Exploring Prescription Practices: Insights from an Antimicrobial Stewardship Program at a Tertiary Healthcare Facility, Rwanda
Abstract
1. Introduction
2. Results
2.1. AMS Activities That Were Working Well
2.1.1. Laboratory Utilization: HCWs across Various Departments (ICU, Pediatrics, and Laboratory) Emphasized the Importance of Laboratory Testing to Guide Antibiotic Selection—This Included Using Culture Results to Adjust Initial Empiric Therapy and following Established Protocols for Requesting and Interpreting Tests
“For us when a patient comes to ICU, we check their diagnosis and if there is a corresponding protocol, especially international, for example, if someone has sepsis, you assess the score then with this you can tell which medicine to give, does he need an antibiotic or not, so we base on the patient’s assessment. You can guess which is the likely microbe, if it is resistant or not and then, so you do laboratory, tests and start antibiotics if you find the pertinent need. Then, you can stop after you get the antibiogram, so I can say that here at King Faisal, there is no problem. I have visited the laboratory, and I didn’t find any problem”.“ICU Doctor”—KFH/HCW/006-
2.1.2. Communication and Guidelines: Several HCWs Mentioned Clear Communication Protocols within the Hospital Regarding AMS—This Included Timely Reporting of Laboratory Results and Adherence to National Guidelines for Antibiotic Use
“Most of the time: when the patient comes with fever, we check sensitivity, but when the patient is severely ill, she is put-on broad-spectrum antibiotic then waits for 2–3 days. Then, adjust antibiotic after getting laboratory results”.“Nurse in ICU”—KFH/HCW/003-
“Well, here in hospital, there are guidelines regarding how we communicate within the Hospital. When we receive something like a blood culture or a swab, we follow those guidelines. If, for instance, a sample like a blood culture is growing, we have to communicate with the doctor. We inform them that the bacteria that’s growing is either gram-negative or gram-positive, and we provide them with a preliminary report. Then, we tell them that after two days, when checking the system, there will be the final result”.“Laboratory technician”—KFH/HCW/004-
2.1.3. Infection Prevention and Control (IPC): The IPC Department Was Highlighted as a Key Player in the AMS Program—HCWs Described Routine Practices Such as Handwashing Education, Environmental Cleaning, and Equipment Maintenance to Prevent the Spread of Resistant Pathogens
“All of us have taken this as a responsibility, And we do practice hand hygiene in the clinical areas. We practice the five moments of hand hygiene. We do monitor that and audit. And any gaps we find, we try to close them”.“IPC Officer”—KFH/HCW/005-
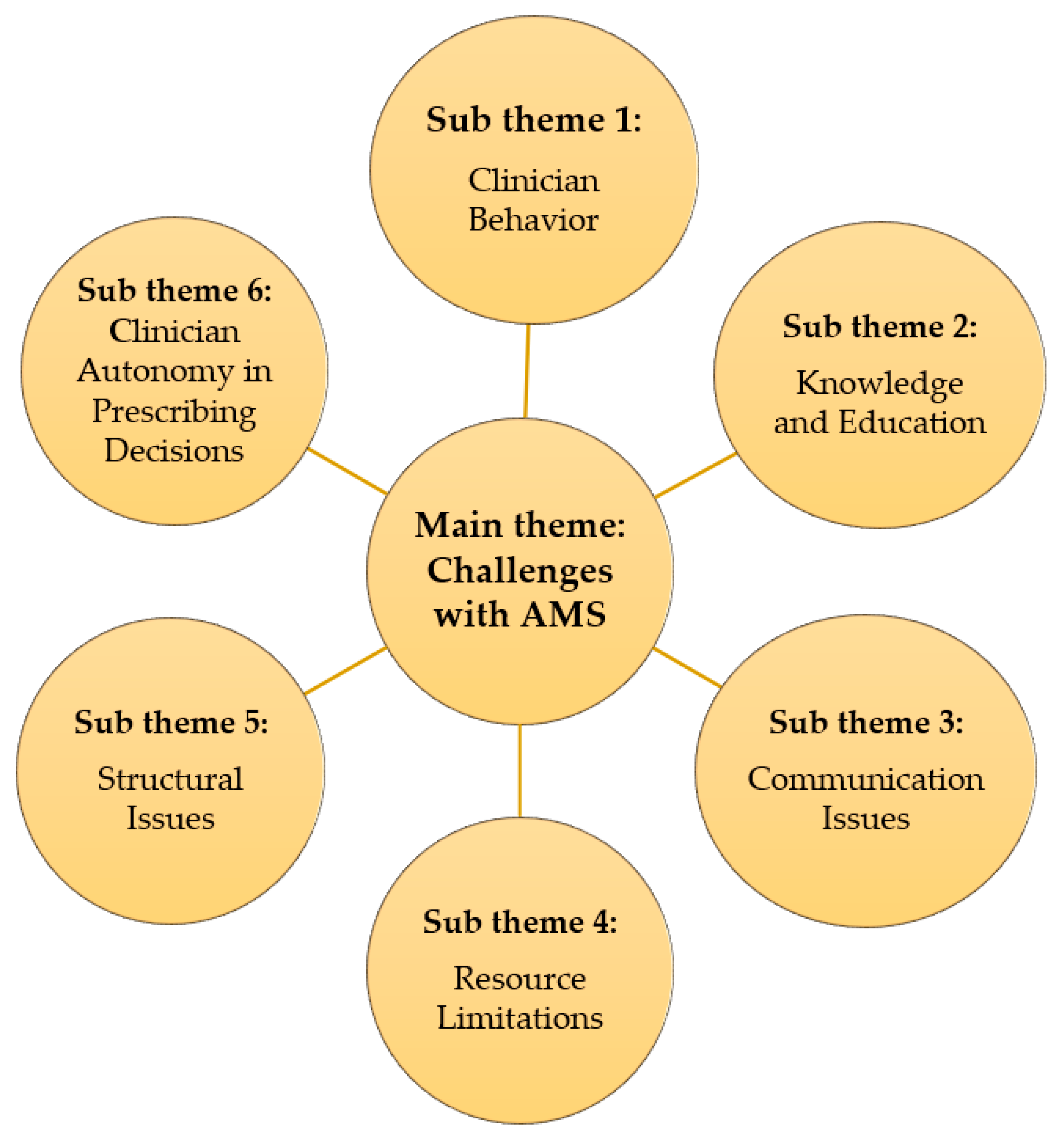
2.2. Challenges with AMS
2.2.1. Clinician Behavior: Some Participants Reported Concerns about Antibiotic Overuse and Lack of Adherence to Established Protocols for Prescribing and Duration of Treatment
“I feel like sometimes, um, we, the health care personnel, we do abuse antibiotics, because sometimes we may order an antibiotic today, tomorrow we are changing it, another day we are changing it, and we don’t follow the protocols, not to start with and not to end with”.“ICU Doctor”—KFH/HCW/006-
2.2.2. Knowledge and Education: Several HCWs Highlighted a Lack of Training and Education on AMR among Staff, Particularly Regarding Interpretation and Utilization of Antimicrobial Resistance Data from the Laboratory
“Um, because it’s willing to assist us. It feels it knows the impact of the infections and it tries to support us. I can also request the Rwanda Biomedical Center (RBC) where you come from that whenever you have these, uh, trainings if you can always invite us so that we can also be updated on what is going on around in the world”.“IPC Officer”—KFH/HCW/005-
2.2.3. Communication Issues: Breakdowns in Communication between Personnel and Departments Were Identified as a Barrier to Timely Delivery and Utilization of Laboratory Results
“There’s no report, no communication that tells us anything, except for us to take the initiative to ask. At that moment, when we identify a germ that is resistant, we tend to call that we’ve encountered a patient with resistance to a certain antibiotic that is spreading rapidly, especially to this antibiotic. Then, we communicate that we have identified a germ that is resistant”.“Laboratory technician”—KFH/HCW/004-
2.2.4. Resource Limitations: Stockouts of Essential Antibiotics and Laboratory Supplies Were Reported as a Significant Challenge
“This is a challenge we usually encounter, some times we request for culture and sensitivity for UTI to which we usually prescribe quinolones but when the results come out you find they did not test the quinolones but instead they tested cephlosporins and you realize that has become a challenge to us and we do not know why in the laboratory they choose to test certain antibiotics and not others but there is a time back they told us that they had a stock out of some antibiotic discs though they usually have them most of the time”.“Emergency Doctor”—KFH/HCW/007-
2.2.5. Structural Issues: The Limited Functionality of the AMS Committee and Lack of a Dedicated Forum for Discussing AMR Issues Were Identified as Weaknesses in the Current System
2.2.6. Additionally, Some Participants Expressed Concerns about Clinician Autonomy in Prescribing Decisions, Suggesting That Some May Not Consistently follow National Guidelines or International Recommendations
“In the pediatric population, our approach to antimicrobial treatment aligns with the guidelines provided by the Ministry of Health or the Rwanda Biomedical Centre. These guidelines outline the use of first, second, and third-line antimicrobials for various conditions. When treating pediatric patients, our strategy for selecting antibiotics follows a similar path. We typically initiate treatment with first-line antimicrobials as empiric prophylactic therapy while awaiting a definitive diagnosis. For instance, in the case of newborns, our current practice recommends starting the initiation of treatment with ampicillin and gentamicin as first-line empiric therapy. However, it is concerning that we are observing high levels of resistance, with rates reportedly reaching up to 80% for these antibiotics. Despite this challenge, our adherence to guidelines mandates the initiation of treatment with ampicillin and gentamicin as the initial approach. If there is no improvement in the patient’s condition or if concerns arise regarding resistance, we then consider transitioning to second-line antimicrobials as per the guidelines”.“Pediatrician Doctor”—KFH/HCW/002-
2.3. Use of Guidelines and Standard Protocols for AMR
2.3.1. Compliance to Guidelines, Standards, or Protocols When It Comes to AMR: Most of Them Understand That if There Are Available Nationally Based Guidelines, Then That Can Help People to Adhere to the Local Hospital-Based Guidelines
“No! When we conduct testing, we need to adhere to established standards. We adhere to these standards and then send the report. It’s up to them to take the initiative to provide the medicine because often, you can see in the treatment that it may need to start. The patient can start the treatment, the patient arrives with symptoms that match the sample taken to the laboratory, and then they start the antibiotic to save time because microbiology results can take like two to three days. So, these days, the patient needs to start the antibiotic. Therefore, when we conduct testing, we follow established standards and I third of them also mentioned antimicrobial stewardship policy”.“Laboratory technician”—KFH/HCW/004-
2.3.2. Departmental Guidelines: Infection Surveillance in ICU Guidelines Outline the Protocol for Any Patient Who Is Admitted in ICU; They Have to Perform a Blood Culture Sensitivity
“Um, we have a policy on infection surveillance whereby every patient who comes into our hospital is expected to have a blood culture done on them. But because of our insurance, it becomes a little bit difficult. But where we have found it’s not very difficult is for our patients who are admitted in ICU. Any patient who is admitted in ICU, they have to do a blood culture sensitivity so that they are able to know what infection the patient has. They came with it from home, or they acquired it from a hospital? And any devices they come with, like the urinary catheters, the IV cannulas, we do remove them from accident and emergency, before they go into the unit. So that we start again and fix ours”.“ICU Doctor”—KFH/HCW/006-
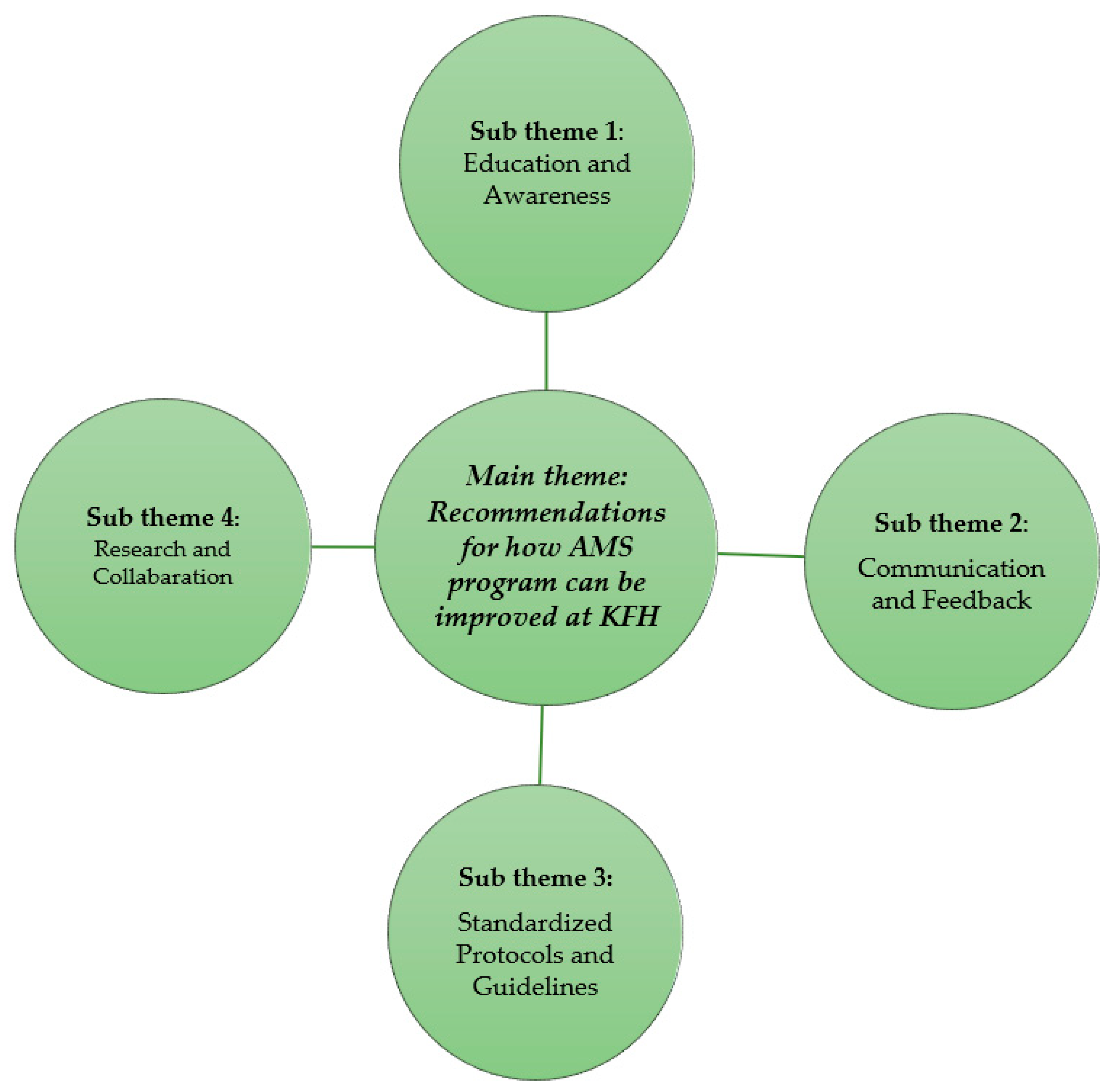
2.4. Recommendations for How AMS Program Can Be Improved at KFH
2.4.1. Education and Awareness: Participants Emphasized the Need for Ongoing Training and Education for All Staff on AMR, Including Interpretation of Antimicrobial Resistance Data and Appropriate Antibiotic Prescribing Practices
“Way forward! Except cases we discuss in session when we see an infection; Also when we send samples (environmental swabs) to the lab, when results are releases, we discuss about the results. For instance, last time in July and August we had training on antibiograms and antibiotic stewardship which can be continuous for smooth running of AMS”.“Pediatrician Doctor”—KFH/HCW/002-
2.4.2. Communication and Feedback: Improved Communication between Laboratory Personnel, Pharmacist, and Clinicians Was Identified as Essential—This Includes Timely Delivery of Test Results and Mechanisms for Laboratory Staff to Understand How Clinicians Are Utilizing This Information
“In our collaboration as a pharmacy, when a doctor prescribes an antibiotic, we often find that certain tests are necessary to support their decision. In such cases, the pharmacy contacts the lab to confirm whether these tests have been conducted. This demonstrates collaboration between the pharmacy and the lab/microbiology department which is recommended for good practice”.“Pharmacist”—KFH/HCW/001-
“As I am the manager, every morning I am supposed to check into the system to know newly admitted patients, sometimes they are like 14 or 13. I just go there as I have the history and the clinician is very busy or we may have critical cases (severely ill patients). So, I have my responsibility to go and check. When I get results, I inform the clinician or sometimes the lab can call the clinician but often we are the ones to check what we sent if results are available for the purpose of guiding clinical decisions”.“Pediatrician Doctor”—KFH/HCW/002-
2.4.3. Standardized Protocols and Guidelines: A Call Was Made for Clearer and More Consistent Use of National and Hospital-Based Guidelines for Antibiotic Prescribing
“Considering the recent trend of antimicrobial resistance among our patients, for instance, some individuals believe that only certain antibiotics can cure them, leading them to prescribe ceftriaxone yet it would have been more prudent to start with Amoxicillin. I believe it is essential to initiate treatment with first-line or lower-class antibiotics rather than higher classes. Additionally, I would recommend that doctors use antibiograms more frequently to guide their prescribing practices”.“Pharmacist”—KFH/HCW/001-
2.4.4. Research and Collaboration: Several Participants Highlighted the Value of Conducting Local Research on AMR Patterns and Fostering Collaboration between Clinicians and Researchers to Inform Best Practices
“Yes, of course, Rwanda is ahead in many things there is no reason to limit ourselves. I believe these things of antibiograms, and antibiotics are not complex, you must train people. I remember when we were in Butare we could see laboratory technicians doing that work and then sharing guidance with clinicians. So, it means that we cannot wait to have specialists everywhere for admitted patients for us to achieve these targets. We all treat patients, and all patients deserve the best care which means no harmful prescriptions. So, I say that I wish that research can be promoted, collaboration of clinicians and researchers in the center like RBC, so we can analyze our own data draw specific conclusions. The problem is that clinicians do not have time for writing grants and they may not have the right skills for that. That is the advice I can give”.“IPC Officer”—KFH/HCW/005-
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Site and Selection
4.3. Study Interview Guide Development
4.4. Recruitment of Participants
4.5. Key Informants’ Interviews (KIIs)
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Rodrigues, A.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Rivard, K.R.; Dumkow, L.E. Antimicrobial Stewardship Interventions to Combat Antibiotic Resistance: An Update on Targeted Strategies. Curr. Infect. Dis. Rep. 2019, 21, 33. [Google Scholar] [PubMed]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Van Schooneveld, T. Antimicrobial stewardship: Attempting to preserve a strategic resource. J. Community Hosp. Intern. Med. Perspect. 2011, 1, 7209. [Google Scholar] [CrossRef] [PubMed]
- Lanckohr, C.; Bracht, H. Antimicrobial stewardship. Curr. Opin. Crit. Care 2022, 28, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lago, J.M.; Lopez-Vazquez, P.; López-Durán, A.; Taracido-Trunk, M.; Figueiras, A. Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: A qualitative study from Spain. Fam. Pr. 2011, 29, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.G.; Lutkemeyer, D.S.; Levin, A.S. Are antimicrobial stewardship programs effective strategies for preventing antibiotic resistance? A systematic review. Am. J. Infect. Control 2018, 46, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [PubMed]
- Gulumbe, B.H.; Haruna, U.A.; Almazan, J.; Ibrahim, I.H.; Faggo, A.A.; Bazata, A.Y. Combating the menace of antimicrobial resistance in Africa: A review on stewardship, surveillance and diagnostic strategies. Biol. Proced. Online 2022, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.W.; Gerber, J.S.; Moehring, R.; Anderson, D.J.; Calderwood, M.S.; Han, J.H.; Mehta, J.M.; Pollack, L.A.; Zaoutis, T.; Srinivasan, A.; et al. Point-of-prescription interventions to improve antimicrobial stewardship. Clin. Infect. Dis. 2015, 60, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Skodvin, B.; Aase, K.; Charani, E.; Holmes, A.; Smith, I. An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrob. Resist. Infect. Control 2015, 4, 24. [Google Scholar] [CrossRef]
- Mbugua, S.M.; Njoroge, G.; Kijogi, C.; Kamita, M.; Kimani, R.; Mwaura, P.; Aidi, B.W.; Gitaka, J. Exploring perspectives on antimicrobial stewardship: A qualitative study of health managers in Kenya. Glob. Health Res. Policy 2020, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Orubu, E.S.F.; Al-Dheeb, N.; Ching, C.; Jawdeh, S.B.; Anderson, J.; Sheikh, R.; Hariri, F.; Basaleem, H.; Zaman, M.H. Assessing Antimicrobial Resistance, Utilization, and Stewardship in Yemen: An Exploratory Mixed-Methods Study. Am. J. Trop. Med. Hyg. 2021, 105, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Kimbowa, I.M.; Eriksen, J.; Nakafeero, M.; Obua, C.; Lundborg, C.S.; Kalyango, J.; Ocan, M. Antimicrobial stewardship: Attitudes and practices of healthcare providers in selected health facilities in Uganda. PLoS ONE 2022, 17, e0262993. [Google Scholar] [CrossRef] [PubMed]
- Adegbite, B.R.; Edoa, J.R.; Schaumburg, F.; Alabi, A.S.; Adegnika, A.A.; Grobusch, M.P. Knowledge and perception on antimicrobial resistance and antibiotics prescribing attitude among physicians and nurses in Lambaréné region, Gabon: A call for setting-up an antimicrobial stewardship program. Antimicrob. Resist. Infect. Control. 2022, 11, 44. [Google Scholar] [CrossRef]
- Balliram, R.; Sibanda, W.; Essack, S.Y. The knowledge, attitudes and practices of doctors, pharmacists and nurses on antimicrobials, antimicrobial resistance and antimicrobial stewardship in South Africa. South. Afr. J. Infect. Dis. 2021, 36, 15. [Google Scholar] [CrossRef] [PubMed]
- Lévin, C.; Thilly, N.; Dousak, M.; Beraud, G.; Klesnik, M.; Uhan, S.; Nathwani, D.; Beovic, B.; Pulcini, C. Perceptions, attitudes, and practices of French junior physicians regarding antibiotic use and resistance. Med. Mal. Infect. 2018, 49, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Baraka, M.A.; Alsultan, H.; Alsalman, T.; Alaithan, H.; Islam, A.; Alasseri, A.A. Health care providers’ perceptions regarding antimicrobial stewardship programs (AMS) implementation—facilitators and challenges: A cross-sectional study in the Eastern province of Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.K.; Shafiq, N.; Singh, G.; Ray, P.; Gautam, V.; Agarwal, R.; Muralidharan, J.; Arora, P. Antimicrobial Stewardship Programs in Resource Constrained Environments: Understanding and Addressing the Need of the Systems. Front. Public Health 2020, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Kpokiri, E.E.; Taylor, D.G.; Smith, F.J. Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals. Antibiotics 2020, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Morency-Potvin, P.; Schwartz, D.N.; Weinstein, R.A. Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship. Clin. Microbiol. Rev. 2016, 30, 381–407. [Google Scholar] [PubMed]
- Gahamanyi, N.; Umuhoza, T.; Saeed, S.I.; Mayigane, L.N.; Hakizimana, J.N. A Review of the Important Weapons against Antimicrobial Resistance in Sub-Saharan Africa. Appl. Biosci. 2023, 2, 136–156. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Fuller, W.F.; Hamzat, O.T.; Aboderin, A.O.; Gahimbare, L.; Kapona, O.; Yahaya, A.A.; Kasambara, W.; Nikiema, J.-B.; Ilboudo, D.W.; Mpundu, M.M. National action plan on antimicrobial resistance: An evaluation of implementation in the World Health Organization Africa region. J. Public Health Afr. 2022, 13, 2000. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Akhtar, Z.; Chowdhury, S.; Islam, M.A.; Parveen, S.; Ghosh, P.K.; Rahman, A.; Khan, Z.H.; Islam, K.; Debnath, N.; et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics 2022, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Buising, K.L.; Thursky, K.A.; Robertson, M.B.; Black, J.F.; Street, A.C.; Richards, M.J.; Brown, G.V. Electronic antibiotic stewardship--reduced consumption of broad-spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J. Antimicrob. Chemother. 2008, 62, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Schuts, E.C.; Boyd, A.; E Muller, A.; Mouton, J.W.; Prins, J.M. The Effect of Antibiotic Restriction Programs on Prevalence of Antimicrobial Resistance: A Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2021, 8, ofab070. [Google Scholar] [CrossRef] [PubMed]
- Kakumba, J.M.; Kindenge, J.M.; Kapepula, P.M.; Iyamba, J.-M.L.; Mashi, M.L.; Mulwahali, J.W.; Kialengila, D.M. Evaluation of Antibiotic Prescribing Pattern Using WHO Access, Watch and Reserve Classification in Kinshasa, Democratic Republic of Congo. Antibiotics 2023, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, A.; Garbovan, L.; Bhattarai, B.; Arjyal, A.; Baral, S.; Cooke, P.; Latham, S.; Barrington, D.J.; Mitchell, J.; King, R. Exploring community insights on antimicrobial resistance in Nepal: A formative qualitative study. BMC Heal. Serv. Res. 2024, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef] [PubMed]
| Study Number | Healthcare Workers Function | Period in Service |
|---|---|---|
| KFH/HCW/001 | Pharmacist | 7 years |
| KFH/HCW/002 | Pediatrician Doctor | 12 years |
| KFH/HCW/003 | Nurse in ICU | 15 years |
| KFH/HCW/004 | Laboratory technician | 12 years |
| KFH/HCW/005 | IPC Officer | 18 years |
| KFH/HCW/006 | ICU Doctor | 11 years |
| KFH/HCW/007 | Emergency Doctor | 8 years |
| KFH/HCW/008 | General Practitioner in the emergency Department | 14 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashegu, M.; Gahamanyi, N.; Ndayambaje, F.X.; Munyemana, J.B.; Ndahindwa, V.; Lukwago, F.; Ingabire, L.; Gambanga, F.; Gashema, P.; Tuyishime, A.; et al. Exploring Prescription Practices: Insights from an Antimicrobial Stewardship Program at a Tertiary Healthcare Facility, Rwanda. Antibiotics 2024, 13, 548. https://doi.org/10.3390/antibiotics13060548
Gashegu M, Gahamanyi N, Ndayambaje FX, Munyemana JB, Ndahindwa V, Lukwago F, Ingabire L, Gambanga F, Gashema P, Tuyishime A, et al. Exploring Prescription Practices: Insights from an Antimicrobial Stewardship Program at a Tertiary Healthcare Facility, Rwanda. Antibiotics. 2024; 13(6):548. https://doi.org/10.3390/antibiotics13060548
Chicago/Turabian StyleGashegu, Misbah, Noel Gahamanyi, François Xavier Ndayambaje, Jean Bosco Munyemana, Vedaste Ndahindwa, Fredrick Lukwago, Lambert Ingabire, Fiona Gambanga, Pierre Gashema, Albert Tuyishime, and et al. 2024. "Exploring Prescription Practices: Insights from an Antimicrobial Stewardship Program at a Tertiary Healthcare Facility, Rwanda" Antibiotics 13, no. 6: 548. https://doi.org/10.3390/antibiotics13060548
APA StyleGashegu, M., Gahamanyi, N., Ndayambaje, F. X., Munyemana, J. B., Ndahindwa, V., Lukwago, F., Ingabire, L., Gambanga, F., Gashema, P., Tuyishime, A., Dzinamarira, T., Dukundane, D., Muvunyi, T. Z., & Muvunyi, C. M. (2024). Exploring Prescription Practices: Insights from an Antimicrobial Stewardship Program at a Tertiary Healthcare Facility, Rwanda. Antibiotics, 13(6), 548. https://doi.org/10.3390/antibiotics13060548









