Stability Studies of the Dilution Series of Different Antibiotic Stock Solutions in Culture Medium Incubated at 37 °C
Abstract
:1. Introduction
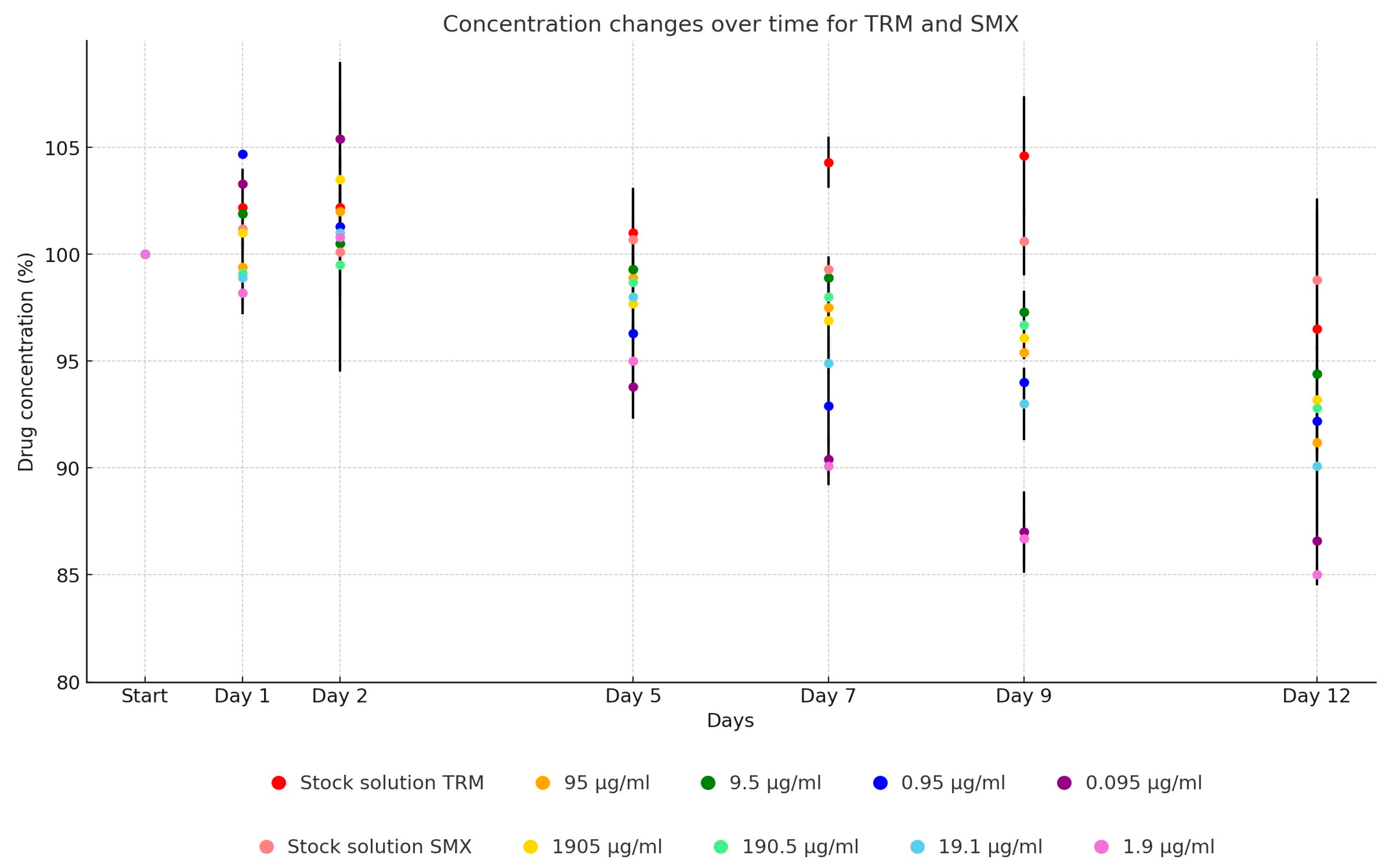
2. Results
3. Discussion
4. Materials and Methods
4.1. Active Substances and Matrices
4.2. Sample Preparation
4.3. Preparation of Calibration Solutions
4.4. Liquid Chromatograph and Mass Spectrometer Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016.
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Hung. Vet. J. 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Kłodzińska, S.N.; Priemel, P.A.; Rades, T.; Nielsen, H.M. Combining Diagnostic Methods for Antimicrobial Susceptibility Testing—A Comparative Approach. J. Microbiol. Methods 2018, 144, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Tests for Bactericidal Effects of Antimicrobial Agents: Technical Performance and Clinical Relevance. Available online: https://pubmed.ncbi.nlm.nih.gov/1423219/ (accessed on 12 February 2024).
- Kerek, Á.; Török, B.; Laczkó, L.; Kardos, G.; Bányai, K.; Somogyi, Z.; Kaszab, E.; Bali, K.; Jerzsele, Á. In Vitro Microevolution and Co-Selection Assessment of Florfenicol Impact on Escherichia coli Resistance Development. Antibiotics 2023, 12, 1728. [Google Scholar] [CrossRef]
- Kerek, Á.; Török, B.; Jerzsele, Á. MEGA-Plate—New Evolutionary and Coselection Microbiological Method. Hung. Vet. J. 2022, 144, 429–439. [Google Scholar]
- Kerek, Á.; Török, B.; Laczkó, L.; Somogyi, Z.; Kardos, G.; Bányai, K.; Kaszab, E.; Bali, K.; Jerzsele, Á. In Vitro Microevolution and Co-Selection Assessment of Amoxicillin and Cefotaxime Impact on Escherichia coli Resistance Development. Antibiotics 2024, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Mátis, G.; Kulcsár, A.; Turowski, V.; Fébel, H.; Neogrády, Z.; Huber, K. Effects of Oral Butyrate Application on Insulin Signaling in Various Tissues of Chickens. Domest. Anim. Endocrinol. 2015, 50, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tráj, P.; Sebők, C.; Mackei, M.; Kemény, Á.; Farkas, O.; Kákonyi, Á.; Kovács, L.; Neogrády, Z.; Jerzsele, Á.; Mátis, G. Luteolin: A Phytochemical to Mitigate S. Typhimurium Flagellin-Induced Inflammation in a Chicken In Vitro Hepatic Model. Animal 2023, 13, 1410. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Hung. Vet. J. 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, H.-D.; Chung, E.G.; Kim, Y.; Lee, J.-K. A Review of Analytical Procedures for the Simultaneous Determination of Medically Important Veterinary Antibiotics in Environmental Water: Sample Preparation, Liquid Chromatography, and Mass Spectrometry. J. Environ. Manag. 2018, 217, 629–645. [Google Scholar] [CrossRef]
- Fanali, S. Nano-Liquid Chromatography Applied to Enantiomers Separation. J. Chromatogr. A 2017, 1486, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Lugoboni, B.; Gazzotti, T.; Zironi, E.; Barbarossa, A.; Pagliuca, G. Development and Validation of a Liquid Chromatography/Tandem Mass Spectrometry Method for Quantitative Determination of Amoxicillin in Bovine Muscle. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Binson, G.; Grignon, C.; Le Moal, G.; Lazaro, P.; Lelong, J.; Roblot, F.; Venisse, N.; Dupuis, A. Overcoming Stability Challenges during Continuous Intravenous Administration of High-Dose Amoxicillin Using Portable Elastomeric Pumps. PLoS ONE 2019, 14, e0221391. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, L.; Sunderland, V.B. Kinetics of Amoxicillin and Clavulanate Degradation Alone and in Combination in Aqueous Solution under Frozen Conditions. Int. J. Pharm. 2007, 342, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, S.; Merzouk, M.; Barton, S.; Nabhani-Gebara, S. Stability of Amoxicillin and Clavulanic Acid in Separate Containers for Administration via a Y-Site. Drug Des. Dev. Ther. 2021, 15, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Albarran, M.; Villafuerte-Robles, L. Assay of Amoxicillin Sustained Release from Matrix Tablets Containing Different Proportions of Carbopol 971P NF. Int. J. Pharm. 2004, 273, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cui, X.; Liu, B.; Zhang, J. Relationship between the Color Stability and Impurity Profile of Cefotaxime Sodium. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, A.; Andrási, M.; Kardos, S. Application of Capillary Zone Electrophoresis to the Analysis and to a Stability Study of Cephalosporins. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 775, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Bahari, M.B.; Darwis, Y.; Iqbal, M.Z.; Hayat, A.; Venkatesh, G. An RP-HPLC-UV Method with SPE for Cefotaxime in All-in-One Total Parenteral Nutritional Admixtures: Application to Stability Studies. J. AOAC Int. 2013, 96, 290–294. [Google Scholar] [CrossRef]
- Qureshi, T.; Memon, N.; Memon, S.Q.; Abro, K.; Shah, S.W. LC/UV Determination of Cefradine, Cefuroxime, and Cefotaxime in Dairy Milk, Human Serum and Wastewater Samples. Springerplus 2013, 2, 575. [Google Scholar] [CrossRef]
- Loeuille, G.; D’Huart, E.; Vigneron, J.; Nisse, Y.-E.; Beiler, B.; Polo, C.; Ayari, G.; Sacrez, M.; Demoré, B.; Charmillon, A. Stability Studies of 16 Antibiotics for Continuous Infusion in Intensive Care Units and for Performing Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2022, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Reubsaet, J.L.; Beijnen, J.H.; Bult, A.; van Maanen, R.J.; Marchal, J.A.; Underberg, W.J. Analytical Techniques Used to Study the Degradation of Proteins and Peptides: Chemical Instability. J. Pharm. Biomed. Anal. 1998, 17, 955–978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Coulthard, K. Stability of Colistin and Colistin Methanesulfonate in Aqueous Media and Plasma as Determined by High-Performance Liquid Chromatography. Antimicrob. Agents Chemother. 2003, 47, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Orwa, J.A.; Govaerts, C.; Gevers, K.; Roets, E.; Van Schepdael, A.; Hoogmartens, J. Study of the Stability of Polymyxins B(1), E(1) and E(2) in Aqueous Solution Using Liquid Chromatography and Mass Spectrometry. J. Pharm. Biomed. Anal. 2002, 29, 203–212. [Google Scholar] [CrossRef]
- Bueno, M.S.; Longhi, M.R.; Garnero, C. Pharmaceutical Systems as a Strategy to Enhance the Stability of Oxytetracycline Hydrochloride Polymorphs in Solution. Pharmaceutics 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- German, R.; Bukowska, B.; Pajchel, G.; Grzybowska, W.; Tyski, S. Extremely Long Time Stability Study of Selected Antibiotic Standards. J. Pharm. Biomed. Anal. 2010, 51, 758–763. [Google Scholar] [CrossRef]
- Okerman, L.; Van Hende, J.; De Zutter, L. Stability of Frozen Stock Solutions of Beta-Lactam Antibiotics, Cephalosporins, Tetracyclines and Quinolones Used in Antibiotic Residue Screening and Antibiotic Susceptibility Testing. Anal. Chim. Acta 2007, 586, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Sample Preservation for the Analysis of Antibiotics in Water. J. Chromatogr. A 2014, 1369, 43–51. [Google Scholar] [CrossRef]
- Sah, H. Degradation Patterns of Tetracycline Antibiotics in Reverse Micelles and Water. Biomed. Chromatogr. 2006, 20, 1142–1149. [Google Scholar] [CrossRef]
- Marx, J.O.; Vudathala, D.; Murphy, L.; Rankin, S.; Hankenson, F.C. Antibiotic Administration in the Drinking Water of Mice. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 301–306. [Google Scholar]
- Petritz, O.A.; Guzman, D.S.-M.; Wiebe, V.J.; Papich, M.G. Stability of Three Commonly Compounded Extemporaneous Enrofloxacin Suspensions for Oral Administration to Exotic Animals. J. Am. Vet. Med. Assoc. 2013, 243, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, T.; Bae, S. The Stability and in Vitro Antibacterial Efficacy of Enrofloxacin and Gentamicin Solutions against Staphylococcus pseudintermedius over 28 Days. Vet. Dermatol. 2023, 34, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Batrawi, N.; Naseef, H.; Al-Rimawi, F. Development and Validation of a Stability-Indicating HPLC Method for the Simultaneous Determination of Florfenicol and Flunixin Meglumine Combination in an Injectable Solution. J. Anal. Methods Chem. 2017, 2017, 1529280. [Google Scholar] [CrossRef] [PubMed]
- Franje, C.A.; Chang, S.-K.; Shyu, C.-L.; Davis, J.L.; Lee, Y.-W.; Lee, R.-J.; Chang, C.-C.; Chou, C.-C. Differential Heat Stability of Amphenicols Characterized by Structural Degradation, Mass Spectrometry and Antimicrobial Activity. J. Pharm. Biomed. Anal. 2010, 53, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, I.; Zaidi, S.T.R.; Shastri, M.D.; Eapen, M.S.; Ming, L.C.; Wanandy, T.; Patel, R.P. Investigations into the Physical and Chemical Stability of Concentrated Co-Trimoxazole Intravenous Infusions. Eur. J. Hosp. Pharm. 2018, 25, e102–e108. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Hung. Vet. J. 2021, 143, 281–282. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Hung. Vet. J. 2022, 144, 285–298. [Google Scholar]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Hung. Vet. J. 2023, 145, 461–475. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antiprotozoal and Antifungal Efficiency of Propolis—Part 2. Hung. Vet. J. 2022, 144, 691–704. [Google Scholar]
- Tsuji, A.; Nakashima, E.; Hamano, S.; Yamana, T. Physicochemical Properties of Amphoteric β-Lactam Antibiotics I: Stability, Solubility, and Dissolution Behavior of Amino Penicillins as a Function of PH. J. Pharm. Sci. 1978, 67, 1059–1066. [Google Scholar] [CrossRef]
- Erah, P.O.; Goddard, A.F.; Barrett, D.A.; Shaw, P.N.; Spiller, R.C. The Stability of Amoxycillin, Clarithromycin and Metronidazole in Gastric Juice: Relevance to the Treatment of Helicobacter pylori Infection. J. Antimicrob. Chemother. 1997, 39, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Hahne, F.; Müller, C.; Yalman, S.; Meißner, J.; Kietzmann, M.; Hamscher, G. Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions. Antibiotics 2023, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Seraissol, P.; Lanot, T.; Baklouti, S.; Mané, C.; Ruiz, S.; Lavit, M.; De Riols, P.; Garrigues, J.-C.; Gandia, P. Evaluation of 4 Quantification Methods for Monitoring 16 Antibiotics and 1 Beta-Lactamase Inhibitor in Human Serum by High-Performance Liquid Chromatography with Tandem Mass Spectrometry Detection. J. Pharm. Biomed. Anal. 2022, 219, 114900. [Google Scholar] [CrossRef] [PubMed]
- Mascher, D.G.; Unger, C.P.; Mascher, H.J. Determination of Neomycin and Bacitracin in Human or Rabbit Serum by HPLC-MS/MS. J. Pharm. Biomed. Anal. 2007, 43, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M.; Eichman, J.; Katz, T.; Gilewicz, R. Stability of Florfenicol in Drinking Water. J. AOAC Int. 2003, 86, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Metry, C.A.; Maddox, C.W.; Dirikolu, L.; Johnson, Y.J.; Campbell, K.L. Determination of Enrofloxacin Stability and in Vitro Efficacy against Staphylococcus pseudintermedius and Pseudomonas aeruginosa in Four Ear Cleaner Solutions over a 28 Day Period. Vet. Dermatol. 2012, 23, 23–28, e6. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.; Noll, S.; Gerecke, H.; Fassauer, G.; Jira, T.; Remane, Y.; Vogel, J.; Frontini, R.; Reinhardt, R. A Stability Study of Amphotericin B, Colistin and Tobramycin in a Hydrophilic Suspension Commonly Used for Selective Decontamination of the Digestive Tract by HPLC and in Vitro Potency Measurements. Eur. J. Hosp. Pharm. 2017, 24, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yu, S.; Chai, G.; Liu, J.; Zhou, Q.T. An LC-MS/MS Method for Simultaneous Analysis of the Cystic Fibrosis Therapeutic Drugs Colistin, Ivacaftor and Ciprofloxacin. J. Pharm. Anal. 2021, 11, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mesini, A.; Barbagallo, L.; Maffia, A.; Tripodi, G.; Pea, F.; Saffioti, C.; Castagnola, E.; Cangemi, G. A Liquid Chromatography-Tandem Mass Spectrometry Platform for the Routine Therapeutic Drug Monitoring of 14 Antibiotics: Application to Critically Ill Pediatric Patients. J. Pharm. Biomed. Anal. 2020, 186, 113273. [Google Scholar] [CrossRef]
- Matar, K.M.; Al-Refai, B. Quantification of Colistin in Plasma by Liquid Chromatography-Tandem Mass Spectrometry: Application to a Pharmacokinetic Study. Sci. Rep. 2020, 10, 8198. [Google Scholar] [CrossRef]
- Rehm, S.; Rentsch, K.M. LC-MS/MS Method for Nine Different Antibiotics. Clin. Chim. Acta 2020, 511, 360–367. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.R.; Lipman, N.S. Amoxicillin-Clavulanic Acid and Trimethoprim- Sulfamethoxazole in Rodent Feed and Water: Effects of Compounding on Antibiotic Stability. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 26–32. [Google Scholar] [PubMed]
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI standards M07. [Google Scholar]
- Thambavita, D.; Galappatthy, P.; Mannapperuma, U.; Jayakody, L.; Cristofoletti, R.; Abrahamsson, B.; Groot, D.W.; Langguth, P.; Mehta, M.; Parr, A.; et al. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Amoxicillin Trihydrate. J. Pharm. Sci. 2017, 106, 2930–2945. [Google Scholar] [CrossRef] [PubMed]
- Cefotaxime. Sodium Salt—ALX-380-271—Enzo Life Sciences. Available online: https://www.enzolifesciences.com/ALX-380-271/cefotaxime-.-sodium-salt/ (accessed on 25 May 2024).
- Neomycin Sulfate Datasheet. Available online: https://www.selleckchem.com/datasheet/Neomycin-sulfate-S256803-DataSheet.html (accessed on 25 May 2024).
- Oxytetracycline. Available online: https://go.drugbank.com/drugs/DB00595 (accessed on 22 May 2024).
- Florfenicol. Available online: https://go.drugbank.com/drugs/DB11413 (accessed on 22 May 2024).
- Enrofloxacin. Available online: https://go.drugbank.com/drugs/DB11404 (accessed on 22 May 2024).
- Colistin. Sulfate—ALX-380-272—Enzo Life Sciences. Available online: https://www.enzolifesciences.com/ALX-380-272/colistin-.-sulfate/ (accessed on 25 May 2024).
- Sulfamethoxazole. Available online: https://go.drugbank.com/drugs/DB01015 (accessed on 22 May 2024).
- Trimethoprim. Available online: https://go.drugbank.com/drugs/DB00440 (accessed on 22 May 2024).
- VICH GL1 Validation of Analytical Procedures: Definition and Terminology—Scientific Guideline | European Medicines Agency. Available online: https://www.ema.europa.eu/en/vich-gl1-validation-analytical-procedures-definition-terminology-scientific-guideline (accessed on 24 May 2024).
- VICH GL2 Validation of Analytical Procedures: Methodology—Scientific Guideline | European Medicines Agency. Available online: https://www.ema.europa.eu/en/vich-gl2-validation-analytical-procedures-methodology-scientific-guideline (accessed on 24 May 2024).


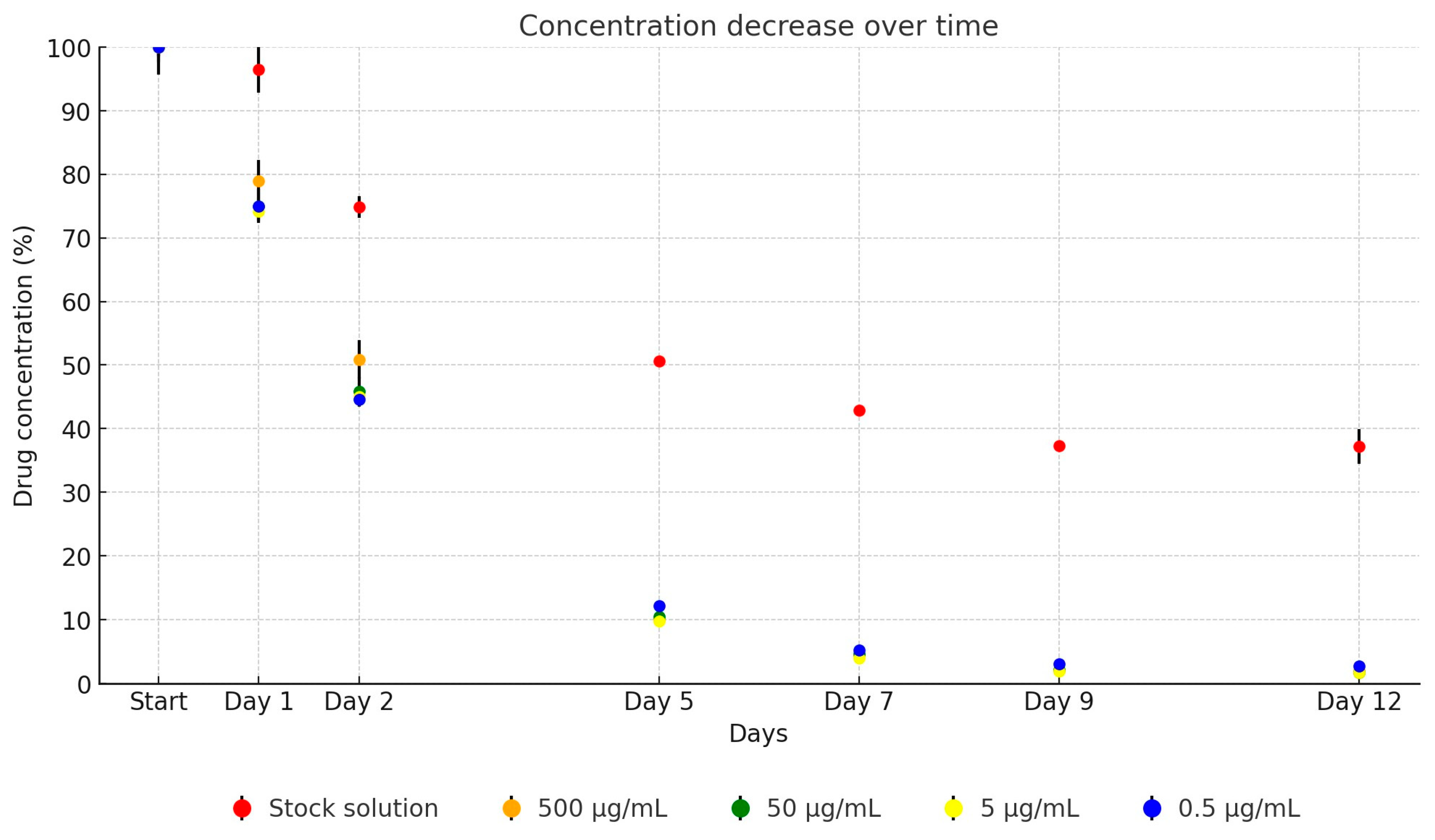
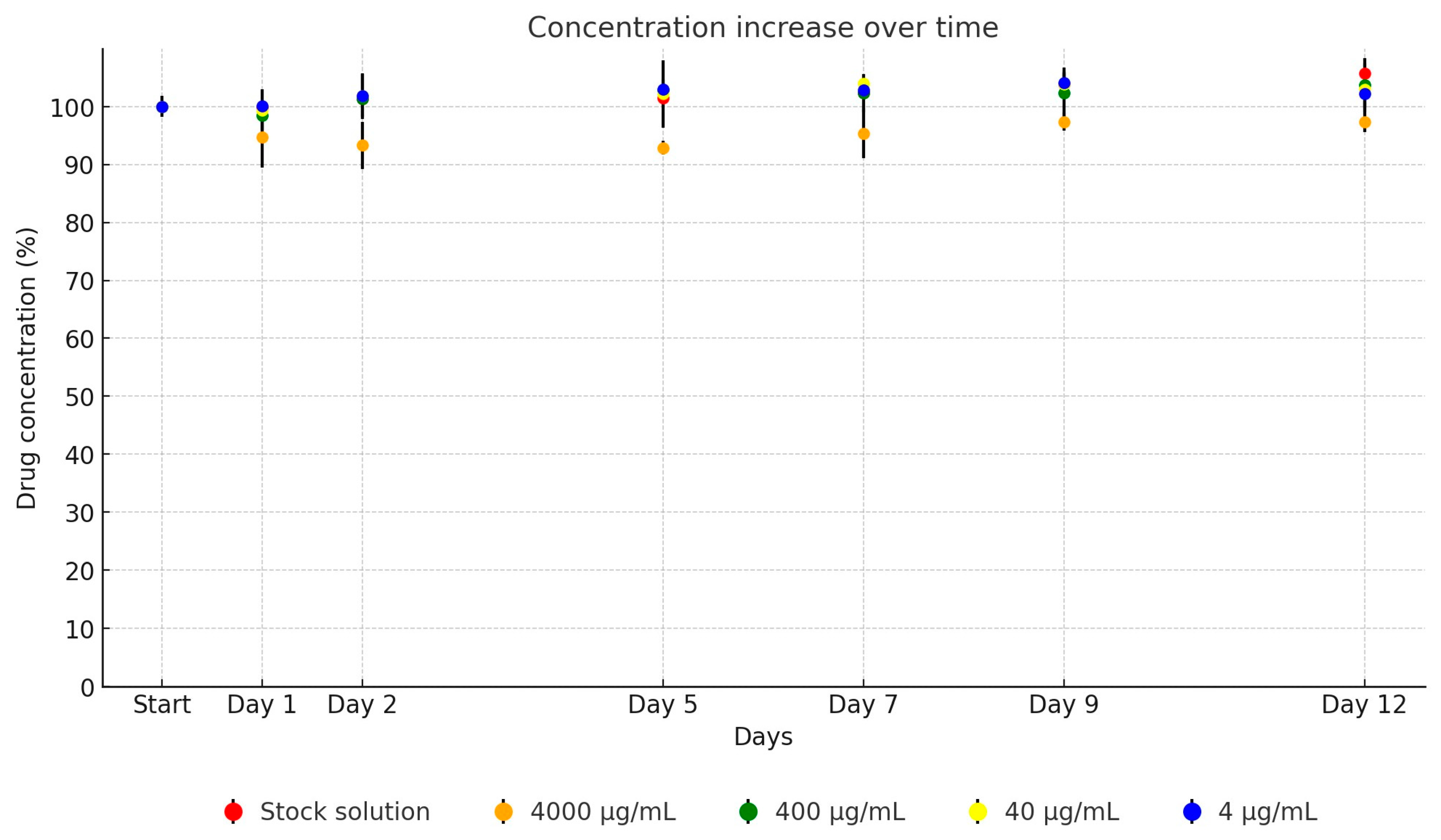
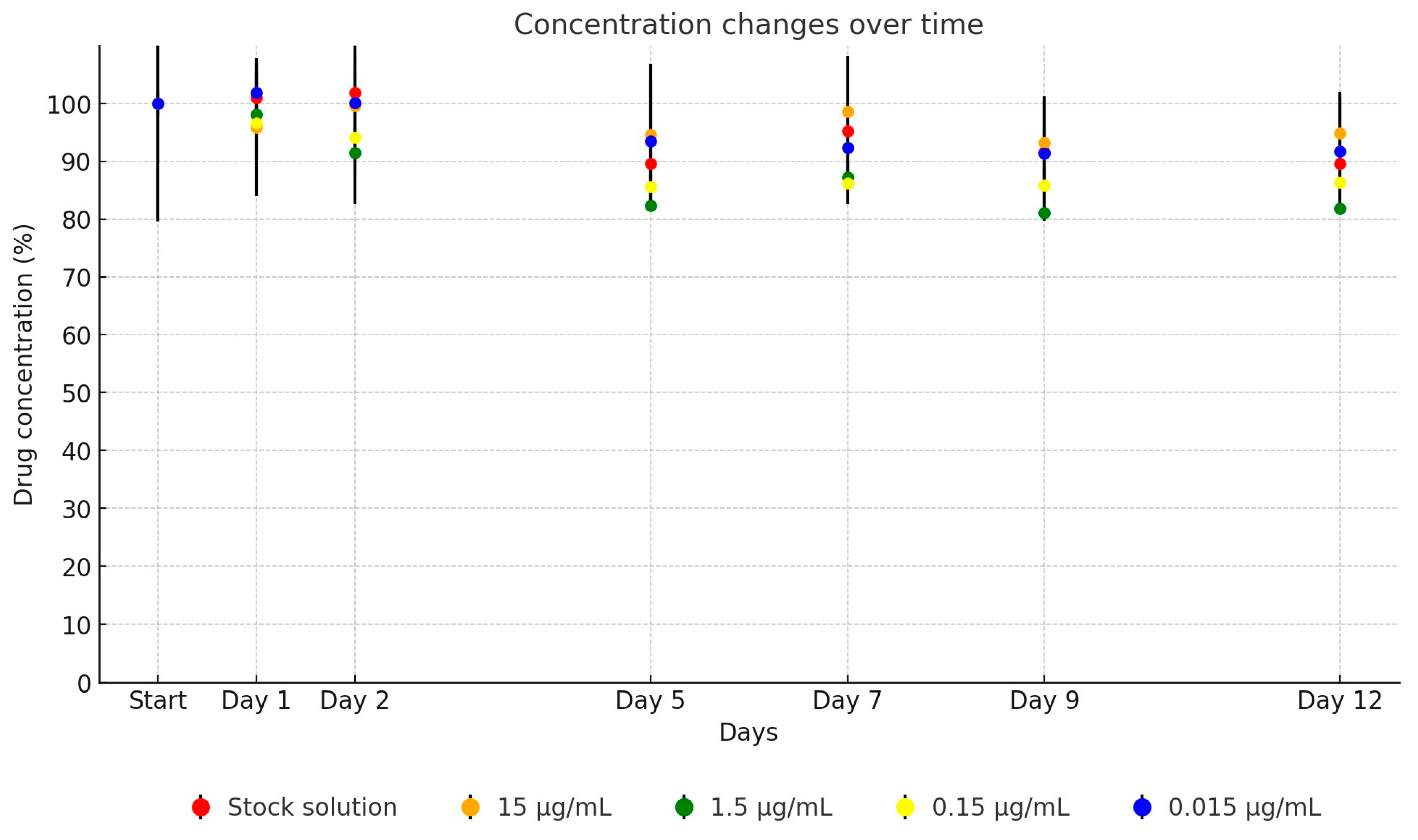

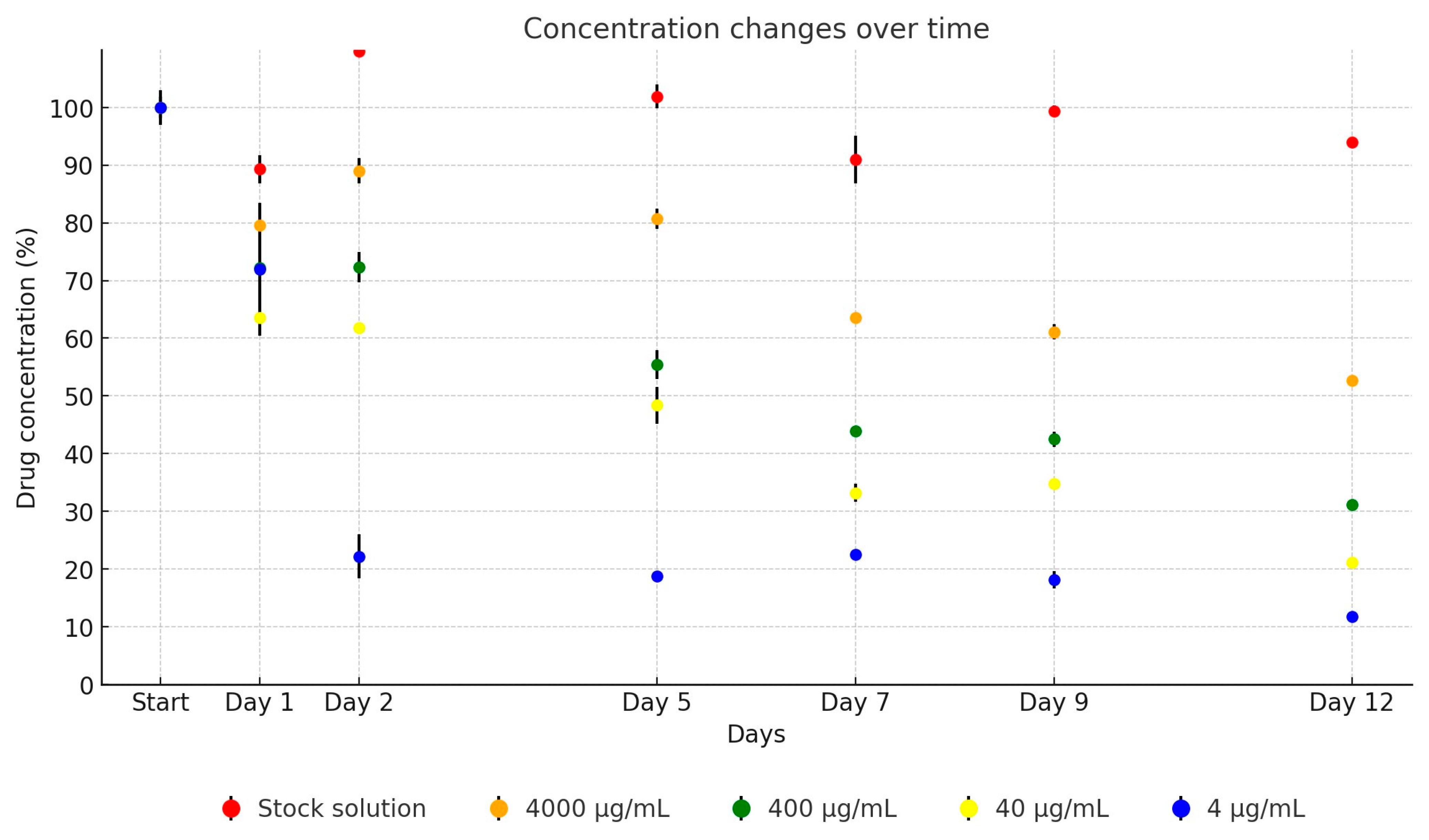
| Active Substance | Equation | Explanatory Power | p-Value | |
|---|---|---|---|---|
| Day | Dilution | |||
| Amoxicillin | y = −0.495ln(x) + 0.9096 | R2 = 0.9363 | <0.0001 * | 0.9784 |
| Cefotaxime | y = −0.548ln(x) + 1.0193 | R2 = 0.9602 | <0.0001 * | 0.5722 |
| Neomycin | y = −0.350ln(x) + 0.9881 | R2 = 0.9930 | 0.0011 * | 0.0001 * |
| Oxytetracycline | y = −0.571ln(x) + 1.0413 | R2 = 0.9468 | <0.0001 * | 0.8379 |
| Florfenicol | y = 0.0133ln(x) + 0.9875 | R2 = 0.4948 | 0.3484 | <0.0001 * |
| Enrofloxacin | y = −0.068ln(x) + 1.0128 | R2 = 0.8609 | 0.0389 * | 0.0028 * |
| Colistin | y = −0.377ln(x) + 0.9575 | R2 = 0.9828 | <0.0001 * | 0.0054 * |
| Sulfamethoxazole | y = −0.051ln(x) + 1.0346 | R2 = 0.6164 | <0.0001 * | 0.3279 |
| Trimethoprim | y = −0.047ln(x) + 1.0232 | R2 = 0.6513 | <0.0001 * | 0.1371 |
| Active Substance | Applied Compound | Purity of the Applied Compound | Target Concentration of Stock Solution | Target Amount of Compound | Compound(s) Suspended for Dissolution |
|---|---|---|---|---|---|
| Amoxicillin | Amoxicillin trihydrate | 86.0% | 10,000 µg/mL | 11,628 µg/mL | 0.1 mol/L phosphate buffer |
| Cefotaxime | Cefotaxime sodium | 89.1% | 5000 µg/mL | 5612.5 µg/mL | UPW |
| Neomycin | Neomycin sulphate | 60.9% | 40,000 µg/mL | 65,680 µg/mL | UPW |
| Oxytetracycline | Oxytetracycline | 100% | 5000 µg/mL | 5000 µg/mL | UPW |
| Florfenicol | Florfenicol | 99.6% | 40,000 µg/mL | 40,160 µg/mL | UPW + 5% 96% ethanol |
| Enrofloxacin | Enrofloxacin | 98% | 150 µg/mL | 153 µg/mL | UPW + 10% 0.1 mol sodium hydroxide |
| Colistin | Colistin sulphate | 71.6% | 2500 µg/mL | 3490 µg/mL | UPW |
| Potential sulfonamide | Sulfamethoxazole | 99.9% | 19,047.5 µg/mL * | 19,066.5 µg/mL | Warm UPW + 5% 2.5 mol NAOH |
| Trimethoprim | 99.9% | 952.5 µg/mL * | 953.8 µg/mL | UPW + 5% 0.05 mol HCl |
| Active Substance | Separation Procedure | Mobile Phase Composition | Column Diameter (mm) | Column Temperature (°C) | Flow Rate (mL/min) | Injection Volume (µL) | Measuring Time (min) | |
|---|---|---|---|---|---|---|---|---|
| “A” | “B” | |||||||
| Amoxicillin | Isocratic; 95% “A”, 5% “B” | UPW + 0.2 V/V% HCOOH + 5 mM NH4OOCH | ACN | 150 × 4.6 | 35 | 1.0 | 50 | 6 |
| Cefotaxime | Isocratic; 80% “A”, 20% “B” | UPW + 0.1 V/V% HCOOH + 5 mM NH4OOCH | MeOH | 150 × 3.0 | 45 | 0.8 | 25 | 7.5 |
| Neomycin | Gradient (t/min) t0, t1: 70% “A”, 30% “B” t11, t12: 5% “A”, 95% “B” t13, t15: 70% “A”, 30% “B” | UPW + 0.1 V/V% HFBA | ACN + 0.1 V/V% HFBA | 100 × 4.6 | 30 | 0.3 | 25 | 15 |
| Oxytetracycline | Isocratic; 75% “A”, 25% “B” | UPW + 0.1 V/V% HCOOH | ACN + 0.1 V/V% HCOOH | 150 × 4.6 | 45 | 1.0 | 20 | 5 |
| Florfenicol | Isocratic; 75% “A”, 25% “B” | UPW + 0.1 V/V% HCOOH + 2 mM NH4OOCH | ACN | 150 × 4.6 | 45 | 1.0 | 10 | 10 |
| Enrofloxacin | Isocratic; 80% “A”, 20% “B” | UPW + 0.1 V/V% HCOOH + 2 mM NH4OOCH | ACN | 100 × 4.6 | 45 | 1.0 | 10 | 6 |
| Colistin | Isocratic; 20% “A”, 80% “B” | UPW + 0.2 V/V% HCOOH | ACN + 0.2 V/V% HCOOH | 100 × 4.6 | 30 | 0.5 | 50 | 6 |
| Sulfamethoxazole + trimethoprim | Isocratic; 70% “A”, 30% “B” | UPW + 0.1 V/V% HCOOH | ACN | 150 × 4.6 | 45 | 1.0 | 25 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Ecsedi, B.G.; Szabó, Á.; Szimrók, Z.; Paliczné Kustán, B.; Jerzsele, Á.; Nagy, G. Stability Studies of the Dilution Series of Different Antibiotic Stock Solutions in Culture Medium Incubated at 37 °C. Antibiotics 2024, 13, 549. https://doi.org/10.3390/antibiotics13060549
Kerek Á, Ecsedi BG, Szabó Á, Szimrók Z, Paliczné Kustán B, Jerzsele Á, Nagy G. Stability Studies of the Dilution Series of Different Antibiotic Stock Solutions in Culture Medium Incubated at 37 °C. Antibiotics. 2024; 13(6):549. https://doi.org/10.3390/antibiotics13060549
Chicago/Turabian StyleKerek, Ádám, Bence G. Ecsedi, Ábel Szabó, Zoltán Szimrók, Bianka Paliczné Kustán, Ákos Jerzsele, and Gábor Nagy. 2024. "Stability Studies of the Dilution Series of Different Antibiotic Stock Solutions in Culture Medium Incubated at 37 °C" Antibiotics 13, no. 6: 549. https://doi.org/10.3390/antibiotics13060549
APA StyleKerek, Á., Ecsedi, B. G., Szabó, Á., Szimrók, Z., Paliczné Kustán, B., Jerzsele, Á., & Nagy, G. (2024). Stability Studies of the Dilution Series of Different Antibiotic Stock Solutions in Culture Medium Incubated at 37 °C. Antibiotics, 13(6), 549. https://doi.org/10.3390/antibiotics13060549







