Incidence of Antibiotic Exposure for Suspected and Proven Neonatal Early-Onset Sepsis between 2019 and 2021: A Retrospective, Multicentre Study
Abstract
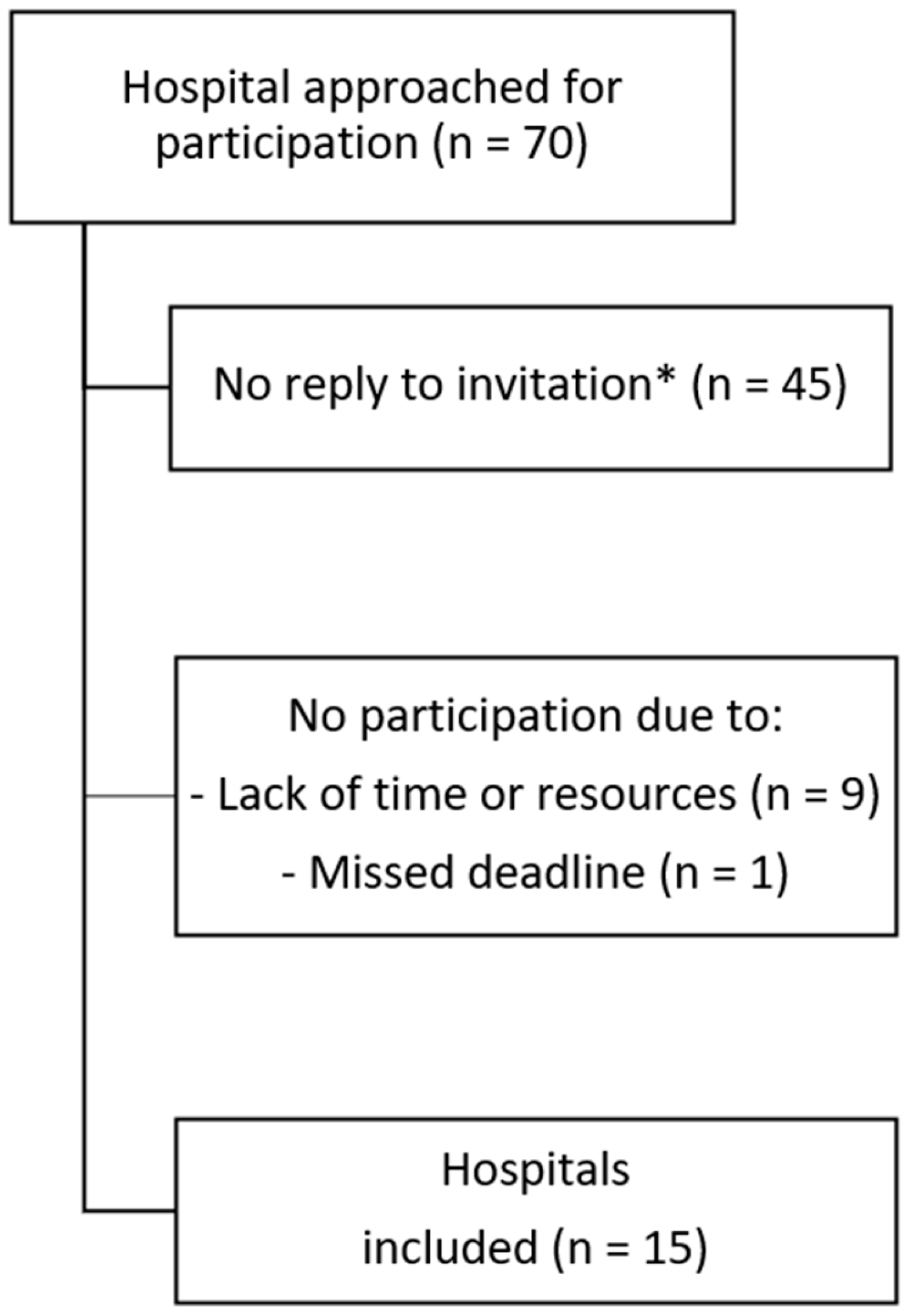
:1. Introduction
2. Results
2.1. Overall Initiation and Continuation of Antibiotic Therapy, and Proven EOS Incidence
2.2. Variation among Hospitals
- Regional Hospitals (Hospitals A–M):
- Academic Hospitals (Hospital N and O):
2.3. Academic vs. Regional Hospitals
2.4. Differences within a Three-Year Period
3. Discussion
3.1. Strengths/Limitations
3.2. Clinical Implications
4. Methods
4.1. Study Design, Population and Period
4.2. Data Collection
4.3. Study Outcomes and Definitions
4.4. Statistical Analysis
4.5. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agyeman, P.K.A.; Schlapbach, L.J.; Giannoni, E.; Stocker, M.; Posfay-Barbe, K.M.; Heininger, U.; Schindler, M.; Korten, I.; Konetzny, G.; Niederer-Loher, A.; et al. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: A population-based cohort study. Lancet Child Adolesc. Health 2017, 1, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Smith, P.B.; Hornik, C.P.; Zimmerman, K.O.; Hornik, C.D.; Pradeep, S.; Clark, R.H.; Benjamin, D.K.; Laughon, M.; Greenberg, R.G. Medication Use in the Neonatal Intensive Care Unit and Changes from 2010 to 2018. J. Pediatr. 2021, 240, 66–71.e4. [Google Scholar] [CrossRef] [PubMed]
- Al-Turkait, A.; Szatkowski, L.; Choonara, I.; Ojha, S. Review of Drug Utilization Studies in Neonatal Units: A Global Perspective. Int. J. Environ. Res. Public Health 2020, 17, 5669. [Google Scholar] [CrossRef]
- Al-Turkait, A.; Szatkowski, L.; Choonara, I.; Ojha, S. Drug utilisation in neonatal units in England and Wales: A national cohort study. Eur. J. Clin. Pharmacol. 2022, 78, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Fjalstad, J.W.; Esaiassen, E.; Juvet, L.K.; Anker, J.N.v.D.; Klingenberg, C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: A systematic review. J. Antimicrob. Chemother. 2017, 73, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Esaiassen, E.; Fjalstad, J.W.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Soh, S.-E.; Lee, Y.; Kwek, K.; Holbrook, J.; Van der Beek, E.; Shek, L.; Goh, A.; Teoh, O.; Godfrey, K.; et al. Ratio of Klebsiella/Bifidobacterium in early life correlates with later development of paediatric allergy. Benef. Microbes 2017, 8, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Oosterloo, B.C.; van Elburg, R.M.; Rutten, N.B.; Bunkers, C.M.; Crijns, C.E.; Meijssen, C.B.; Oudshoorn, J.H.; Rijkers, G.T.; van der Ent, C.K.; Vlieger, A.M. Wheezing and infantile colic are associated with neonatal antibiotic treatment. Pediatr. Allergy Immunol. 2018, 29, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged Initial Empirical Antibiotic Treatment is Associated with Adverse Outcomes in Premature Infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, K.; Vlieger, A.M.; Oosterloo, B.C.; Waarlo, S.; van Elburg, R.M. Higher risk of allergies at 4–6 years of age after systemic antibiotics in the first week of life. Allergy 2021, 76, 2599–2602. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, K.; Oosterloo, B.C.; Riet, E.V.; Reichwein, L.C.; Vlieger, A.M.; van Elburg, R.M. The association between exposure to antibiotics in the first week of life and later otitis media: The INCA study. Int. J. Pediatr. Otorhinolaryngol. 2023, 164, 111415. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, K.; Van Daele, E.; Vlieger, A.M.; Daams, J.G.; Knol, J.; van Elburg, R.M. Early life antibiotics and childhood gastrointestinal disorders: A systematic review. BMJ Paediatr. Open 2021, 5, e001028. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, K.; Oosterloo, B.C.; Vlieger, A.M.; Rutten, N.B.; Bunkers, C.M.; Wit, E.C.; van Elburg, R.M. Antibiotic Treatment in the First Week of Life Impacts the Growth Trajectory in the First Year of Life in Term Infants. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 131–136. [Google Scholar] [CrossRef]
- Uzan-Yulzari, A.; Turta, O.; Belogolovski, A.; Ziv, O.; Kunz, C.; Perschbacher, S.; Neuman, H.; Pasolli, E.; Oz, A.; Ben-Amram, H.; et al. Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization. Nat. Commun. 2021, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; van Houten, M.A.; Watson, R.L.; Chu, M.L.J.N.; Arp, K.; de Waal, W.J.; Schiering, I.; Plötz, F.B.; Willems, R.J.L.; van Schaik, W.; et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: A randomized trial. Nat. Commun. 2022, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; el Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; Dimopoulou, V.; et al. Less is more: Antibiotics at the beginning of life. Nat. Commun. 2023, 14, 2423. [Google Scholar] [CrossRef] [PubMed]
- Flannery, D.D.; Horbar, J.D. Metrics of neonatal antibiotic use. Semin. Perinatol. 2020, 44, 151329. [Google Scholar] [CrossRef]
- Schulman, J.; Benitz, W.E.; Profit, J.; Lee, H.C.; Dueñas, G.; Bennett, M.V.; Jocson, M.A.; Schutzengel, R.; Gould, J.B. Newborn Antibiotic Exposures and Association with Proven Bloodstream Infection. Pediatrics 2019, 144, e20191105. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Dimopoulou, V.; Klingenberg, C.; Navér, L.; Nordberg, V.; Berardi, A.; el Helou, S.; Fusch, G.; Bliss, J.M.; Lehnick, D.; et al. Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia. JAMA Netw. Open 2022, 5, e2243691. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Cannell, S.; Davies, G.; Natti, M.S.; Kirupaalar, V.; Abelian, A.; Saeed, S.; Smith, R.; Manikonda, R.; Pitchaikani, P.K.; et al. Implementation of an adapted Sepsis Risk Calculator algorithm to reduce antibiotic usage in the management of early onset neonatal sepsis: A multicentre initiative in Wales, UK. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 107, 303–310. [Google Scholar] [CrossRef]
- Payton, K.S.E.; Bennett, M.V.; Schulman, J.; Benitz, W.E.; Stellwagen, L.; Darmstadt, G.L.; Quinn, J.; Kristensen-Cabrera, A.I.; Breault, C.C.; Bolaris, M.; et al. 28 NICUs participating in a quality improvement collaborative targeting early-onset sepsis antibiotic use. J. Perinatol. 2024, 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, B.M.; Achten, N.B.; Bekhof, J.; Evers, E.E.; Berk, M.; Kamps, A.W.A.; Rijpert, M.; Tusscher, G.W.T.; van Houten, M.A.; Plotz, F.B. Multicentre study found that adherence to national antibiotic recommendations for neonatal early-onset sepsis was low. Acta Paediatr. 2021, 110, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Schrag, S.J.; Farley, M.M.; Petit, S.; Reingold, A.; Weston, E.J.; Pondo, T.; Jain, J.H.; Lynfield, R. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics 2016, 138, e20162013. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Puopolo, K.M.; Hansen, N.I.; Sánchez, P.J.; Bell, E.F.; Carlo, W.A.; Cotten, C.M.; D’angio, C.T.; Kazzi, S.N.J.; Poindexter, B.B.; et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020, 174, e200593. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Agyeman, P.K.; Stocker, M.; Posfay-Barbe, K.M.; Heininger, U.; Spycher, B.D.; Bernhard-Stirnemann, S.; Niederer-Loher, A.; Kahlert, C.R.; Donas, A.; et al. Neonatal Sepsis of Early Onset, and Hospital-Acquired and Community-Acquired Late Onset: A Prospective Population-Based Cohort Study. J. Pediatr. 2018, 201, 106–114.e4. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.J.; Pondo, T.M.; Lewis, M.M.; Martell-Cleary, P.M.; Morin, C.; Jewell, B.; Daily, P.; Apostol, M.; Petit, S.; Farley, M.; et al. The Burden of Invasive Early-onset Neonatal Sepsis in the United States, 2005–2008. Pediatr. Infect. Dis. J. 2011, 30, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Vatne, A.; Klingenberg, C.; Rettedal, S.; Øymar, K. Early-Onset Sepsis in Neonates—A Population-Based Study in South-West Norway From 1996 to 2018. Front. Pediatr. 2021, 9, 634798. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, B.M.; van Dorth, J.R.; Achten, N.B.; Plötz, F.B. Factors Associated with Prolonged Antibiotic Therapy in Neonates with Suspected Early-Onset Sepsis. Antibiotics 2024, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Mundal, H.S.; Rønnestad, A.; Klingenberg, C.; Stensvold, H.J.; Størdal, K. Antibiotic Use in Term and Near-Term Newborns. Pediatrics 2021, 148, e2021051339. [Google Scholar] [CrossRef]
- Gyllensvärd, J.; Studahl, M.; Gustavsson, L.; Hentz, E.; Åkesson, K.; Li, H.; Norman, M.; Elfvin, A.; Håkansson, S.; SWENAB Study Group. Antibiotic Use in Late Preterm and Full-Term Newborns Key Points + Supplemental content. JAMA Netw. Open 2024, 7, e243362. [Google Scholar] [CrossRef] [PubMed]
- NVOG (Nederlands Vereniging voor Obstetrie en Gynaecologie); NVK (Nederlandse Vereniging voor Kindergeneeskunde). Preventie en Behandeling van Early-Onset Neonatale Infecties; NVK Richtlijnen: Utrecht, The Netherlands, 2017; pp. 1–94. [Google Scholar]
- Vatne, A.; Klingenberg, C.; Øymar, K.; E Rønnestad, A.; Manzoni, P.; Rettedal, S. Reduced Antibiotic Exposure by Serial Physical Examinations in Term Neonates at Risk of Early-onset Sepsis. Pediatr. Infect. Dis. J. 2020, 39, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Sloane, A.; Carola, D.; Lafferty, M.; Edwards, C.; Greenspan, J.; Aghai, Z. Management of infants born to mothers with chorioamnionitis: A retrospective comparison of the three approaches recommended by the committee on fetus and newborn. J. Neonatal-Perinatal Med. 2021, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Joshi, N.S.; Frymoyer, A.; Achepohl, G.D.; Dang, R.; Taylor, N.K.; Salomon, J.A.; Goldhaber-Fiebert, J.D.; Owens, D.K. Resource Utilization and Costs Associated with Approaches to Identify Infants with Early-Onset Sepsis. MDM Policy Pr. 2024, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Achten, N.B.; Klingenberg, C.; Benitz, W.E.; Stocker, M.; Schlapbach, L.J.; Giannoni, E.; Bokelaar, R.; Driessen, G.J.A.; Brodin, P.; Uthaya, S.; et al. Association of Use of the Neonatal Early-Onset Sepsis Calculator with Reduction in Antibiotic Therapy and Safety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Cantey, J.B.; Baird, S.D. Ending the Culture of Culture-Negative Sepsis in the Neonatal ICU. Pediatrics 2017, 140, e20170044. [Google Scholar] [CrossRef] [PubMed]
- Schelonka, R.L.; Chai, M.K.; Yoder, B.A.; Hensley, D.; Brockett, R.M.; Ascher, D.P. Volume of blood required to detect common neonatal pathogens. J. Pediatr. 1996, 129, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sabui, T.; Tudehope, D.; Tilse, M. Clinical significance of quantitative blood cultures in newborn infants. J. Paediatr. Child Health 1999, 35, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gray, J.E. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J. Perinatol. 2021, 41, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Dhudasia, M.B.; Benitz, W.E.; Flannery, D.D.; Christ, L.; Rub, D.; Remaschi, G.; Puopolo, K.M.; Mukhopadhyay, S. Diagnostic Performance and Patient Outcomes With C-Reactive Protein Use in Early-Onset Sepsis Evaluations. J. Pediatr. 2023, 256, 98. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; van Herk, W.; el Helou, S.; Dutta, S.; Schuerman, F.A.B.A.; van den Tooren-de Groot, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; Moonen, R.; et al. C-Reactive Protein, Procalcitonin, and White Blood Count to Rule Out Neonatal Early-onset Sepsis Within 36 Hours: A Secondary Analysis of the Neonatal Procalcitonin Intervention Study. Clin. Infect. Dis. 2020, 73, e383–e390. [Google Scholar] [CrossRef] [PubMed]
- Stocker, M.; van Herk, W.; el Helou, S.; Dutta, S.; Fontana, M.S.; Schuerman, F.A.; van den Tooren-de, R.K.; Wieringa, J.W.; Janota, J.; van der Meer-Kappelle, L.H.; et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: A multicentre, randomised controlled trial (NeoPIns). Lancet 2017, 390, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fetus and Newborn; Barfield, W.D.; Papile, L.-A.; Baley, J.E.; Benitz, W.; Cummings, J.; Carlo, W.A.; Kumar, P.; Polin, R.A.; Tan, R.C.; et al. Levels of Neonatal Care. Pediatrics 2012, 130, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Perined. Available online: https://wwwperistatNl/2022 (accessed on 22 December 2023).

| All Hospitals N = 15 n (%, 95% CI) | Academic Hospitals (Level IV Facilities) N = 2 n (%, 95% CI) | Regional Hospitals (Level I–II Facilities) N = 13 n (%, 95% CI) | Academic vs. Regional | |
|---|---|---|---|---|
| Relative Risk (95% CI) e | ||||
| Number of births | 103492 | 10912 | 92580 | NA |
| Culture-proven EOS a | 117 (0.11%, 0.09–0.14) | 30 (0.27%, 0.19–0.39) | 87 (0.09%, 0.08–0.12) | 2.93 (1.93–4.42) |
| Antibiotic therapy initiation b | 4755 (4.6%, 4.5–4.7) | 1240 (11.4%, 10.8–12.0) | 3515 (3.8%, 3.7–3.9) | 2.99 (2.81–3.18) |
| Antibiotic therapy continuation >48 h/total number of neonates initiated | 2399 (50.5%, 49.0–51.9) | 323 (26.0%, 23.6–28.6) | 2076 (59.1%, 57.4–60.7) | 0.44 (0.40–0.48) |
| Antibiotic therapy continuation >48 h/total number of births | 2399 (2.3%, 2.2–2.4) | 323 (3.0%, 2.7–3.3) | 2076 (2.2%, 2.2–2.3) | 1.32 (1.18–1.48) |
| Number initiated per culture-proven sepsis case c | 40.6 | 41.3 | 40.4 | 1.00 (0.99–1.01) |
| Number continued per culture-proven sepsis case d | 20.5 | 10.8 | 23.9 | 0.94 (0.91–0.98) |
| Pathogen Determination | All Positive Blood Cultures | Academic Hospitals | Regional Hospitals |
|---|---|---|---|
| N = 117 | N = 30 | N = 87 | |
| Streptococcus agalactiae | 47 (40.1%) | 9 (30.0%) | 38 (43.7%) |
| Escherichia coli | 25 (23.3%) | 7 (23.3%) | 18 (20.7%) |
| Staphylococcus aureus | 13 (11.1%) | 5 (16.7%) | 8 (9.2%) |
| Streptococcus mitis | 5 (4.3%) | 0 (0.0%) | 5 (5.7%) |
| Streptococcus anginosus | 4 (3.4%) | 2 (6.7%) | 2 (2.3%) |
| Enterococcus faecalis | 4 (3.4%) | 1 (3.3%) | 3 (3.4%) |
| Staphylococcus epidermidis * | 3 (2.6%) | 1 (3.3%) | 2 (2.3%) |
| Listeria monocytogenes | 3 (2.6%) | 3 (10.0%) | 0 (0.0%) |
| Other pathogens ** | 13 (11.1%) | 3 (10.0%) | 10 (11.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Veen, L.E.J.; van der Weijden, B.M.; Achten, N.B.; van der Lee, L.; Hol, J.; van Rossem, M.C.; Rijpert, M.; Oorthuys, A.O.J.; van Beek, R.H.T.; Dubbink-Verheij, G.H.; et al. Incidence of Antibiotic Exposure for Suspected and Proven Neonatal Early-Onset Sepsis between 2019 and 2021: A Retrospective, Multicentre Study. Antibiotics 2024, 13, 537. https://doi.org/10.3390/antibiotics13060537
van Veen LEJ, van der Weijden BM, Achten NB, van der Lee L, Hol J, van Rossem MC, Rijpert M, Oorthuys AOJ, van Beek RHT, Dubbink-Verheij GH, et al. Incidence of Antibiotic Exposure for Suspected and Proven Neonatal Early-Onset Sepsis between 2019 and 2021: A Retrospective, Multicentre Study. Antibiotics. 2024; 13(6):537. https://doi.org/10.3390/antibiotics13060537
Chicago/Turabian Stylevan Veen, Liesanne E. J., Bo M. van der Weijden, Niek B. Achten, Lotte van der Lee, Jeroen Hol, Maaike C. van Rossem, Maarten Rijpert, Anna O. J. Oorthuys, Ron H. T. van Beek, Gerdien H. Dubbink-Verheij, and et al. 2024. "Incidence of Antibiotic Exposure for Suspected and Proven Neonatal Early-Onset Sepsis between 2019 and 2021: A Retrospective, Multicentre Study" Antibiotics 13, no. 6: 537. https://doi.org/10.3390/antibiotics13060537





