Development of a High-Throughput Analytical Method for Antimicrobials in Wastewater Using an Automated Pipetting and Solid-Phase Extraction System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Analytical Procedures for Antimicrobials in the Environmental Water Based on the Manual Analysis
| Classification | CAS Registry Number | Antimicrobials | Molecular Formula | Molecular Mass (g/mol) | Structure | pKa | LogP |
|---|---|---|---|---|---|---|---|
| β-lactams | 69-53-4 | Ampicillin (APL) | C16H19N3O4S | 349.4 | 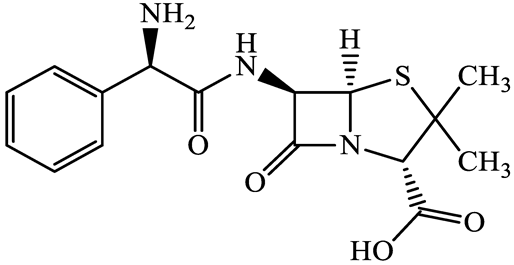 | 2.4 | 1.4 |
| 61-33-6 | Benzylpenicillin (BZP) | C16H18N2O4S | 334.4 |  | 2.5 | 1.7 | |
| 91832-40-5 | Cefdinir (CDN) | C14H13N5O5S2 | 395.4 | 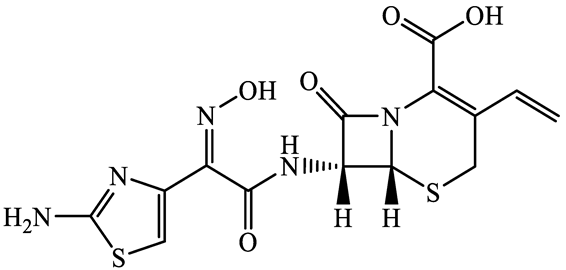 | 2.8 | −1.8 | |
| 80210-62-4 | Cefpodoxime (CPX) | C17H19N5O6S2 | 453.5 |  | 2.8 (Acid) 1.7 (Base) | 0.4 | |
| 87239-81-4 | Cefpodoxime proxetil (CPXP) | C21H27N5O9S2 | 557.6 | 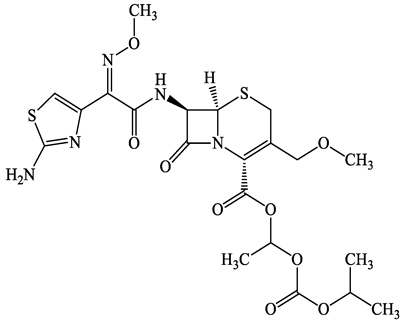 | 8.1 (Acid) 1.7 (Base) | 2.9 | |
| 80370-57-6 | Ceftiofur (CTF) | C19H17N5O7S3 | 523.6 |  | 2.6 | 1.7 | |
| New quinolones | 85721-33-1 | Ciprofloxacin (CFX) | C17H18FN3O3 | 331.3 |  | 6.0 | 1.3 |
| 93106-60-6 | Enrofloxacin (EFX) | C19H22FN3O3 | 359.4 | 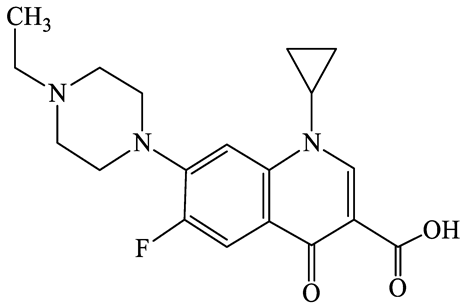 | 6.4 | 2.3 | |
| 100986-85-4 | Levofloxacin (LFX) | C18H20FN3O4 | 361.4 |  | 5.2 | 1.6 | |
| Macrolides | 83905-01-5 | Azithromycin (ATM) | C38H72N2O12 | 749.0 | 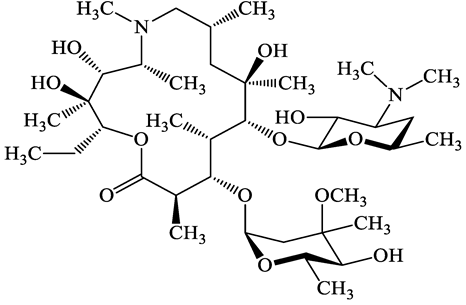 | 13.3 | 3.3 |
| 81103-11-9 | Clarithromycin (CTM) | C38H69NO13 | 748.0 | 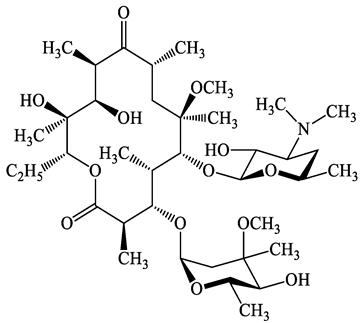 | 13.1 | 3.2 | |
| Tetracyclines | 57-62-5 | Chlortetracycline (CTCL) | C22H23ClN2O8 | 478.9 | 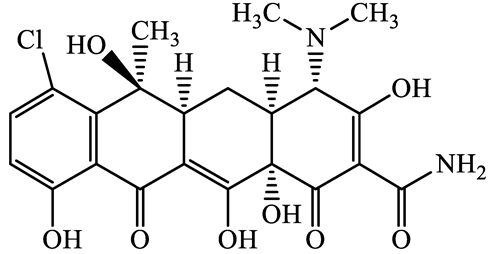 | 4.5 | 4.8 |
| 564-25-0 | Doxycycline (DCL) | C22H24N2O8 | 444.4 | 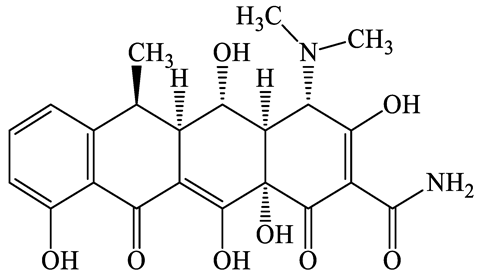 | 4.5 (Acid) 10.8 (Base) | 1.8 | |
| 10118-90-8 | Minocycline (MCL) | C23H27N3O7 | 457.5 | 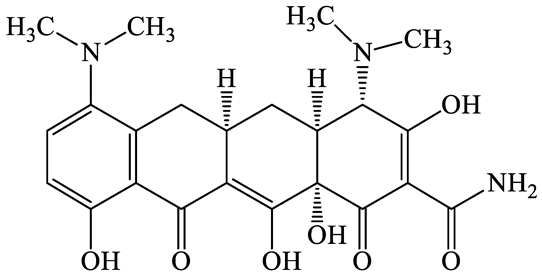 | 4.5 (Acid) 11.1 (Base) | 2.2 | |
| 79-57-2 | Oxytetracycline (OTCL) | C22H24N2O9 | 460.4 |  | 4.5 | −1.5 | |
| 60-54-8 | Tetracycline (TCL) | C22H24N2O8 | 444.4 | 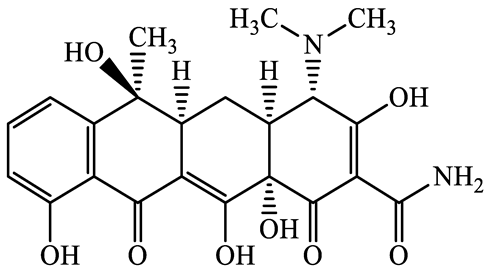 | 4.5 | −1.5 | |
| Glycopeptide | 1404-90-6 | Vancomycin (VMC) | C66H75Cl2N9O24 | 1449.3 | 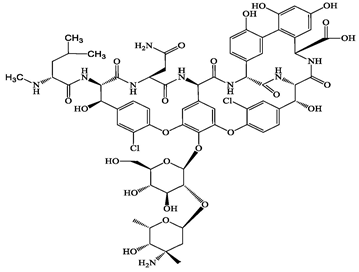 | 3.0 | −1.4 |
2.3. Development of Analytical Procedures for Antimicrobials in Environmental Water Based on the HTA Systems Using Automated Pipetting Equipped with Automated SPE
2.4. Method Validation
2.5. Monitoring Antimicrobials in Hospital Wastewater
2.6. Statistical Analysis
3. Results and Discussion
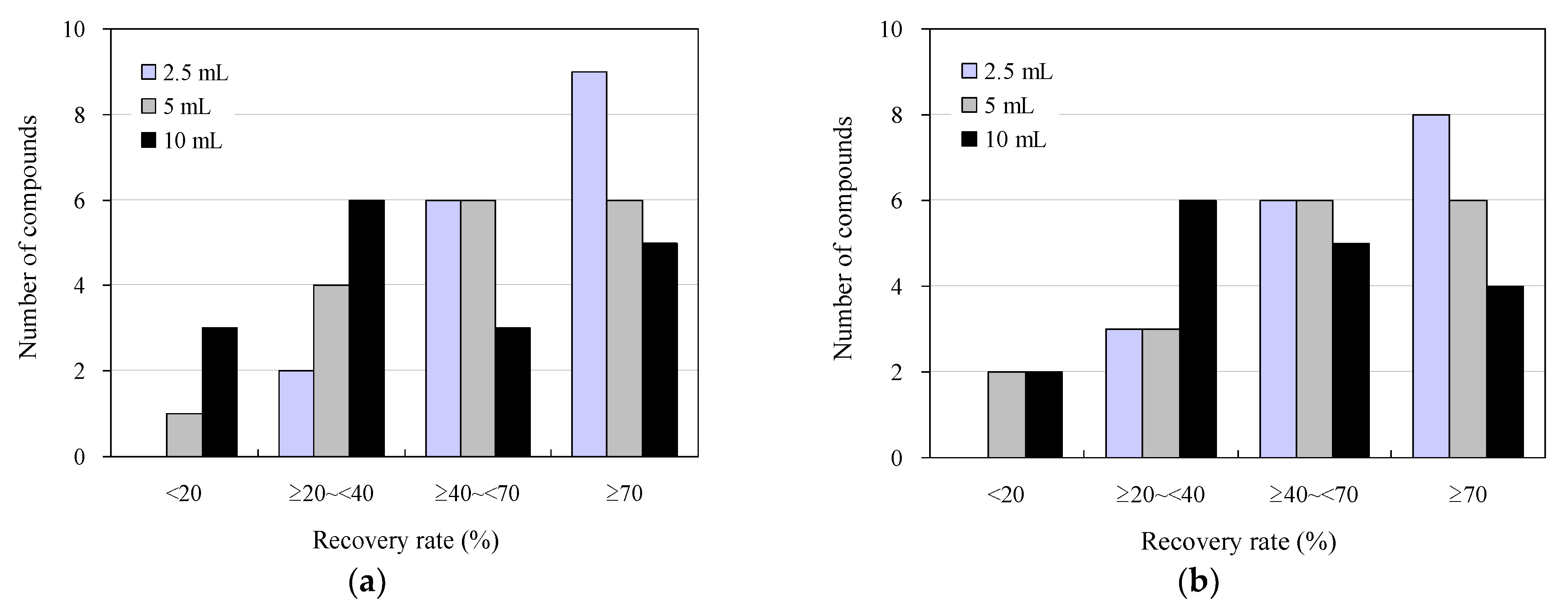
3.1. Effect of Solid-Phase Sample Loading on the Analysis of Antimicrobials in Wastewater Using a 96-Well SPE Plate
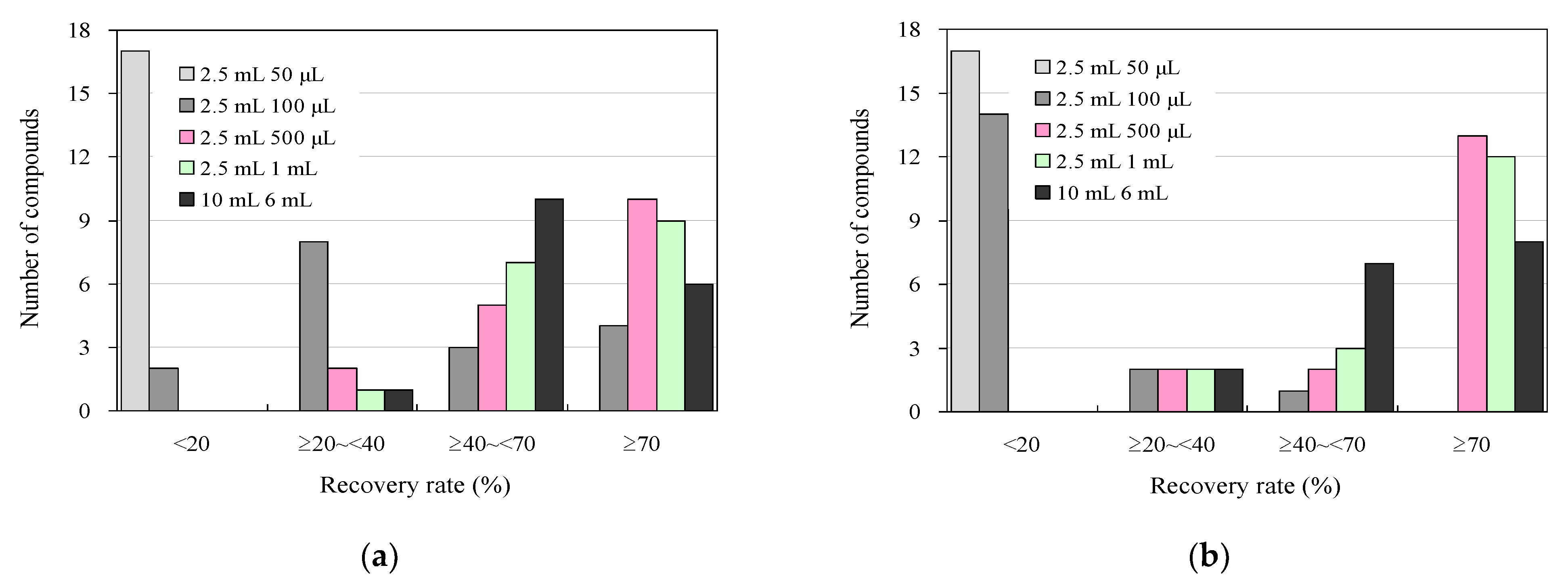
3.2. Effect of Organic Solvent Composition on Eluent Volume and UPLC–MS/MS Analysis in the Detection of Antimicrobials in Wastewater Using a 96-Well SPE Plate
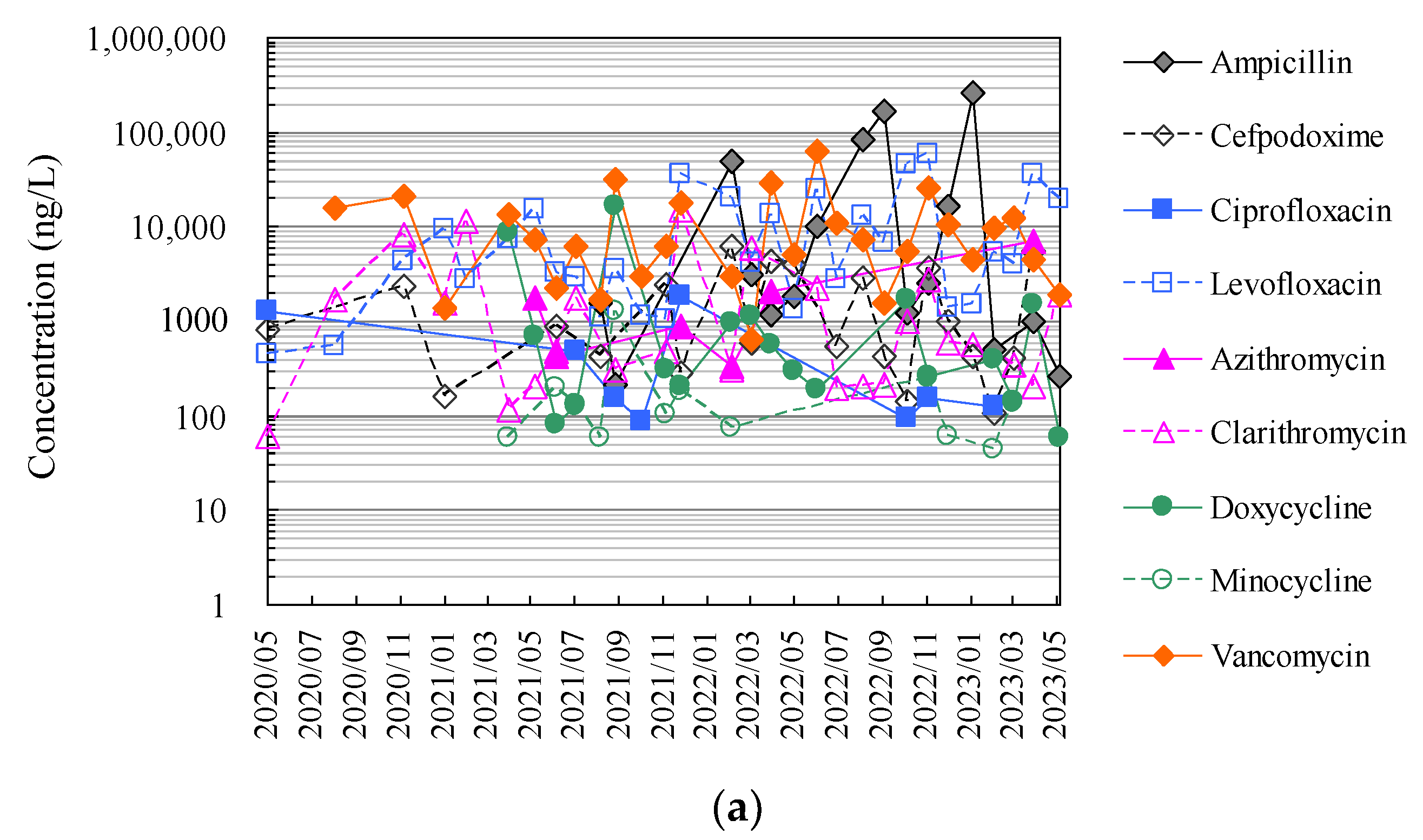
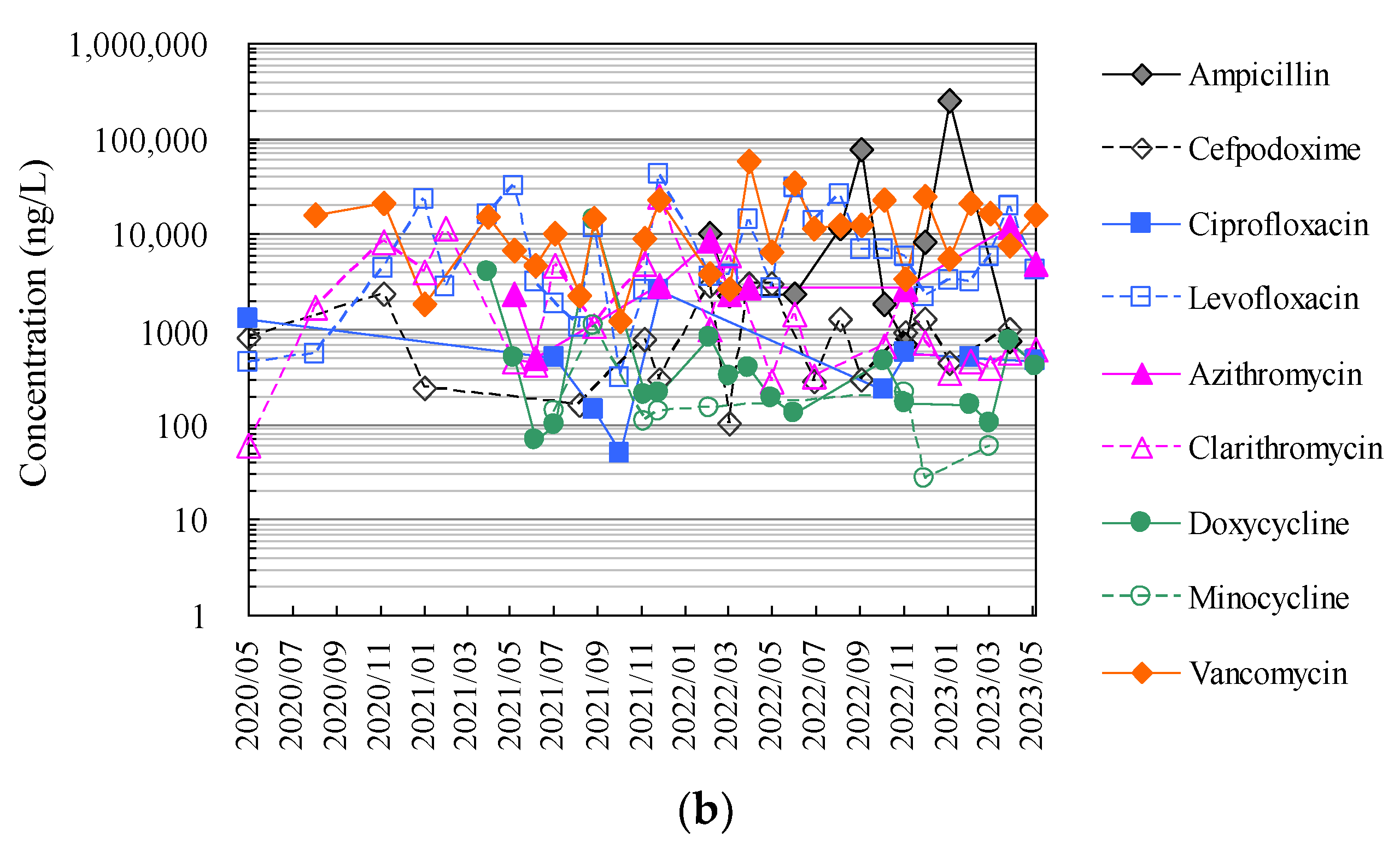
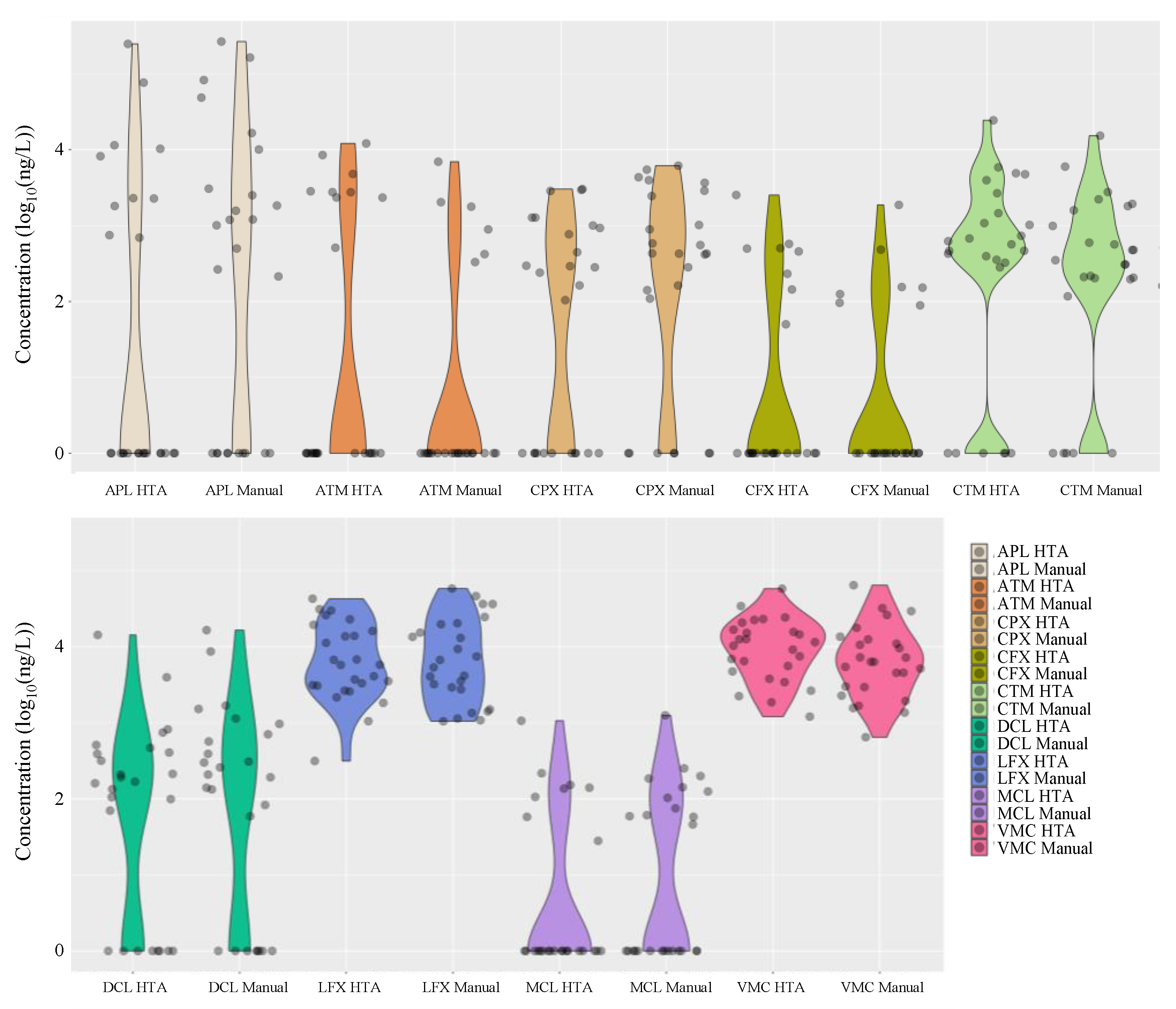
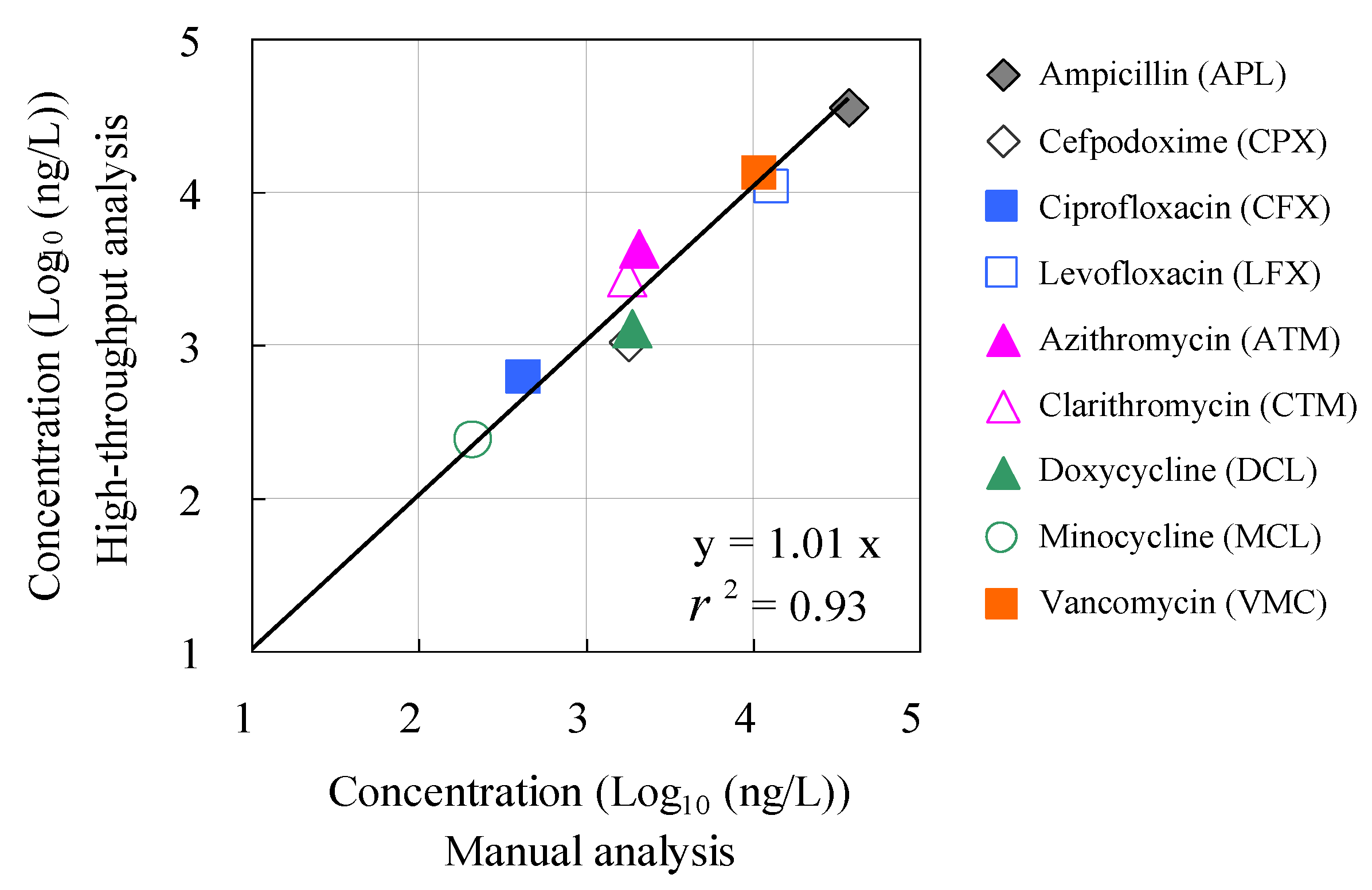
3.3. Application to Monitoring of Antimicrobials in Hospital Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Bhandari, G.; Chaudhary, P.; Gangola, S.; Gupta, S.; Gupta, A.; Rafatullah, M.; Chen, S. A review on hospital wastewater treatment technologies: Current management practices and future prospects. J. Water Process Eng. 2023, 56, 104516. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Han, Q.; Yu, Q.; Wanyan, R.; Li, H. Bibliometric analysis of papers on antibiotic resistance genes in aquatic environments on a global scale from 2012 to 2022: Evidence from universality, development and harmfulness. Sci. Total Environ. 2024, 909, 168597. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Khiadani, M.; Foroughi, M.; Alizade Siuki, H.; Mehrfar, H. Wastewater treatment plants: The missing link in global One-Health surveillance and management of antibiotic resistance. J. Infect. Public Health 2023, 16, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Z.; Guo, J.; de la Fuente-Nunez, C.; Wang, J.; Han, B.; Tao, H.; Liu, J.; Wang, X. Bacterial resistance to antibacterial agents: Mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023, 860, 160461. [Google Scholar] [CrossRef]
- Miłobedzka, A.; Ferreira, C.; Vaz-Moreira, I.; Calderón-Franco, D.; Gorecki, A.; Purkrtova, S.; Jan, B.; Dziewit, L.; Singleton, C.M.; Nielsen, P.H.; et al. Monitoring antibiotic resistance genes in wastewater environments: The challenges of filling a gap in the one-health cycle. J. Hazard. Mater. 2022, 424, 127407. [Google Scholar]
- Wang, C.; Yang, H.; Liu, H.; Zhang, X.X.; Ma, L. Anthropogenic contributions to antibiotic resistance gene pollution in household drinking water revealed by machine-learning-based source-tracking. Water Res. 2023, 246, 120682. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shan, X.; Chen, J.; Zhang, Y.; Wang, J.; Chen, H. Fate, risk and sources of antibiotic resistome and its attenuation dynamics in the river water-sediment system: Field and microcosm study. Environ. Pollut. 2024, 340, 122853. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, V.; Manageiro, V.; Rosado, T.; Bandarra, N.M.; Botelho, M.J.; Dias, E.; Caniça, M. Snapshot of resistome, virulome and mobilome in aquaculture. Sci. Total Environ. 2023, 905, 166351. [Google Scholar] [CrossRef]
- Xu, N.; Qiu, D.; Zhang, Z.; Wang, Y.; Chen, B.; Zhang, Q.; Wang, T.; Hong, W.; Zhou, N.Y.; Penuelas, J.; et al. A global atlas of marine antibiotic resistance genes and their expression. Water Res. 2023, 244, 120488. [Google Scholar] [CrossRef]
- Sambaza, S.S.; Naicker, N. Contribution of wastewater to antimicrobial resistance: A review article. J. Glob. Antimicrob. Resist. 2023, 34, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Siri, Y.; Bumyut, A.; Precha, N.; Sirikanchana, K.; Haramoto, E.; Makkaew, P. Multidrug antibiotic resistance in hospital wastewater as a reflection of antibiotic prescription and infection cases. Sci. Total Environ. 2024, 908, 168453. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, E.; Solimini, A.; Pantanella, F.; Cummins, E. The potential human exposure to antibiotic resistant-Escherichia coli through recreational water. Sci. Total Environ. 2019, 650, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Schoen, M.E.; Jahne, M.A.; Garland, J.; Ramirez, L.; Lopatkin, A.J.; Hamilton, K.A. Quantitative microbial risk assessment of antimicrobial resistant and susceptible Staphylococcus aureus in reclaimed wastewaters. Environ. Sci. Technol. 2021, 55, 15246–15255. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.G.; Haller, L.; Ng, C.; Charles, F.R.; Jitxin, L.; Chen, H.; He, Y.; Gin, K.Y.-H. Assessing the additional health burden of antibiotic resistant enterobacteriaceae in surface waters through an integrated QMRA and DALY approach. J. Hazard. Mater. 2023, 458, 132058. [Google Scholar] [CrossRef]
- Mangla, D.; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment—A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Cephalosporin antibiotics in the aquatic environment: A critical review of occurrence, fate, ecotoxicity and removal technologies. Environ. Pollut. 2018, 241, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Yu, C.; Pang, H.; Wang, J.H.; Chi, Z.Y.; Zhang, Q.; Kong, F.T.; Xu, Y.P.; Li, S.Y.; Che, J. Occurrence of antibiotics in waters, removal by microalgae-based systems, and their toxicological effects: A review. Sci. Total Environ. 2022, 813, 151891. [Google Scholar] [CrossRef]
- Cacace, D.; Fatta-Kassinos, D.; Manaia, C.M.; Cytryn, E.; Kreuzinger, N.; Rizzo, L.; Karaolia, P.; Schwartz, T.; Alexander, J.; Merlin, C.; et al. Antibiotic resistance genes in treated wastewater and in the receiving water bodies: A pan-european survey of urban settings. Water Res. 2019, 162, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.P.; Brinkman, N.E.; Wheaton, E.A.; Jahne, M.A.; Siefring, S.D.; Varma, M.; Hill, R.A.; Leibowitz, S.G.; Martin, R.W.; Garland, J.L.; et al. Geospatial patterns of antimicrobial resistance genes in the US EPA national rivers and streams assessment survey. Environ. Sci. Technol. 2022, 56, 14960–14971. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Abramova, A.; Berendonk, T.U.; Coelho, L.P.; Forslund, S.K.; Gschwind, R.; Heikinheimo, A.; Jarquín-Díaz, V.H.; Khan, A.A.; Klümper, U.; et al. Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environ. Int. 2023, 178, 108089. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.; Kasprzyk-Hordern, B. Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020, 139, 105689. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Nishiyama, M.; Watanabe, T.; Kanamori, H. Review of antimicrobial resistance in wastewater in Japan: Current challenges and future perspectives. Antibiotics 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Ploy, M.C.; Berendonk, T.U. Editorial overview: AMR in the environment—Too complex for surveillance? Curr. Opin. Microbiol. 2022, 65, xi–xiii. [Google Scholar] [CrossRef] [PubMed]
- Javvadi, Y.; Mohan, S.V. Understanding the distribution of antibiotic resistance genes in an urban community using wastewater-based epidemiological approach. Sci. Total Environ. 2023, 868, 161419. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Kurittu, P.; Al-Mustapha, A.I.; Heljanko, V.; Johansson, V.; Thakali, O.; Mishra, S.K.; Lehto, K.M.; Lipponen, A.; Oikarinen, S.; et al. Wastewater surveillance of antibiotic-resistant bacterial pathogens: A systematic review. Front. Microbiol. 2022, 13, 977106. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Yousef, A.F.; Alsafar, H.; Hasan, S.W. Surveillance, distribution, and treatment methods of antimicrobial resistance in water: A review. Sci. Total Environ. 2023, 890, 164360. [Google Scholar] [CrossRef]
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Tutu, H.; Richards, H.; Newman, B.; Ndungu, K.; Chimuka, L. Pharmaceuticals and their metabolites in the marine environment: Sources, analytical methods and occurrence. Trends Environ. Anal. Chem. 2020, 28, e00104. [Google Scholar] [CrossRef]
- Lozano, I.; Pérez-Guzmán, C.J.; Mora, A.; Mahlknecht, J.; Aguilar, C.L.; Cervantes-Avilés, P. Pharmaceuticals and personal care products in water streams: Occurrence, detection, and removal by electrochemical advanced oxidation processes. Sci. Total Environ. 2022, 827, 154348. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Rodríguez-Mozaz, S.; Corominas, L.; Covaci, A.; Petrovic, M. Applicability of an on-line solid-phase extraction liquid chromatography—Tandem mass spectrometry for the wastewater-based assessment of human exposure to chemicals from personal care and household products. Sci. Total Environ. 2022, 845, 157309. [Google Scholar] [CrossRef] [PubMed]
- Suseela, M.N.L.; Viswanadh, M.K.; Mehata, A.K.; Priya, V.; Setia, A.; Malik, A.K.; Gokul, P.; Selvin, J.; Muthu, M.S. Advances in solid-phase extraction techniques: Role of nanosorbents for the enrichment of antibiotics for analytical quantification. J. Chromatogr. A 2023, 1695, 463937. [Google Scholar] [CrossRef] [PubMed]
- An, X.L.; Su, J.Q.; Li, B.; Ouyang, W.Y.; Zhao, Y.; Chen, Q.L.; Cui, L.; Chen, H.; Gillings, M.R.; Zhang, T.; et al. Tracking antibiotic resistome during wastewater treatment using high throughput quantitative PCR. Environ. Int. 2018, 117, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fontenele, R.S.; Kraberger, S.; Hadfield, J.; Driver, E.M.; Bowes, D.; Holland, L.A.; Faleye, T.O.C.; Adhikari, S.; Kumar, R.; Inchausti, R.; et al. High-throughput sequencing of SARS-CoV-2 in wastewater provides insights into circulating variants. Water Res. 2021, 205, 117710. [Google Scholar] [CrossRef] [PubMed]
- Mailepessov, D.; Arivalan, S.; Kong, M.; Griffiths, J.; Low, S.L.; Chen, H.; Hapuarachchi, H.C.; Gu, X.; Lee, W.L.; Alm, E.J.; et al. Development of an efficient wastewater testing protocol for high-throughput country-wide SARS-CoV-2 monitoring. Sci. Total Environ. 2022, 826, 154024. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, M.; Deng, Y.; Xu, X.; Lin, D.; Zhang, Y.; Li, S.; Ding, J.; Shi, X.; Yau, C.I.; et al. A rapid, high-throughput, and sensitive peg-precipitation method for SARS-CoV-2 wastewater surveillance. Water Res. 2023, 230, 119560. [Google Scholar] [CrossRef] [PubMed]
- Srathongneam, T.; Sresung, M.; Paisantham, P.; Ruksakul, P.; Singer, A.C.; Sukchawalit, R.; Satayavivad, J.; Mongkolsuk, S.; Sirikanchana, K. High throughput qPCR unveils shared antibiotic resistance genes in tropical wastewater and river water. Sci. Total Environ. 2024, 908, 167867. [Google Scholar] [CrossRef]
- Zwart, N.; Nio, S.L.; Houtman, C.J.; de Boer, J.; Kool, J.; Hamers, T.; Lamoree, M.H. High-throughput effect-directed analysis using downscaled in vitro reporter gene assays to identify endocrine disruptors in surface water. Environ. Sci. Technol. 2018, 52, 4367–4377. [Google Scholar] [CrossRef]
- Limayem, A.; Wasson, S.; Mehta, M.; Pokhrel, A.R.; Patil, S.; Nguyen, M.; Chen, J.; Nayak, B. High-throughput detection of bacterial community and its drug-resistance profiling from local reclaimed wastewater plants. Front. Cell. Infect. Microbiol. 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, I.; Nagasawa, K.; Suzuki, M.; Kurisu, F.; Furumai, H. High-throughput screening of antimicrobial resistance genes and their association with class 1 integrons in urban rivers in Japan. Front. Environ. Sci. 2022, 10, 825372. [Google Scholar] [CrossRef]
- Boogaerts, T.; Quireyns, M.; Maes, F.; Laimou-Geraniou, M.; Van Wichelen, N.; Heath, E.; Pussig, B.; Aertgeerts, B.; Covaci, A.; van Nuijs, A.L.N. Optimization, validation and application of a high-throughput 96-well elution protocol for the quantification of psychoactive substances in influent wastewater. Drug Test. Anal. 2023, 15, 240–246. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.; Botero-Coy, A.M.; Nieto-Juárez, J.I.; Torres-Palma, R.A.; Hernández, F. Searching for pharmaceutically active products and metabolites in environmental waters of Peru by HRMS-based screening: Proposal for future monitoring and environmental risk assessment. Chemosphere 2023, 337, 139375. [Google Scholar] [CrossRef] [PubMed]
- Liguori, K.; Keenum, I.; Davis, B.C.; Calarco, J.; Milligan, E.; Harwood, V.J.; Pruden, A. Antimicrobial resistance monitoring of water environments: A framework for standardized methods and quality control. Environ. Sci. Technol. 2022, 56, 9149–9160. [Google Scholar] [CrossRef] [PubMed]
- Tarek, M.H.; Garner, E. A proposed framework for the identification of indicator genes for monitoring antibiotic resistance in wastewater: Insights from metagenomic sequencing. Sci. Total Environ. 2023, 854, 158698. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, P.; Horak, G.; Skinner, N. Highly versatile cloud-based automation solution for the remote design and execution of experiment protocols during the COVID-19 pandemic. SLAS Technol. 2021, 26, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Vargas Medina, D.A.; Maciel, E.V.S.; Lanças, F.M. Modern automated sample preparation for the determination of organic compounds: A review on robotic and on-flow systems. TrAC Trends Anal. Chem. 2023, 166, 117171. [Google Scholar] [CrossRef]
- Mary, T.; Jonathan, P.D. Fully automated solid phase extraction sample preparation, using the Andrew+™ pipetting robot configured with the extraction+ connected device. Waters™ Appl. Note 2022, 720007711, 1–23. [Google Scholar]
- Palát, J.; Kukučka, P.; Codling, G.P.; Price, E.J.; Janků, P.; Klánová, J. Application of 96-well plate spe method for analysis of persistent organic pollutants in low volume blood serum samples. Chemosphere 2022, 287, 132300. [Google Scholar] [CrossRef]
- Braun, G.; Krauss, M.; Escher, B.I. Recovery of 400 chemicals with three extraction methods for low volumes of human plasma quantified by instrumental analysis and in vitro bioassays. Environ. Sci. Technol. 2023, 57, 19363–19373. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Katagiri, M.; Sasaki, N.; Kuroda, M.; Watanabe, M. Performance of a pilot-scale continuous flow ozone-based hospital wastewater treatment system. Antibiotics 2023, 12, 932. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, S.; Zhao, K.; Song, G.; Zhao, S.; Liu, R. Risk control of antibiotics, antibiotic resistance genes (ARGs) and antibiotic resistant bacteria (ARB) during sewage sludge treatment and disposal: A review. Sci. Total Environ. 2023, 877, 162772. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare, Japan, Ministry of Health Labour and Welfare, Japan. Japan Nosocomial Infections Surveillance (JANIS), Nosocomial Infections Surveillance for Drug-Resistant Bacteria. 2023. Available online: https://janis.mhlw.go.jp/english/index.asp (accessed on 25 March 2024).
- Ministry of Health Labour and Welfare, Japan, Annual Report on Statistics of Production by Pharmaceutical Industry in 2022 (in Japanese). 2023. Available online: https://www.mhlw.go.jp/toukei/list/105-1c.html (accessed on 25 March 2024).
- Azuma, T.; Nakano, T.; Koizumi, R.; Matsunaga, N.; Ohmagari, N.; Hayashi, T. Evaluation of the correspondence between the concentration of antimicrobials entering sewage treatment plant influent and the predicted concentration of antimicrobials using annual sales, shipping, and prescriptions data. Antibiotics 2022, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Usui, M.; Hayashi, T. Inactivation of antibiotic-resistant bacteria in hospital wastewater by ozone-based advanced water treatment processes. Sci. Total Environ. 2024, 906, 167432. [Google Scholar] [CrossRef] [PubMed]
- American Chemical Society Chemical Abstracts Service (CAS). Scifindern. American Chemical Society, Chemical Abstracts Service (CAS). SciFindern Online Database (Subscription Database). 2024. Available online: https://scifinder-n.cas.org (accessed on 25 March 2024).
- Waters Corporation (USA). Vials, Plates, and Certified Containers. 2024. Available online: https://www.waters.com/nextgen/us/en/products/vials--plates--and-certified-containers.html (accessed on 25 March 2024).
- Daughton, C.G. Pharmaceuticals and the environment (PiE): Evolution and impact of the published literature revealed by bibliometric analysis. Sci. Total Environ. 2016, 562, 391–426. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.K.; Choi, S.D. Review of the quechers method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, S.; Bao, Y.; Daniels, K.D.; How, Z.T.; El-Din, M.G.; Wang, J.; Tang, L. Fully-automated SPE coupled to UHPLC-MS/MS method for multiresidue analysis of 26 trace antibiotics in environmental waters: SPE optimization and method validation. Environ. Sci. Pollut. Res. 2022, 29, 16973–16987. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, L.; Wang, B.; Liu, C.S.; Jia, Y.; Zhai, N.; Blaney, L.; Yu, G. Efficient multiresidue determination method for 168 pharmaceuticals and metabolites: Optimization and application to raw wastewater, wastewater effluent, and surface water in Beijing, China. Environ. Pollut. 2020, 261, 114113. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Jiménez-Rodríguez, M.G.; Martínez-Ruiz, M.; Peña-Benavides, S.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Sosa-Hernández, J.E. Antidepressants surveillance in wastewater: Overview extraction and detection. Case Stud. Chem. Environ. Eng. 2021, 3, 100074. [Google Scholar] [CrossRef]
- Petrović, M.; Škrbić, B.; Živančev, J.; Ferrando-Climent, L.; Barcelo, D. Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole–linear ion trap in different types of water in Serbia. Sci. Total Environ. 2014, 468–469, 415–428. [Google Scholar] [CrossRef]
- Wu, D.; Sui, Q.; Yu, X.; Zhao, W.; Li, Q.; Fatta-Kassinos, D.; Lyu, S. Identification of indicator PPCPs in landfill leachates and livestock wastewaters using multi-residue analysis of 70 PPCPs: Analytical method development and application in Yangtze River Delta, China. Sci. Total Environ. 2021, 753, 141653. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Ishida, M.; Hisamatsu, K.; Yunoki, A.; Mino, Y.; et al. Environmental fate of pharmaceutical compounds and antimicrobial-resistant bacteria in hospital effluents, and contributions to pollutant loads in the surface waters in Japan. Sci. Total Environ. 2019, 657, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, S.; Shao, P.; Wang, J.; Liu, Y.; Wei, W.; He, C.; Zhang, L. Occurrence and removal rate of typical pharmaceuticals and personal care products (ppcps) in an urban wastewater treatment plant in Beijing, China. Chemosphere 2023, 339, 139644. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Y.; Chan, C.L.; On, H.; Wai, H.K.F.; Shekhawat, S.S.; Gupta, A.B.; Varshney, A.K.; Chuanchuen, R.; Zhou, X.; et al. Metagenomic survey reveals more diverse and abundant antibiotic resistance genes in municipal wastewater than hospital wastewater. Front. Microbiol. 2021, 12, 712843. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (part i). TrAC Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (part ii). TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Pashaei, Y.; Mehrabi, M.; Shekarchi, M. A review on various analytical methods for determination of anthracyclines and their metabolites as anti–cancer chemotherapy drugs in different matrices over the last four decades. TrAC Trends Anal. Chem. 2020, 130, 115991. [Google Scholar] [CrossRef]
- Azuma, T.; Katagiri, M.; Sekizuka, T.; Kuroda, M.; Watanabe, M. Inactivation of bacteria and residual antimicrobials in hospital wastewater by ozone treatment. Antibiotics 2022, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Masiá, A.; Suarez-Varela, M.M.; Llopis-Gonzalez, A.; Picó, Y. Determination of pesticides and veterinary drug residues in food by liquid chromatography-mass spectrometry: A review. Anal. Chim. Acta 2016, 936, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Kanu, A.B. Recent developments in sample preparation techniques combined with high-performance liquid chromatography: A critical review. J. Chromatogr. A 2021, 1654, 462444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Luan, T.; Jiang, R.; Ouyang, G. Sample preparation and instrumental methods for illicit drugs in environmental and biological samples: A review. J. Chromatogr. A 2021, 1640, 461961. [Google Scholar] [CrossRef]
- Fu, C.; Xu, B.; Chen, H.; Zhao, X.; Li, G.; Zheng, Y.; Qiu, W.; Zheng, C.; Duan, L.; Wang, W. Occurrence and distribution of antibiotics in groundwater, surface water, and sediment in Xiong’an new area, China, and their relationship with antibiotic resistance genes. Sci. Total Environ. 2022, 807, 151011. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Meng, F.; Qi, H. Simultaneous determination of fourteen pharmaceuticals in sewage sludge using online solid-phase extraction-liquid chromatography-tandem mass spectrometry combined with accelerated solvent extraction. Environ. Sci. Pollut. Res. 2023, 30, 62522–62531. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from east China sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef] [PubMed]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Bouwman, H.; Palm, W.U.; Kümmerer, K. Spatial trends and ecotoxic risk assessment of selected pharmaceuticals in sediments from Lake Victoria, Uganda, East Africa. Sci. Total Environ. 2024, 906, 167348. [Google Scholar] [CrossRef] [PubMed]
- Meirinho, S.; Rodrigues, M.; Fortuna, A.; Falcão, A.; Alves, G. Liquid chromatographic methods for determination of the new antiepileptic drugs stiripentol, retigabine, rufinamide and perampanel: A comprehensive and critical review. J. Pharm. Anal. 2021, 11, 405–421. [Google Scholar] [CrossRef]
- Steinborner, S.; Henion, J. Liquid−liquid extraction in the 96-well plate format with SRM LC/MS quantitative determination of methotrexate and its major metabolite in human plasma. Anal. Chem. 1999, 71, 2340–2345. [Google Scholar] [CrossRef]
- Zhang, H.; Henion, J. Comparison between liquid chromatography–time-of-flight mass spectrometry and selected reaction monitoring liquid chromatography–mass spectrometry for quantitative determination of idoxifene in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Pahade, P.; Durgbanshi, A.; Carda-Broch, S.; Peris-Vicente, J.; Bose, D. Direct injection green chromatographic method for simultaneous quantification of amoxicillin and amikacin in maternity hospital wastewater (Sagar, India). Environ. Pollut. 2022, 296, 118719. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Eaglesham, G.; Shimko, K.M.; Mueller, J. Quantification of new psychoactive substances in australian wastewater utilising direct injection liquid chromatography coupled to tandem mass spectrometry. Talanta 2023, 251, 123767. [Google Scholar] [CrossRef] [PubMed]
- Gehring, T.; Deineko, E.; Hobus, I.; Kolisch, G.; Lübken, M.; Wichern, M. Effect of sewage sampling frequency on determination of design parameters for municipal wastewater treatment plants. Water Sci. Technol. 2020, 84, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Cristóvão, M.B.; Bento-Silva, A.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J. Detection of anticancer drugs in wastewater effluents: Grab versus passive sampling. Sci. Total Environ. 2021, 786, 147477. [Google Scholar] [CrossRef]
- Lima, L.M.; Silva, B.N.M.d.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review. Animals 2022, 12, 60. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, K.; Wang, Z.; Zhang, Y.; Yang, M.; Zhu, Y.G.; Virta, M.P.J.; Deng, Y. High-throughput single-cell technology reveals the contribution of horizontal gene transfer to typical antibiotic resistance gene dissemination in wastewater treatment plants. Environ. Sci. Technol. 2021, 55, 11824–11834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dong, Q.; Yuan, Z.; Huang, X.; Liu, Y. Fate characteristics, exposure risk, and control strategy of typical antibiotics in chinese sewerage system: A review. Environ. Int. 2022, 167, 107396. [Google Scholar] [CrossRef]
- Givens, C.E.; Kolpin, D.W.; Hubbard, L.E.; Meppelink, S.M.; Cwiertny, D.M.; Thompson, D.A.; Lane, R.F.; Wilson, M.C. Simultaneous stream assessment of antibiotics, bacteria, antibiotic resistant bacteria, and antibiotic resistance genes in an agricultural region of the united states. Sci. Total Environ. 2023, 904, 166753. [Google Scholar] [CrossRef]
- Akbari, M.Z.; Xu, Y.; Lu, Z.; Peng, L. Review of antibiotics treatment by advance oxidation processes. Environ. Adv. 2021, 5, 100111. [Google Scholar] [CrossRef]
- Yuan, Q.; Sui, M.; Qin, C.; Zhang, H.; Sun, Y.; Luo, S.; Zhao, J. Migration, transformation and removal of macrolide antibiotics in the environment: A review. Environ. Sci. Pollut. Res. 2022, 29, 26045–26062. [Google Scholar] [CrossRef] [PubMed]
- Fatimazahra, S.; Latifa, M.; Laila, S.; Monsif, K. Review of hospital effluents: Special emphasis on characterization, impact, and treatment of pollutants and antibiotic resistance. Environ. Monit. Assess. 2023, 195, 393. [Google Scholar] [CrossRef] [PubMed]
- Pariente, M.I.; Segura, Y.; Álvarez-Torrellas, S.; Casas, J.A.; de Pedro, Z.M.; Diaz, E.; García, J.; López-Muñoz, M.J.; Marugán, J.; Mohedano, A.F.; et al. Critical review of technologies for the on-site treatment of hospital wastewater: From conventional to combined advanced processes. J. Environ. Manag. 2022, 320, 115769. [Google Scholar] [CrossRef] [PubMed]

| Classification | Compound | Ionisation Mode | Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | Manual Analysis | HTA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD (ng/L) | LOQ (ng/L) | Reproducibility (%) | LOD (ng/L) | LOQ (ng/L) | Reproducibility (%) | |||||||
| β-lactams | Ampicillin | ESI+ | 350.2 | 105.9, 192.0 | 29 | 24 | 1.6 | 5.4 | 8.7 | 3.2 | 10.7 | 6.6 |
| Benzylpenicillin | ESI+ | 335.2 | 160.1, 174.0 | 32 | 23 | 0.5 | 1.7 | 8.3 | 1.0 | 3.3 | 4.6 | |
| Cefdinir | ESI+ | 369.2 | 170.0, 227.0 | 30 | 20 | 0.2 | 0.8 | 8.6 | 0.5 | 1.6 | 5.7 | |
| Cefpodoxime | ESI+ | 427.8 | 240.5, 395.9 | 31 | 15 | 0.3 | 1.1 | 6.1 | 0.6 | 2.1 | 13.1 | |
| Cefpodoxime proxetil | ESI+ | 557.5 | 409.8, 525.2 | 30 | 18 | 1.2 | 3.9 | 6.9 | 2.4 | 7.9 | 5.7 | |
| Ceftiofur | ESI+ | 524.1 | 240.9 | 38 | 18 | 0.6 | 2.1 | 4.4 | 1.3 | 4.3 | 1.6 | |
| New quinolones | Ciprofloxacin | ESI+ | 332.2 | 288.2, 314.2 | 40 | 25 | 0.7 | 2.2 | 5.0 | 1.3 | 4.5 | 6.0 |
| Enrofloxacin | ESI+ | 360.0 | 316.2, 245.2 | 37 | 19 | 0.2 | 0.8 | 7.8 | 0.5 | 1.5 | 5.8 | |
| Levofloxacin | ESI+ | 362.2 | 261.2, 318.2 | 40 | 21 | 0.3 | 1.0 | 11.5 | 0.6 | 2.0 | 5.2 | |
| Macrolides | Azithromycin | ESI+ | 350.2 | 105.9, 192.0 | 29 | 24 | 0.3 | 1.1 | 8.3 | 0.6 | 2.1 | 7.8 |
| Clarithromycin | ESI+ | 748.2 | 316.6, 558.3 | 38 | 18 | 0.5 | 1.6 | 8.4 | 0.9 | 3.1 | 9.0 | |
| Tetracyclines | Chlortetracycline | ESI+ | 479.2 | 443.5, 461.5 | 36 | 20 | 0.3 | 1.1 | 2.1 | 0.6 | 2.1 | 1.0 |
| Doxycycline | ESI+ | 445.2 | 428.3 | 32 | 18 | 0.3 | 1.0 | 3.1 | 0.6 | 2.0 | 3.8 | |
| Minocycline | ESI+ | 458.3 | 441.0 | 36 | 21 | 0.3 | 1.0 | 2.2 | 0.6 | 2.0 | 10.3 | |
| Oxytetracycline | ESI+ | 461.2 | 425.8 | 28 | 19 | 0.3 | 1.1 | 2.5 | 0.6 | 2.1 | 8.7 | |
| Tetracycline | ESI+ | 445.2 | 409.9, 427.1 | 28 | 20 | 0.4 | 1.3 | 3.1 | 0.8 | 2.5 | 9.6 | |
| Glycopeptide | Vancomycin | ESI+ | 724.2 | 82.9, 100.2 | 17 | 18 | 0.6 | 1.9 | 9.4 | 1.1 | 3.8 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azuma, T.; Matsunaga, N.; Ohmagari, N.; Kuroda, M. Development of a High-Throughput Analytical Method for Antimicrobials in Wastewater Using an Automated Pipetting and Solid-Phase Extraction System. Antibiotics 2024, 13, 335. https://doi.org/10.3390/antibiotics13040335
Azuma T, Matsunaga N, Ohmagari N, Kuroda M. Development of a High-Throughput Analytical Method for Antimicrobials in Wastewater Using an Automated Pipetting and Solid-Phase Extraction System. Antibiotics. 2024; 13(4):335. https://doi.org/10.3390/antibiotics13040335
Chicago/Turabian StyleAzuma, Takashi, Nobuaki Matsunaga, Norio Ohmagari, and Makoto Kuroda. 2024. "Development of a High-Throughput Analytical Method for Antimicrobials in Wastewater Using an Automated Pipetting and Solid-Phase Extraction System" Antibiotics 13, no. 4: 335. https://doi.org/10.3390/antibiotics13040335






