. Gentamicin-Loaded Chitosan Nanoparticles Improve Its Therapeutic Effects on Brucella-Infected J774A.1 Murine Cells. Galen. Med. J. 2019, 8, e1296. [Google Scholar] [CrossRef]
- Shakya, A.K.; Al-Sulaibi, M.; Naik, R.R.; Nsairat, H.; Suboh, S.; Abulaila, A. Review on PLGA Polymer Based Nanoparticles with Antimicrobial Properties and Their Application in Various Medical Conditions or Infections. Polymers 2023, 15, 3597. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Zhou, W.-Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly(DL-lactide-coglycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, A.M.; Brugnano, J.L.; Seal, B.L.; Knight, F.C.; Panitch, A. Synthesis and characterization of a poly (lactic-co-glycolic acid) core + poly (N-isopropylacrylamide) shell nanoparticle system. Biomatter 2012, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Bandyopadhyay, S.; Das, P.; Samanta, I.; Mukherjee, P.; Roy, S.; Kundu, B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv. 2016, 34, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Worku, S.; Abebe, T.; Alemu, A.; Seyoum, B.; Swedberg, G.; Abdissa, A.; Mihret, A.; Beyene, G.T. Bacterial profile of surgical site infection and antimicrobial resistance patterns in Ethiopia: A multicentre prospective cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 96. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liang, A. Current research progress of local drug delivery systems based on biodegradable polymers in treating chronic osteomyelitis. Front. Bioeng. Biotechnol. 2022, 10, 1042128. [Google Scholar] [CrossRef]
- Flores, C.; Degoutin, S.; Chai, F.; Raoul, G.; Hornez, J.-C.; Martel, B.; Siepmann, J.; Ferri, J.; Blanchemain, N. Gentamicin-loaded poly(lactic-co-glycolic acid) microparticles for the prevention of maxillofacial and orthopedic implant infections. Mater. Sci. Engineering C 2016, 64, 108–116. [Google Scholar] [CrossRef]
- Akhtar, B.; Muhammad, F.; Aslam, B.; Saleemi, M.K.; Sharif, A. Biodegradable nanoparticle based transdermal patches for gentamicin delivery: Formulation, characterization and pharmacokinetics in rabbits. J. Drug Deliv. Sci. Technol. 2020, 57, 101680. [Google Scholar] [CrossRef]
- Dhal, C.; Mishra, R. In vitro and in vivo evaluation of gentamicin sulphate-loaded PLGA nanoparticle-based film for the treatment of surgical site infection. Drug Deliv. Transl. Res. 2020, 10, 1032–1043. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Lu, E.; Franzblau, S.; Onyuksel, H.; Popescu, C. Preparation of aminoglycoside-loaded chitosan nanoparticles using dextran sulphate as a counterion. J. Microencapsul. 2009, 26, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Hao, S.; Wu, D.; Huang, R.; Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial proanthocyanidin-chitosan composite nanoparticles loaded with gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508. [Google Scholar] [CrossRef]
- Asgarirad, H.; Ebrahimnejad, P.; Mahjoub, M.A.; Jalalian, M.; Morad, H.; Ataee, R.; Hosseini, S.S.; Farmoudeh, A. A promising technology for wound healing; in-vitro and in-vivo evaluation of chitosan nano-biocomposite films containing gentamicin. J. Microencapsul. 2021, 38, 100–107. [Google Scholar] [CrossRef]
- Abdel-Hakeem, M.A.; Abdel Maksoud, A.I.; Aladhadh, M.A.; Almuryif, K.A.; Elsanhoty, R.M.; Elebeedy, D. Gentamicin-Ascorbic Acid Encapsulated in Chitosan Nanoparticles Improved In Vitro Antimicrobial Activity and Minimized Cytotoxicity. Antibiotics 2022, 11, 1530. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M. Chitosan nanoparticles: Polyphosphates cross-linking and protein delivery properties. Int. J. Biol. Macromol. 2019, 136, 133–142. [Google Scholar] [CrossRef]
- Pan, C.; Qian, J.; Zhao, C.; Yang, H.; Zhao, X.; Guo, H. Study on the relationship between crosslinking degree and properties of TPP crosslinked chitosan nanoparticles. Carbohydr. Polym. 2020, 241, 116349. [Google Scholar] [CrossRef] [PubMed]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, D.; Sui, G.; Wang, D.; Wu, M.; Han, L.; Mu, H.; Duan, J. Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria. Int. J. Biol. Macromol. 2020, 156, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In Vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The Future of Drug Delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) acid-controlled-release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Chavan, Y.R.; Tambe, S.M.; Jain, D.D.; Khairnar, S.V.; Amin, P.D. Redefining the importance of polylactide-co-glycolide acid (PLGA) in drug delivery. Ann. Pharm. Fr. 2022, 80, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.T.T.; Mariatti, M.; Badrul, H.Y.; Masakazu, K.; Nguyen, X.T.T.; Zuratul, A.A.H. Drug Release Profile Study of Gentamicin Encapsulated Poly (lactic Acid) Microspheres for Drug Delivery. Mater. Today Proc. 2019, 17, 836–845. [Google Scholar] [CrossRef]
- Ivković, B.; Milutinović, I.; Čudina, O.; Marković, B. A new simple liquid chromatographic assay for gentamicin in presence of methylparaben and propylparaben. Acta Chromatogr. 2023, 35, 81–87. [Google Scholar] [CrossRef]
- Ismail, A.F.H.; Mohamed, F.; Rosli, L.M.M.; Shafri, M.A.M.; Haris, M.S.; Adina, A.B. Spectrophotometric Determination of Gentamicin Loaded PLGA Microparticles and Method Validation via Ninhydrin-Gentamicin Complex as a Rapid Quantification Approach. J. Appl. Pharm. Sci. 2016, 6, 007–014. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 304757. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to Assess in Vitro Drug Release from Injectable Polymeric Particulate Systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef]
- Gosau, M.; Müller, B.W. Release of gentamicin sulphate from biodegradable PLGA-implants produced by hot melt extrusion. Pharmazie 2010, 65, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Imbuluzqueta, E.; Gamazo, C.; Lana, H.; Campanero, M.; Salas, D.; Gil, A.G.; Elizondo, E.; Ventosa, N.; Veciana, J.; Blanco-Prieto, M.J. Hydrophobic gentamicin-loaded nanoparticles are effective against Brucella melitensis infection in mice. Antimicrob. Agents Chemother. 2013, 57, 3326–3333. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, K.; Brzychczy-Włoch, M.; Pamuła, E. Antibiotics modified by hydrophobic ion-pairing—A solution world’s problems with resistant bacteria? Sustain. Mater. Technol. 2023, 37, e00662. [Google Scholar] [CrossRef]
- Imbuluzqueta, E.; Elizondo, E.; Gamazo, C.; Moreno-Calvo, E.; Veciana, J.; Ventosa, N.; Blanco-Prieto, M.J. Novel bioactive hydrophobic gentamicin carriers for the treatment of intracellular bacterial infections. Acta Biomater. 2011, 7, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Imbuluzqueta, E.; Lemaire, S.; Gamazo, C.; Elizondo, E.; Ventosa, N.; Veciana, J.; Van Bambeke, F.; Blanco-Prieto, M.J. Cellular pharmacokinetics and intracellular activity against Listeria monocytogenes and Staphylococcus aureus of chemically modified and nanoencapsulated gentamicin. J. Antimicrob. Chemother. 2012, 67, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Pudełko, I.; Moskwik, A.; Kwiecień, K.; Kriegseis, S.; Krok-Borkowicz, M.; Schickle, K.; Ochońska, D.; Dobrzyński, P.; Brzychczy-Włoch, M.; Gonzalez-Julian, J.; et al. Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. Int. J. Mol. Sci. 2023, 24, 8400. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, K.; Pudełko, I.; Knap, K.; Reczyńska-Kolman, K.; Krok-Borkowicz, M.; Ochońska, D.; Brzychczy-Włoch, M.; Pamuła, E. Insight in Superiority of the Hydrophobized Gentamycin in Terms of Antibiotics Delivery to Bone Tissue. Int. J. Mol. Sci. 2022, 23, 12077. [Google Scholar] [CrossRef]
- Rotman, S.G.; Thompson, K.; Grijpma, D.W.; Richards, R.G.; Moriarty, T.F.; Eglin, D.; Guillaume, O. Development of bone seeker–functionalised microspheres as a targeted local antibiotic delivery system for bone infections. J. Orthop. Translat 2020, 21, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.G.; Moriarty, T.F.; Nottelet, B.; Grijpma, D.W.; Eglin, D.; Guillaume, O. Poly(Aspartic Acid) Functionalized Poly(ϵ-Caprolactone) Microspheres with Enhanced Hydroxyapatite Affinity as Bone Targeting Antibiotic Carriers. Pharmaceutics 2020, 12, 885. [Google Scholar] [CrossRef]
- Elizondo, E.; Sala, S.; Imbuluzqueta, E.; González, D.; Blanco-Prieto, M.J.; Gamazo, C.; Ventosa, N.; Veciana, J. High loading of gentamicin in bioadhesive PVM/MA nanostructured microparticles using compressed carbon-dioxide. Pharm. Res. 2011, 28, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Sande, M.A.; Courtney, K.B. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis. J. Lab. Clin. Med. 1976, 88, 118–124. [Google Scholar] [PubMed]
- Andriole, V.T. Antibiotic synergy in experimental infection with Pseudomonas. II. The effect of carbenicillin, cephalothin, or cephanone combined with tobramycin or gentamicin. J. Infect. Dis. 1974, 129, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.; Fekety, F.R., Jr. Experimental Endocarditis Due to Pseudomonas aeruginosa. II. Therapy with Carbenicillin and Gentamicin. J. Infect. Dis. 1977, 136, 327–335. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Treatment of Neisseria Gonorrhoeae. Available online: https://www.who.int/publications/i/item/9789241549691 (accessed on 19 February 2024).
- Kirkcaldy, R.D.; Weinstock, H.S.; Moore, P.C.; Philip, S.S.; Wiesenfeld, H.C.; Papp, J.R.; Kerndt, P.R.; Johnson, S.; Ghanem, K.G.; Hook, E.W., III; et al. The Efficacy and Safety of Gentamicin Plus Azithromycin and Gemifloxacin Plus Azithromycin as Treatment of Uncomplicated Gonorrhea. Clin. Infect. Dis. 2014, 59, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, Y.; Zhou, J.; Long, Y.; Liu, C.; Xia, B.; Shi, J.; Fan, Z.; Liang, Y.; Chen, S.; et al. Combination of Azithromycin and Gentamicin for Efficient Treatment of Pseudomonas aeruginosa Infections. J. Infect. Dis. 2019, 220, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Svedholm, E.; Bruce, B.; Parcell, B.J.; Coote, P.J. Repurposing Mitomycin C in Combination with Pentamidine or Gentamicin to Treat Infections with Multi-Drug-Resistant (MDR) Pseudomonas aeruginosa. Antibiotics 2024, 13, 177. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin against Escherichia coli and Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef]
- Oliva, A.; Tafin, U.F.; Maiolo, E.M.; Jeddari, S.; Bétrisey, B.; Trampuz, A. Activities of Fosfomycin and Rifampin on Planktonic and Adherent Enterococcus faecalis Strains in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2014, 58, 1284–1293. [Google Scholar] [CrossRef]
- Luther, M.K.; Arvanitis, M.; Mylonakis, E.; LaPlante, K.L. Activity of Daptomycin or Linezolid in Combination with Rifampin or Gentamicin against Biofilm-Forming Enterococcus faecalis or E. faecium in an In Vitro Pharmacodynamic Model Using Simulated Endocardial Vegetations and an In Vivo Survival Assay Using Galleria mellonella Larvae. Antimicrob. Agents Chemother. 2014, 58, 4612–4620. [Google Scholar] [CrossRef]
- Usman, M.; Marcus, A.; Fatima, A.; Aslam, B.; Zaid, M.; Khattak, M.; Bashir, S.; Masood, S.; Rafaque, Z.; Dasti, J.I. Synergistic Effects of Gentamicin, Cefepime, and Ciprofloxacin on Biofilm of Pseudomonas aeruginosa. Infect. Drug Resist. 2023, 16, 5887–5898. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hidalgo, N.; Almirante, B.; Gavaldà, J.; Gurgui, M.; Peña, C.; de Alarcón, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; et al. Ampicillin Plus Ceftriaxone Is as Effective as Ampicillin Plus Gentamicin for Treating Enterococcus faecalis Infective Endocarditis. Clin. Infect. Dis. 2013, 56, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Pericas, J.M.; Cervera, C.; del Rio, A.; Moreno, A.; Garcia de la Maria, C.; Almela, M.; Falces, C.; Ninot, S.; Castañeda, X.; Armero, Y.; et al. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: From ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin. Microbiol. Infect. 2014, 20, O1075–O1083. [Google Scholar] [CrossRef] [PubMed]
- Bustos, P.S.; Deza-Ponzio, R.; Páez, P.L.; Cabrera, J.L.; Virgolini, M.B.; Ortega, M.G. Flavonoids as protective agents against oxidative stress induced by gentamicin in systemic circulation. Potent protective activity and microbial synergism of luteolin. Food Chem. Toxicol. 2018, 118, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kang, O.H.; Choi, J.G.; Oh, Y.C.; Chae, H.S.; Kim, J.H.; Park, H.; Sohn, D.H.; Wang, Z.T.; Kwon, D.Y. Synergistic effects of the combination of galangin with gentamicin against methicillin-resistant Staphylococcus aureus. J. Microbiol. 2008, 46, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Macedo, I.; Silva, J.; Silva, P.; Cruz, B.; Vale, J.; Santos, H.; Bandeira, P.; Souza, E.; Xavier, R.; Coutinho, H.; et al. Structural and Microbiological Characterization of 5-Hydroxy-3,7,4′-Trimethoxyflavone: A Flavonoid Isolated from Vitex gardneriana Schauer Leaves. Microb. Drug Resist. 2019, 25, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Oakes, E.; Soren, O.; Moussa, C.; Rathor, G.; Liu, Y.; Coates, A.; Hu, Y. Nordihydroguaiaretic acid enhances the activities of aminoglycosides against methicillin- sensitive and resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2015, 6, 1195. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Kang, X.; Luo, X.; Li, C.; Wang, G. Study on the inhibitory effect of quercetin combined with gentamicin on the formation of Pseudomonas aeruginosa and its bioenvelope. Microb. Pathog. 2023, 182, 106274. [Google Scholar] [CrossRef]
- Elssaig, E.; Alnour, T.; Ahmed, E.; Ullah, M.; Abu-Duhier, F. Antimicrobial synergistic effects of dietary flavonoids rutin and quercetin in combination with antibiotics gentamicin and ceftriaxone against E. coli (MDR) and P. mirabilis (XDR) strains isolated from human infections: Implications for food–medicine interactions. Ital. J. Food Sci. 2022, 34, 34–42. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, A.; Sandhu, P.; Daware, A.; Das, M.C.; Akhter, Y.; Bhattacharjee, S. Potentiation of antibiotic against Pseudomonas aeruginosa biofilm: A study with plumbagin and gentamicin. J. Appl. Microbiol. 2017, 123, 246–261. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined effect of a natural flavonoid rutin from Citrus sinensis and conventional antibiotic gentamicin on Pseudomonas aeruginosa biofilm formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Mun, S.H.; Kang, O.H.; Joung, D.K.; Kim, S.B.; Seo, Y.S.; Choi, J.G.; Lee, Y.S.; Cha, S.W.; Ahn, Y.S.; Han, S.H.; et al. Combination Therapy of Sophoraflavanone B against MRSA: In Vitro Synergy Testing. Evid. Based Complement. Alternat Med. 2013, 2013, 823794. [Google Scholar] [CrossRef]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef]
- Bustos, P.S.; Deza-Ponzio, R.; Páez, P.L.; Albesa, I.; Cabrera, J.L.; Virgolini, M.B.; Ortega, M.G. Protective effect of quercetin in gentamicin-induced oxidative stress in vitro and in vivo in blood cells. Effect on gentamicin antimicrobial activity. Environ. Toxicol. Pharmacol. 2016, 48, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis antibacterial and antioxidant synergisms with gentamicin and honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [CrossRef] [PubMed]
- Sookkhee, S.; Sakonwasun, C.; Mungkornasawakul, P.; Khamnoi, P.; Wikan, N.; Nimlamool, W. Synergistic Effects of Some Methoxyflavones Extracted from Rhizome of Kaempferia parviflora Combined with Gentamicin against Carbapenem-Resistant Strains of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii. Plants 2022, 11, 3128. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, O.; Stankovic, M.; Čomić, L. In vitro antibacterial efficacy of Clinopodium vulgare L. extracts and their synergistic interaction with antibiotics. J. Med. Plant Res. 2011, 5, 4074–4079. [Google Scholar]
- Kuok, C.F.; Hoi, S.O.; Hoi, C.F.; Chan, C.H.; Fong, I.H.; Ngok, C.K.; Meng, L.R.; Fong, P. Synergistic antibacterial effects of herbal extracts and antibiotics on methicillin-resistant Staphylococcus aureus: A computational and experimental study. Exp. Biol. Med. 2017, 242, 731–743. [Google Scholar] [CrossRef]

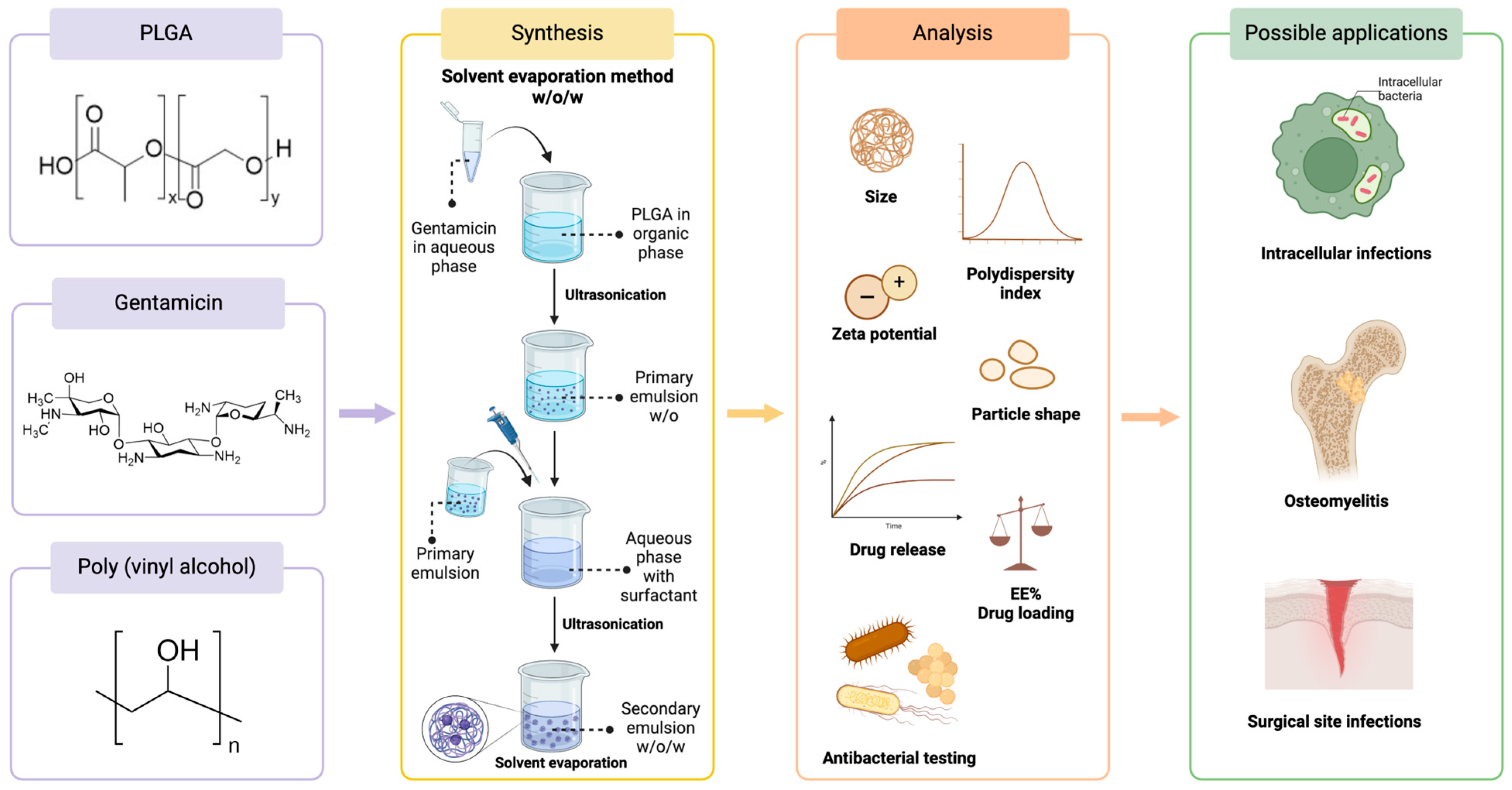
| PLGA Type | Surfactant | Formulation Method | Size (nm) | PDI | Zeta Potential (mV) | Particle Characterization Methods | EE (%) | Drug Loading | Drug Release (In Vitro) | Bacteria (In Vitro Tests) | Potential Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50:50 (13.7 kDa) | PVA (15 kDa) | w/o/w solvent evaporation | 320 | NA | −15.5 ± 0.2 | DLS ELS SEM XRD DSC | 13.12–58.76 | 3.24–7.35 # | 28 days | NT | Intracellular pathogens | [63] |
| 50:50 (13.7 kDa) | PVA (15 kDa) | w/o/w solvent evaporation | 310 ± 2.00 | NA | NT | DLS | NT | 6.2 * | NT | NT | Intracellular pathogens | [64] |
| 50:50 (12 kDa) | PVA | w/o/w s/o/w solvent evaporation | 241.3–358.5 | 0.10–0.23 | −0.4–2.3 | DLS ELS | NT | 6.4–22.4 * | Over 16 days | P. aeruginosa | Planktonic- and biofilm-based infections | [65] |
| 85:15 (80 kDa) | PVA (31 kDa) | w/o/w solvent evaporation | 219–391 | 0.21–0.38 | −7.2–−1.1 | DLS ELS AFM | 2.7–52.4 | 0.06–10.28% | 35 days | S. aureus S. epidermidis | Osteomyelitis | [66] |
| 50:50 (7–17 kDa) | PVA (85–124 kDa) | w/o/w solvent evaporation | 280 ± 12.04 | 0.15 ± 0.01 | −4.9 ± 0.84 | DLS ELS SEM TEM | NT | 60% | 216 h | P. aeruginosa S. aureus | Surgical site infections | [67] |
| 50:50 (7–17 kDa) | PVA | w/o/w solvent evaporation | 227 | 0.162 | −1.67 | DLS ELS SEM | NT | 135 * | 120 h | K. pneumoniae | Intracellular pathogens | [68] |
| PLGA-PEG (70 kDa) | PVA (85–124 kDa) | s/o/w solvent evaporation | 140.0–919.3 | 0.104–1.230 | −5.54–0.36 | DLS ELS TEM | 43.97–64.61 | 2.9–7.9% | 10 h | P. mirabilis E. coli P. aeruginosa S. aureus | Intracellular pathogens | [11] |
| 75:25 (4–15 kDa) | PVA (89–98 kDa) | w/o/w solvent evaporation | 32–2400 | NA | NT | DLS SEM | NT | NT | 10 h | E. coli | Wound treatment | [69] |
| Chitosan Type | Crosslinker | Drugs | Formulation Method | Size (nm) | PDI | Zeta Potential (mV) | Particle Characterization Methods | EE (%) | Drug Loading (%) | Bacteria (In Vitro Tests) | Potential Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 kDa, deacetylation degree 85.6% | TPP | Gentamicin | Ionic gelation | 779.37 ± 51.79 | NT | 1.9 ± 0.5 | DLS ELS | 78.06 ± 2.13 | 63.10 ± 1.54 | NT | Intracellular pathogens | [95] |
| 80 kDa, deacetylation degree 95% | TPP | Gentamicin + salicylic acid | Ionic gelation | 148–345 | 0.234–0.428 | 32.45–42.43 | DLS ELS TEM SEM FTIR XRD | 61.70–87.20 | 13.56–26.64 | NT | Reduction in toxicity | [96] |
| 140 kDa, deacetylation degree 85% | TPP | Gentamicin | Ionic gelation | 100 | NT | 28 | DLS ELS SEM | 72 | 22 | B. abortus B. melitensis | Intracellular pathogens | [75] |
| 304 kDa, deacetylation degree >84% | - | Gentamicin + proanthocyanidin | Hydrogen bonding | 242.9–277.4 | 0.344–0.391 | 34.5–38.5 | DLS ELS SEM FTIR TGA | 94 | NT | E. coli S. aureus P. aeruginosa | Enhanced antimicrobial activity | [97] |
| Low molecular weight, deacetylation ≥ 75% | TPP | Gentamicin | Ionic gelation | 151–212 | 0.21–0.29 | 37.2–51.1 | DLS ELS TEM SEM DSC | 36.6–42.7 | NT | NT | Wound healing | [98] |
| NA | TPP | Gentamicin + ascorbic acid | Ionic gelation | 278 | NT | 30.01 | DLS ELS TEM FTIR | 89 | 22 | S. aureus P. aeruginosa | Reduction in toxicity | [99] |
| Polyphenol | Bacteria | Bacterial Strain Type | Antibacterial Effect on Gentamicin | FICI | Synergy/Partial Synergy | Ref. |
|---|---|---|---|---|---|---|
| Caffeic acid | P. aeruginosa | Clinical isolates | MIC reduced from 625 μg/mL to 24.61 μg/mL | NA | Synergy | [148] |
| Epigallocatechin gallate | A. baumannii | Reference | MIC reduced from 27 μg/mL to 4 μg/mL | 0.65 | Partial synergy | [146] |
| S. aureus | Clinical isolates | MIC reduced from 32 μg/mL to 6.4 μg/mL | 0.325 | Synergy | [149] | |
| E. coli | Clinical isolates | MIC reduced from 32 μg/mL to 6.4 μg/mL | 0.325 | Synergy | [149] | |
| Daidzein | A. baumannii | Reference | MIC reduced from 27 μg/mL to 8 μg/mL | 0.42 | Synergy | [146] |
| Galangin | S. aureus (methicillin-resistant) | Clinical isolates/reference | Reduced MIC | 0.18–0.25 | Synergy | [150] |
| Gallic acid | S. aureus | Clinical isolates | MIC reduced from 49.21 μg/mL to 2.44 μg/mL | NA | Synergy | [148] |
| Genistein | A. baumannii | Reference | MIC reduced from 27 μg/mL to 4 μg/mL | 0.4 | Synergy | [146] |
| 5-Hydroxy-3,7,4′-trimethoxyflavone | S. aureus | Clinical isolates | Reduced MIC | NA | Synergy | [151] |
| E. coli | Clinical isolates | Reduced MIC | NA | Synergy | [151] | |
| Kaempferol 7-O-β-D-(6″-O-cumaroyl)-glucopyranoside | S. aureus | Reference | MIC reduced from 16 μg/mL to 4 μg/mL | NA | Synergy | [3] |
| E. coli | Reference | MIC reduced from 16 μg/mL to 8 μg/mL | NA | Synergy | [3] | |
| Luteolin | S. aureus | Reference | MIC reduced 4-fold | 0.258 | Synergy | [143] |
| E. coli | Reference | Reduced MIC | 0.504 | Additive | [143] | |
| Nordihydroguaiaretic acid | S. aureus (methicillin-sensitive) | Clinical isolates | Several-fold MIC reduction | <0.5 | Synergy | [152] |
| S. aureus (methicillin-resistant) | Clinical isolates | Several-fold MIC reduction | <0.5 | Synergy | [152] | |
| Quercetin | P. aeruginosa | Clinical isolates | MIC reduced from 128 μg/mL to 32 μg/mL | 0.28–0.53 | Synergy/Partial synergy | [153] |
| P. aeruginosa | Clinical isolates/reference | Reduced MIC | 0.375–0.75 | Synergy/Partial synergy | [2] | |
| P. mirabilis | Clinical isolates | Restored antibacterial activity | NA | Synergy | [154] | |
| Plumbagin | P. aeruginosa | Reference | Reduced MIC | 0.152–0.485 | Synergy | [155] |
| Pyrogallol | S. aureus | Clinical isolates | MIC reduced from 49.21 μg/mL to 2.44 μg/mL | NA | Synergy | [148] |
| Rutin | P. aeruginosa | Reference | MIC reduced from 10 μg/mL to 2.5 μg/mL | 0.5 | Synergy | [156] |
| Sophoraflavanone B | S. aureus (methicillin-resistant) | Clinical isolates/reference | MIC reduced 8- to 32-fold | 0.25–0.375 | Synergy | [157] |
| Vitexin | P. aeruginosa | Reference | Reduced MIC | 0.078 | Synergy | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bārzdiņa, A.; Plotniece, A.; Sobolev, A.; Pajuste, K.; Bandere, D.; Brangule, A. From Polymeric Nanoformulations to Polyphenols—Strategies for Enhancing the Efficacy and Drug Delivery of Gentamicin. Antibiotics 2024, 13, 305. https://doi.org/10.3390/antibiotics13040305
Bārzdiņa A, Plotniece A, Sobolev A, Pajuste K, Bandere D, Brangule A. From Polymeric Nanoformulations to Polyphenols—Strategies for Enhancing the Efficacy and Drug Delivery of Gentamicin. Antibiotics. 2024; 13(4):305. https://doi.org/10.3390/antibiotics13040305
Chicago/Turabian StyleBārzdiņa, Ance, Aiva Plotniece, Arkadij Sobolev, Karlis Pajuste, Dace Bandere, and Agnese Brangule. 2024. "From Polymeric Nanoformulations to Polyphenols—Strategies for Enhancing the Efficacy and Drug Delivery of Gentamicin" Antibiotics 13, no. 4: 305. https://doi.org/10.3390/antibiotics13040305
APA StyleBārzdiņa, A., Plotniece, A., Sobolev, A., Pajuste, K., Bandere, D., & Brangule, A. (2024). From Polymeric Nanoformulations to Polyphenols—Strategies for Enhancing the Efficacy and Drug Delivery of Gentamicin. Antibiotics, 13(4), 305. https://doi.org/10.3390/antibiotics13040305






