The Impact of SARS-CoV-2 Pandemic on Antibiotic Prescriptions and Resistance in a University Hospital from Romania
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blumenthal, K.G.; Peter, J.G.; Trubiano, J.A.; Phillips, E.J. Antibiotic allergy. Lancet 2019, 393, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Wu-Wu, J.W.F.; Guadamuz-Mayorga, C.; Oviedo-Cerdas, D.; Zamora, W.J. Antibiotic Resistance and Food Safety: Perspectives on New Technologies and Molecules for Microbial Control in the Food Industry. Antibiotics 2023, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Stewardship Programs in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit. World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/329404 (accessed on 20 November 2023).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. Wellcome Trust and HM Government. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 15 November 2023).
- Lagadinou, M.; Tsami, E.; Deligakis, A.; Paraskevas, T.; Michailides, C.; Velissaris, D.; Gkentzi, D.; Marangos, M. Knowledge and Attitudes of Healthcare Workers towards Antibiotic Use and Antimicrobial Resistance in Two Major Tertiary Hospitals in Western Greece. Antibiotics 2023, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Chaw, P.S.; Höpner, J.; Mikolajczyk, R. The knowledge, attitude and practice of health practitioners towards antibiotic prescribing and resistance in developing countries-A systematic review. J. Clin. Pharm. Ther. 2018, 43, 606–613. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 10 Global Health Issues to Track in 2021. Available online: https://www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021 (accessed on 10 September 2023).
- The World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. Available online: https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future (accessed on 11 December 2023).
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Neidell, M.J.; Cohen, B.; Furuya, Y.; Hill, J.; Jeon, C.Y.; Glied, S.; Larson, E.L. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin. Infect. Dis. 2012, 55, 807–815. [Google Scholar] [CrossRef]
- Nelson, E.R.; Hatfield, K.M.; Wolford, H. National estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin. Infect. Dis. 2021, 72, S17–S26. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobialresistance-europe-2019 (accessed on 10 September 2023).
- Högberg, L.D.; Vlahović-Palčevski, V.; Pereira, C.; Weist, K.; Monnet, D.L.; ESAC-Net Study Group. Decrease in community antibiotic consumption during the COVID-19 pandemic, EU/EEA, 2020. Eurosurveillance 2021, 26, 2101020. [Google Scholar] [CrossRef]
- Krockow, E.M.; Harvey, E.J.; Ashiru-Oredope, D. Addressing long- term and repeat antibiotic prescriptions in primary care: Considerations for a behavioural approach. BMJ Qual. Saf. 2022, 31, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Van Staa, T.; Li, Y.; Gold, N.; Chadborn, T.; Welfare, W.; Palin, V.; Ashcroft, D.M.; Bircher, J. Comparing antibiotic prescribing between clinicians in UK primary care: An analysis in a cohort study of eight different measures of antibiotic prescribing. BMJ Qual. Saf. 2022, 31, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Dolk, F.C.K.; Pouwels, K.B.; Smith, D.R.M.; Robotham, J.V.; Smieszek, T. Antibiotics in primary care in England: Which antibiotics are prescribed and for which conditions? J. Antimicrob. Chemother. 2018, 73 (Suppl. 2), ii2–ii10. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). The CDC COVID-19: U.S. Impact on Antimicrobial Resistance; Special Report; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2022.
- 2019-Ar-Threats-Report-508. 2019. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 15 September 2023).
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. [Google Scholar] [CrossRef]
- Filip, R.; Puscaselu, R.G.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Islam, T.; Islam, M.R. New Coronavirus Variants are Creating More Challenges to Global Healthcare System: A Brief Report on the Current Knowledge. Clin. Pathol. 2022, 15, 2632010X221075584. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Mundra, S. Increasing Consumption of Antibiotics during the COVID-19 Pandemic: Implications for Patient Health and Emerging Anti-Microbial Resistance. Antibiotics 2022, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Bednarčuk, N.; Golić Jelić, A.; Stoisavljević Šatara, S.; Stojaković, N.; Marković Peković, V.; Stojiljković, M.P.; Popović, N.; Škrbić, R. Antibiotic Utilization during COVID-19: Are We Over-Prescribing? Antibiotics 2023, 12, 308. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, X.; Liu, X.; Wen, X.; Wu, R.; Cui, D.; Mao, Z. Antibiotic Use in China’s Public Healthcare Institutions During the COVID-19 Pandemic: An Analysis of Nationwide Procurement Data, 2018–2020. Front. Pharmacol. 2022, 13, 813213. [Google Scholar] [CrossRef] [PubMed]
- Murgadella-Sancho, A.; Coloma-Conde, A.; Oriol-Bermúdez, I. Impact of the strategies implemented by an antimicrobial stewardship program on the antibiotic consumption in the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Khor, W.P.; Olaoye, O.; D’Arcy, N.; Krockow, E.M.; Elshenawy, R.A.; Rutter, V.; Ashiru-Oredope, D. The need for ongoing antimicrobial stewardship during the COVID-19 pandemic and actionable recommendations. Antibiotics 2020, 9, 904. [Google Scholar] [CrossRef]
- Smith, D.R.M.; Dolk, F.C.K.; Smieszek, T.; Robotham, J.V.; Pouwels, K.B. Understanding the gender gap in antibiotic prescribing: A cross-sectional analysis of English primary care. BMJ Open 2018, 8, e020203. [Google Scholar] [CrossRef] [PubMed]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Han, C.; Jang, H.; Oh, J. Excess mortality during the Coronavirus disease pandemic in Korea. BMC Public Health 2023, 23, 1698. [Google Scholar] [CrossRef]
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Sadeq, A.A.; Hasan, S.S.; AbouKhater, N.; Conway, B.R.; Abdelsalam, A.E.; Shamseddine, J.M.; Babiker, Z.O.E.; Nsutebu, E.F.; Bond, S.E.; Aldeyab, M.A. Exploring Antimicrobial Stewardship Influential Interventions on Improving Antibiotic Utilization in Outpatient and Inpatient Settings: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1306. [Google Scholar] [CrossRef]
- Macera, M.; Onorato, L.; Calò, F.; Monari, C.; Annibale, R.; Signoriello, G.; Donnarumma, G.; Montemurro, M.V.; Galdiero, M.; Coppola, N. The Impact of the SARS-Cov2 Pandemic on a Persuasive Educational Antimicrobial Stewardship Program in a University Hospital in Southern Italy: A Pre-Post Study. Antibiotics 2021, 10, 1405. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Model List of Essential Medicines—22nd List (2021); World Health Organization (WHO): Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 15 September 2023).
- Ryu, S.; Hwang, Y.; Ali, S.T.; Kim, D.S.; Klein, E.Y.; Lau, E.H.Y.; Cowling, B.J. Decreased Use of Broad-Spectrum Antibiotics During the Coronavirus Disease 2019 Epidemic in South Korea. J. Infect. Dis. 2021, 224, 949–955. [Google Scholar] [CrossRef]
- Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef]
- Hamidi, A.A.; Yilmaz, Ş. Antibiotic consumption in the hospital during COVID-19 pandemic, distribution of bacterial agents and antimicrobial resistance: A single-center study. J. Surg. Med. 2021, 5, 124–127. [Google Scholar] [CrossRef]
- Gonzalez-Zorn, B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 2021, 27, 646–647. [Google Scholar] [CrossRef]
- Wang, T.; Shen, L.; Yin, J.; Zhou, L.; Sun, Q. Antibiotic use in township hospitals during the COVID-19 pandemic in Shandong, China. Antimicrob. Resist. Infect. Control 2022, 11, 164. [Google Scholar] [CrossRef]
- Fjelltveit, E.B.; Cox, R.J.; Kittang, B.R.; Blomberg, B.; Buanes, E.A.; Group Bergen COVID-19 Research; Langeland, N.; Mohn, K.G. Lower antibiotic prescription rates in hospitalized COVID-19 patients than influenza patients, a prospective study. Infect. Dis. 2022, 54, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Buehrle, D.J.; Decker, B.K.; Wagener, M.M.; Adalja, A.; Singh, N.; McEllistrem, M.C.; Nguyen, M.H.; Clancy, C.J. Antibiotic Consumption and Stewardship at a Hospital outside of an Early Coronavirus Disease 2019 Epicenter. Antimicrob. Agents Chemother. 2020, 64, e01011-20. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, M.; Izadi, H.; Chouhdari, A.; Rezaeifard, M. Anti-inflammatory Effect of Metronidazole in Hospitalized Patients with Pneumonia due to COVID-19. Iran. J. Pharm. Res. 2021, 20, 532–540. [Google Scholar] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Pellegrino, M.; Giuzio, F.; Marra, M.; Rosano, C.; Saturnino, C.; Sinicropi, M.S.; Aquaro, S. Antibiotic-Resistant ESKAPE Pathogens and COVID-19: The Pandemic beyond the Pandemic. Viruses 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.Á.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Márquez-Valdelamar, L.M.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control 2023, 51, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.B.; Bahwerth, F.S.; Alsafi, R.; Elsebaei, E.A.; Ebid, G.T.; Theyab, A.; Assaggaf, H.; Bahwerth, F.; Labah, E.; Theyab, A. The Prevalence and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus Before and After the COVID-19 Pandemic in a Tertiary Saudi Hospital. Cureus 2024, 16, e54809. [Google Scholar] [CrossRef]
- Zarfel, G.; Schmidt, J.; Luxner, J.; Grisold, A.J. No Changes in the Occurrence of Methicillin-Resistant Staphylococcus aureus (MRSA) in South-East Austria during the COVID-19 Pandemic. Pathogens 2023, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2022. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 10 August 2023).
- World Health Organization (WHO). The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization (WHO): Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 15 November 2023).
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: https://www.eucast.org/2024 (accessed on 25 January 2024).
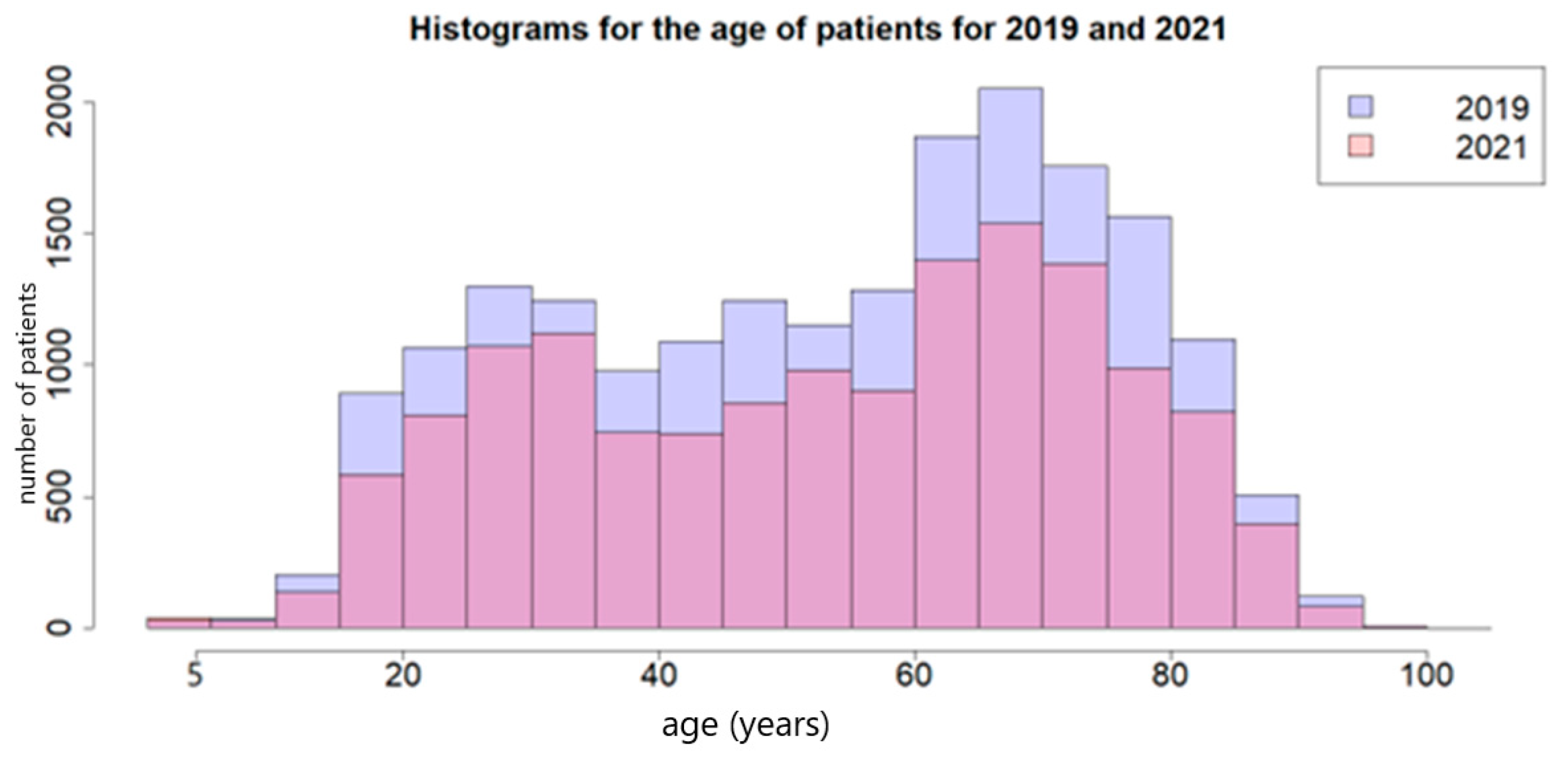
| Patient Characteristics | 2019 N (%) | 2021 N (%) | p-Value |
|---|---|---|---|
| Number of patients | 36,076 | 23,903 | |
| Number of patients treated with antibiotics | 19,502 (54.06%) | 14,660 (61.33%) | p < 0.00001 * |
| Gender | |||
| Male | 7872 (40.37%) | 5753 (39.24%) | p < 0.05 * |
| Female | 11,630 (59.63%) | 14,255 (60.76%) | |
| Age in years | |||
| Mean | 54.15 | 53.72 | p > 0.05 ** |
| SD | 20.64 | 20.66 | |
| Outcome | |||
| Died | 902 (4.63%) | 1179 (8.04%) | p < 0.05 * |
| Discharged | 18,600 (95.37%) | 13,481 (91.96%) |
| Type of Infection/Condition | 2019 | 2021 | p * | ||
|---|---|---|---|---|---|
| Genito-urinary | 2841 | 14.57% | 1498 | 10.22% | <0.00001 |
| Multiple localization | 2384 | 12.22% | 1877 | 12.80% | 0.11 |
| Wound | 2092 | 10.73% | 897 | 6.12% | <0.00001 |
| Digestive | 1126 | 5.77% | 705 | 4.81% | <0.00001 |
| Heart diseases | 1384 | 7.10% | 1050 | 7.16% | 0.83 |
| Respiratory | 986 | 5.06% | 815 | 5.56% | <0.05 |
| Systemic | 744 | 3.81% | 565 | 3.85% | 0.87 |
| Surgical complications | 213 | 1.09% | 443 | 3.02% | <0.00001 |
| COVID-19 | 0 | 0 | 109 | 0.74% | - |
| COVID-19 and associated bacterial infection | 0 | 0 | 475 | 3.24% | - |
| Other viral aetiology | 30 | 0.15% | 298 | 2.03% | <0.00001 |
| Osteoarthritis | 49 | 0.25% | 36 | 0.25% | 0.99 |
| Nervous system | 11 | 0.06% | 16 | 0.11% | 0.13 |
| Tuberculosis | 5 | 0.03% | 7 | 0.05% | 0.43 |
| Other conditions without infectious disease | 1205 | 6.18% | 651 | 4.44% | <0.00001 |
| Prophylactic administration | 6432 | 32.98% | 5218 | 35.59% | <0.00001 |
| Total | 19,502 | 14,660 | 34,162 | ||
| Ward | 2019 Number (%) | 2021 Number (%) | p * |
|---|---|---|---|
| Surgical | 11,453 (58.73) | 7875 (53.72) | p < 0.00001 |
| Medical | 4462 (22.88) | 2751 (18.77) | |
| Intensive care unit | 3587 (18.39) | 4034 (27.52) |
| Ward | 2019 | 2021 | p * |
|---|---|---|---|
| Surgical | 933.13 (56.06) | 4421.46 (73.91) | <0.00001 |
| Medical | 350.21 (21.04) | 1215.75 (20.34) | 0.54 |
| Intensive care unit | 381.04 (22.89) | 344.59 (5.76) | <0.00001 |
| AWaRe Group | 2019 Number (%) | 2021 Number (%) | p * |
|---|---|---|---|
| Watch | 1055.81 (63.44) | 3287.62 (54.96) | <0.00001 |
| Access | 555.26 (33.36) | 2500.74 (41.81) | |
| Reserve | 19.26 (1.16) | 47.83 (0.8) |
| Access Group | 2019 | 2021 | p * |
|---|---|---|---|
| Metronidazole | 104.81 | 1216.38 | <0.00001 |
| Amikacin | 34.24 | 308.83 | <0.00001 |
| Ampicillin | 132.46 | 297.06 | <0.00001 |
| Gentamicin | 89.45 | 116.54 | <0.00001 |
| Clindamycin | 42.85 | 89.07 | <0.01 |
| Benzylpenicillin | 22.71 | 2.38 | <0.00001 |
| Amoxicillin/clavulanic acid | 61.47 | 211.72 | 0.82 |
| Amoxicillin | 43.03 | 170.93 | 0.61 |
| Doxycycline | 8.76 | 54.61 | 0.16 |
| Oxacillin | 9.78 | 12.13 | 0.05 |
| Ampicillin/sulbactam | 5.33 | 11.71 | 0.51 |
| Cefazolin | 0.37 | - | - |
| Watch Group | 2019 | 2021 | p * |
|---|---|---|---|
| Ceftriaxone | 450.90 | 1438.52 | <0.05 |
| Cefuroxime | 255.52 | 449.36 | <0.00001 |
| Cefixime | 9.95 | 434.70 | <0.00001 |
| Rifaximin | 67.63 | 123.53 | <0.00001 |
| Teicoplanin | 22.20 | 20.06 | <0.00001 |
| Ciprofloxacin | 88.42 | 288.43 | 0.45 |
| Meropenem | 31.90 | 145.61 | 0.25 |
| Ceftazidime | 25.21 | 59.86 | 0.1 |
| Cefoperazone | 21.57 | 55.75 | 0.24 |
| Vancomycin | 18.22 | 57.63 | 0.73 |
| Levofloxacin | 10,54 | 59.81 | 0.21 |
| Moxifloxacin | 15.85 | 35.20 | 0.15 |
| Clarithromycin | 7.77 | 31.24 | 0.93 |
| Ertapenem | 4.81 | 18.11 | 1 |
| Cefepime | 2.34 | 19.09 | 0.34 |
| Rifampicin | 4.46 | 15.92 | 1 |
| Piperacillin/tazobactam | 3.36 | 10.29 | 1 |
| Norfloxacin | 3.17 | 7.07 | 0.74 |
| Erythromycin | 2.6 | 7.13 | 1 |
| Fosfomycin | 2.12 | 5.93 | 1 |
| Imipenem/Cilastin | 3.04 | 4.93 | 0.49 |
| Ofloxacin | 2.31 | 3.89 | 0.65 |
| Azithromycin | 0.25 | 4.87 | 0.69 |
| Cefaclor | 1.67 | - | - |
| Reserve Group | 2019 | 2021 | p * |
|---|---|---|---|
| Colistin | 8.98 | 21.54 | 0.42 |
| Tigecycline | 4.1 | 12.38 | 0.99 |
| Linezolid | 5.35 | 6.74 | 0.12 |
| Ceftazidime/avibactam | 0.83 | 6.91 | 0.75 |
| Imipenem/cilastatin/relebactam | - | 0.23 | - |
| Class | 2019 | 2021 | p * |
|---|---|---|---|
| Cephems | 767.53 | 2457.28 | <0.001 |
| Imidazoles | 104.81 | 1216.38 | <0.00001 |
| Penicillins | 207.98 | 482.50 | <0.00001 |
| Rifamycins | 67.63 | 123.53 | <0.00001 |
| Lincosamides | 42.85 | 89.07 | <0.01 |
| Glycopeptides | 40.42 | 77.69 | <0.01 |
| Aminoglycosides | 123.69 | 425.37 | 0.69 |
| Quinolones | 120.29 | 394.40 | 0.39 |
| Beta-lactam/Inhibitors | 105.05 | 386.32 | 0.87 |
| Penems | 39.75 | 168.88 | 0.38 |
| Tetracyclines | 12.86 | 67 | 0.27 |
| Macrolides | 10.62 | 43.24 | 0.84 |
| Lipopeptides | 8.98 | 21.54 | 0.41 |
| Ansamycins | 4.46 | 15.92 | 1 |
| Oxazolidinones | 5.35 | 6.75 | 0.12 |
| Fosfomycin | 2.12 | 5.93 | 1 |
| Folate pathway inhibitors | - | - | - |
| Nitrofurans | - | - | - |
| Antibiotic Name | Cumulative Sensitivity Rate (%) | p * | |
|---|---|---|---|
| 2019 | 2021 | ||
| Amikacin | 84.68 | 78.39 | <0.00001 |
| Piperacillin/tazobactam | 64.80 | 53.60 | <0.00001 |
| Ceftazidime/avibactam | 71.86 | 46.82 | <0.00001 |
| Ceftazidime | 58.40 | 55.69 | <0.05 |
| Cefepime | 65.29 | 59.29 | <0.00001 |
| Cefotaxime | 59.98 | 56.70 | <0.05 |
| Cefixime | 44.40 | 36.00 | <0.05 |
| Trimethoprim/sulfamethoxazole | 56.61 | 52.99 | <0.01 |
| Fosfomycin | 88.99 | 91.39 | <0.05 |
| Vancomycin | 79.57 | 88.79 | <0.00001 |
| Clindamycin | 55.33 | 62.94 | <0.01 |
| Colistin | 71.70 | 81.26 | <0.00001 |
| Imipenem | 65.22 | 56.32 | <0.00001 |
| Meropenem | 74.01 | 60.50 | <0.00001 |
| Ertapenem | 84.10 | 77.42 | <0.00001 |
| Penicillin G | 38.08 | 26.02 | <0.00001 |
| Azithromycin | 50.00 | 20.83 | <0.05 |
| Ciprofloxacin | 48.91 | 42.59 | <0.00001 |
| Levofloxacin | 49.40 | 43.00 | <0.05 |
| Ofloxacin | 51.19 | 67.84 | <0.001 |
| Gentamicin | 65.60 | 66.50 | 0.39 |
| Tobramycin | 37.58 | 32.97 | 0.06 |
| Rifampin | 82.89 | 88.04 | 0.056 |
| Amoxicillin/clavulanic acid | 45.99 | 44.20 | 0.22 |
| Ampicillin/sulbactam | 41.51 | 38.96 | 0.61 |
| Cefoperazone | 58.31 | 51.19 | 0.28 |
| Ceftriaxone | 52.57 | 49.83 | 0.34 |
| Cefuroxime | 58.06 | 57.40 | 0.56 |
| Teicoplanin | 92.14 | 93.97 | 0.13 |
| Erythromycin | 32.33 | 31.09 | 0.55 |
| Nitrofurantoin | 61.19 | 63.81 | 0.12 |
| Linezolid | 91.54 | 91.56 | 1 |
| Ampicillin | 27.02 | 28.30 | 0.32 |
| Oxacillin | 35.07 | 33.13 | 0.61 |
| Moxifloxacin | 73.99 | 71.85 | 0.35 |
| Tetracycline | 34.04 | 38.56 | 0.07 |
| Strain/Resistance Phenotype | 2019 | 2021 | p * |
|---|---|---|---|
| Acinetobacter baumannii | 501 | 751 | |
| AB-CR | 365 | 691 | <0.00001 |
| Enterobacter sp. | 142 | 126 | |
| E-ESBL | 42 | 62 | <0.01 |
| E-CR | 5 | 15 | <0.05 |
| Escherichia coli | 1368 | 1261 | |
| EC-ESBL | 218 | 302 | <0.00001 |
| EC-CR | 41 | 63 | <0.05 |
| Enterococcus faecium | 140 | 178 | |
| VRE | 46 | 60 | 0.9681 |
| Klebsiella pneumoniae | 833 | 1039 | |
| KP-ESBL | 450 | 571 | 0.721 |
| KP-CR | 333 | 520 | <0.00001 |
| Pseudomonas aeruginosa | 521 | 575 | |
| PA-CR | 219 | 299 | <0.01 |
| Staphylococcus aureus | 626 | 312 | |
| MRSA | 426 | 125 | <0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaha, D.C.; Ilea, C.D.N.; Dorobanțu, F.R.; Pantiș, C.; Pop, O.N.; Dascal, D.G.; Dorobanțu, C.D.; Manole, F. The Impact of SARS-CoV-2 Pandemic on Antibiotic Prescriptions and Resistance in a University Hospital from Romania. Antibiotics 2024, 13, 477. https://doi.org/10.3390/antibiotics13060477
Zaha DC, Ilea CDN, Dorobanțu FR, Pantiș C, Pop ON, Dascal DG, Dorobanțu CD, Manole F. The Impact of SARS-CoV-2 Pandemic on Antibiotic Prescriptions and Resistance in a University Hospital from Romania. Antibiotics. 2024; 13(6):477. https://doi.org/10.3390/antibiotics13060477
Chicago/Turabian StyleZaha, Dana Carmen, Codrin Dan Nicolae Ilea, Florica Ramona Dorobanțu, Carmen Pantiș, Ovidiu Nicolae Pop, Dorina Gabriela Dascal, Cătălin Dorin Dorobanțu, and Felicia Manole. 2024. "The Impact of SARS-CoV-2 Pandemic on Antibiotic Prescriptions and Resistance in a University Hospital from Romania" Antibiotics 13, no. 6: 477. https://doi.org/10.3390/antibiotics13060477
APA StyleZaha, D. C., Ilea, C. D. N., Dorobanțu, F. R., Pantiș, C., Pop, O. N., Dascal, D. G., Dorobanțu, C. D., & Manole, F. (2024). The Impact of SARS-CoV-2 Pandemic on Antibiotic Prescriptions and Resistance in a University Hospital from Romania. Antibiotics, 13(6), 477. https://doi.org/10.3390/antibiotics13060477








