A Study on the Effect of Quaternization of Polyene Antibiotics’ Structures on Their Activity, Toxicity, and Impact on Membrane Models
Abstract
1. Introduction
2. Results
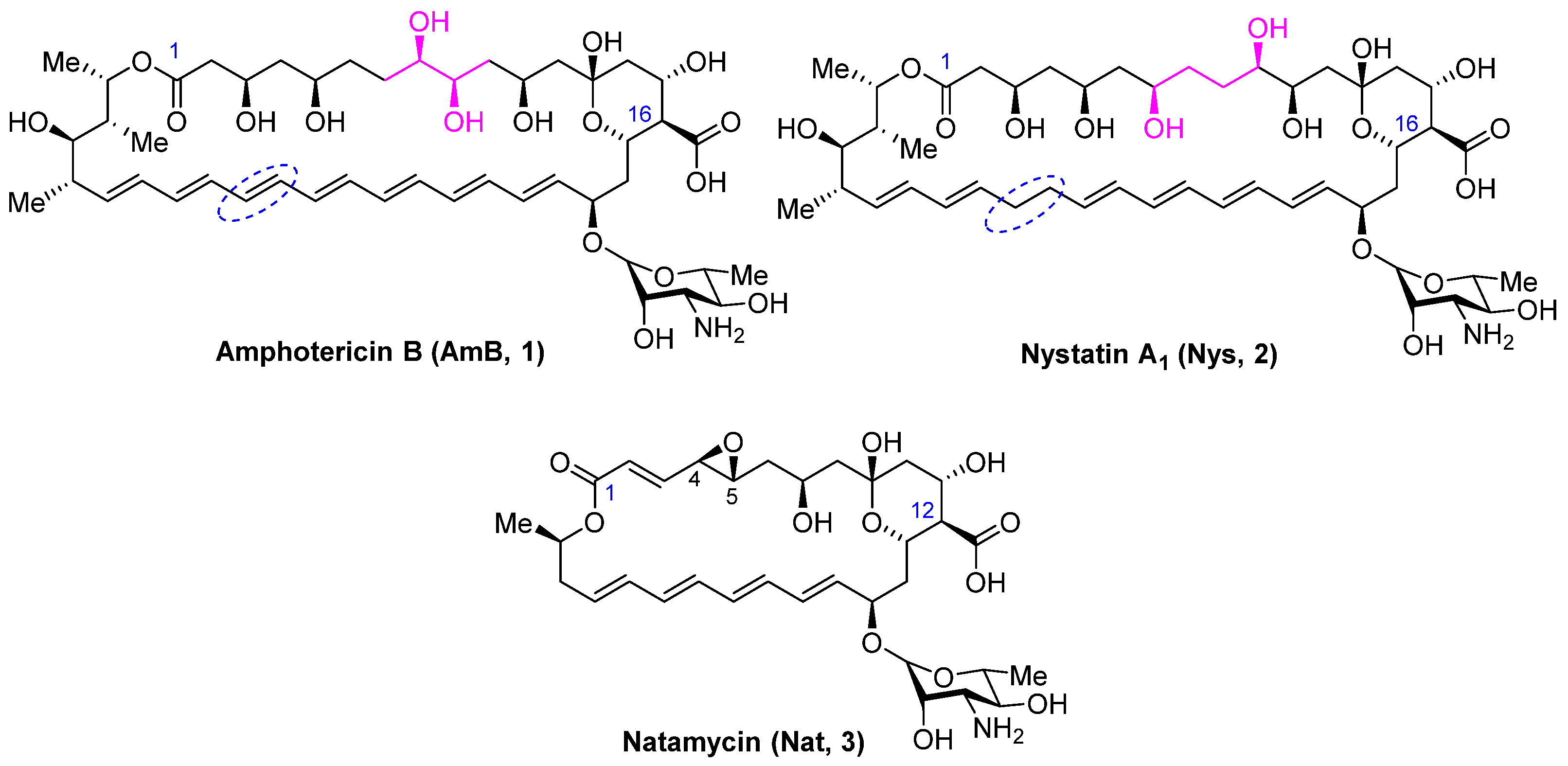
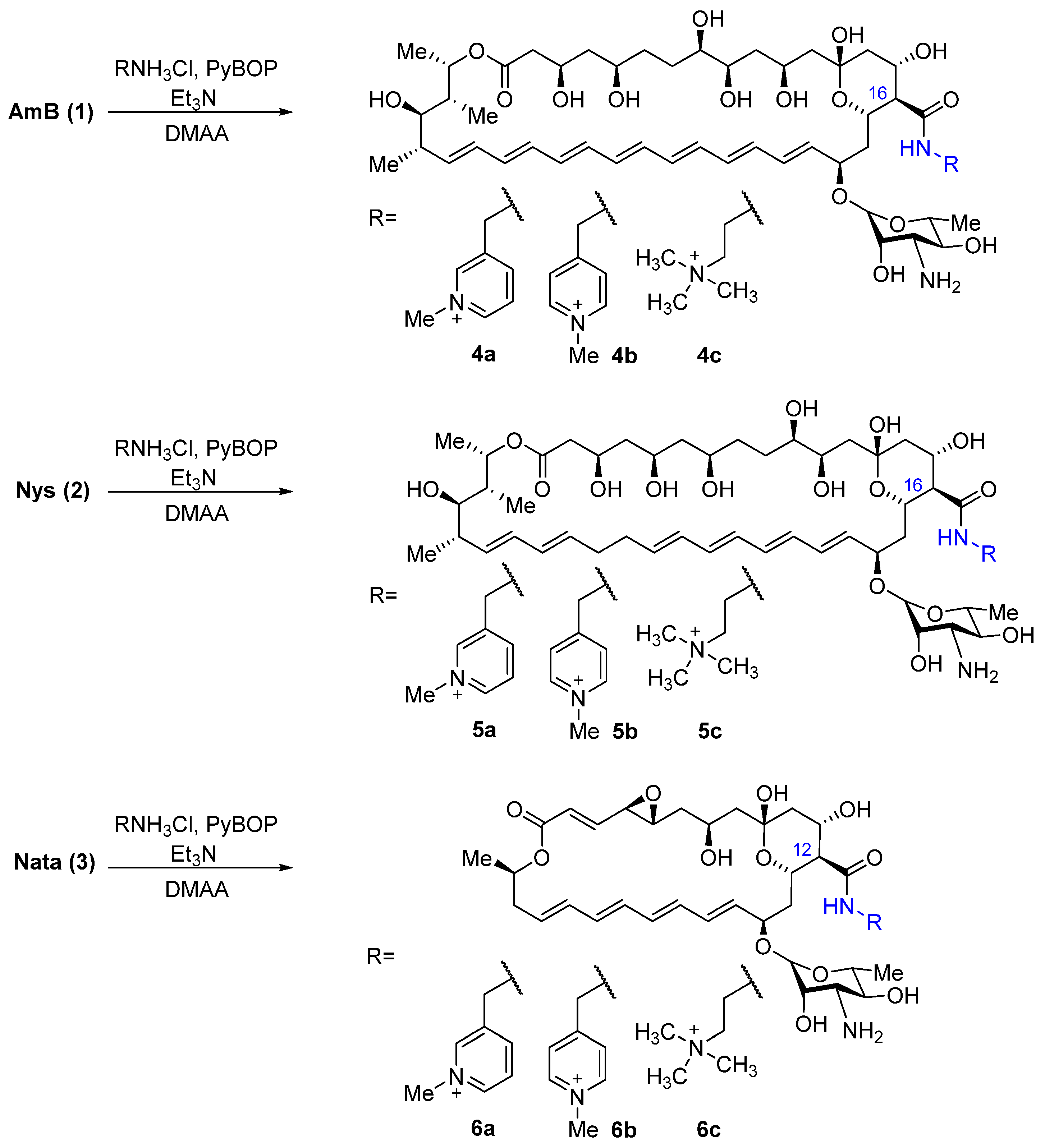
2.1. Synthesis of Polyene Amides
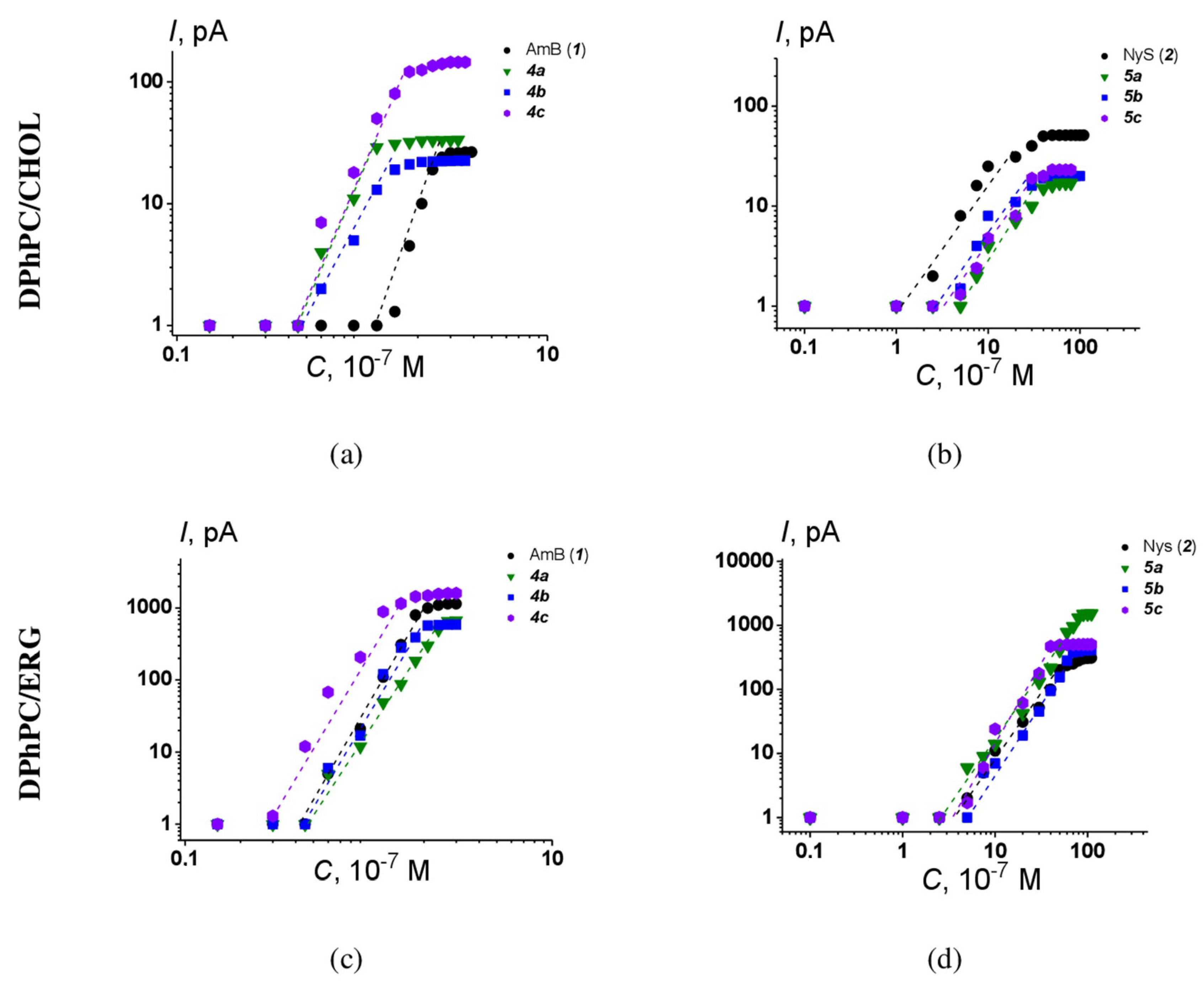
2.2. Spectrum of Antifungal Activity of Quaternized Semisynthetic Polyenes
2.3. Cytoxicity Assays
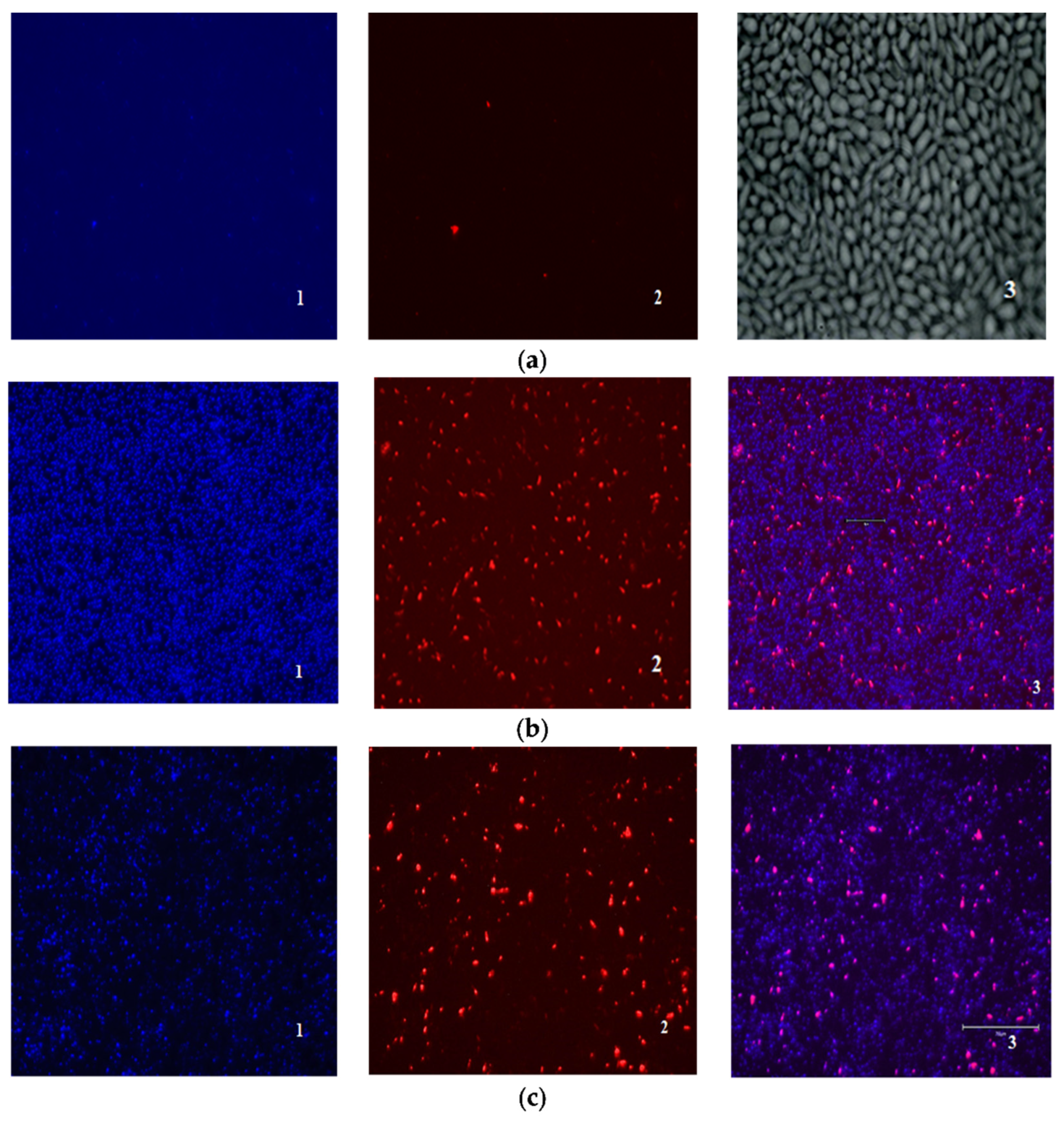
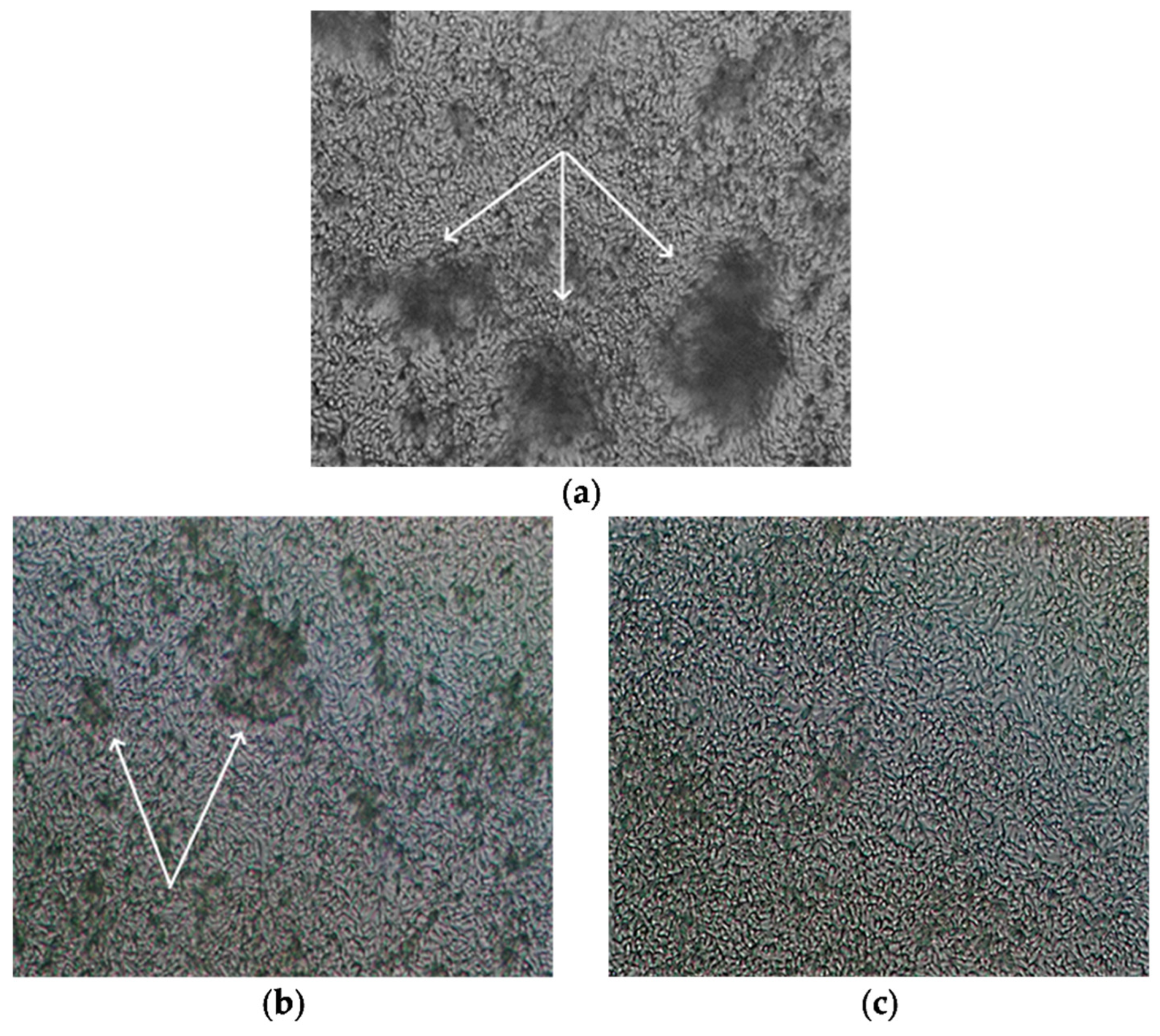
2.4. Antibiofilm Activity
2.5. Calcein Assay
2.6. The Confocal Fluorescence Microscopy of Giant Unilamellar Vesicles
2.7. Differential Scanning Microcalorimetry
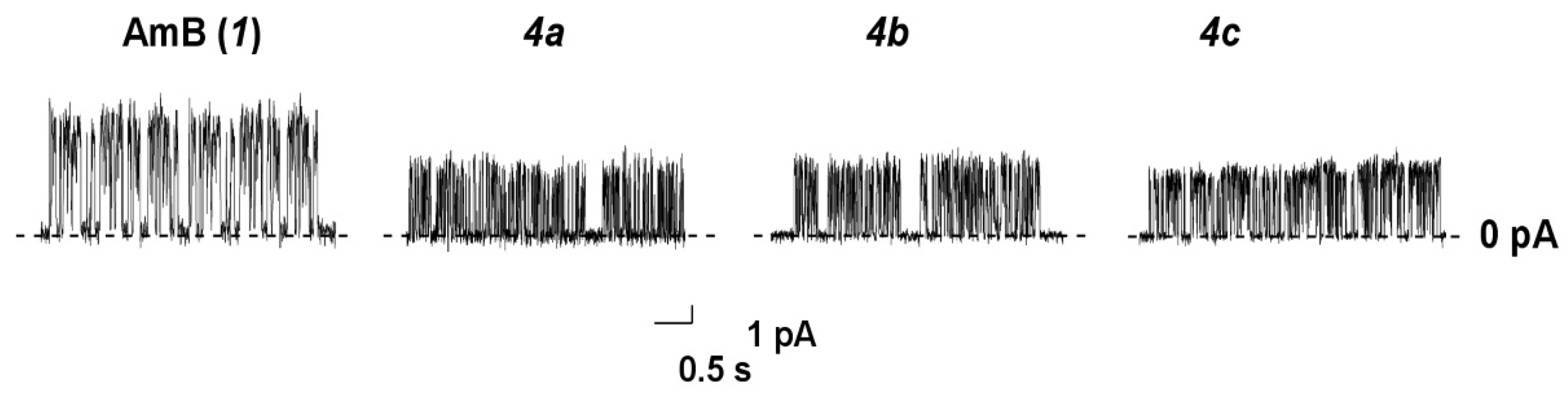
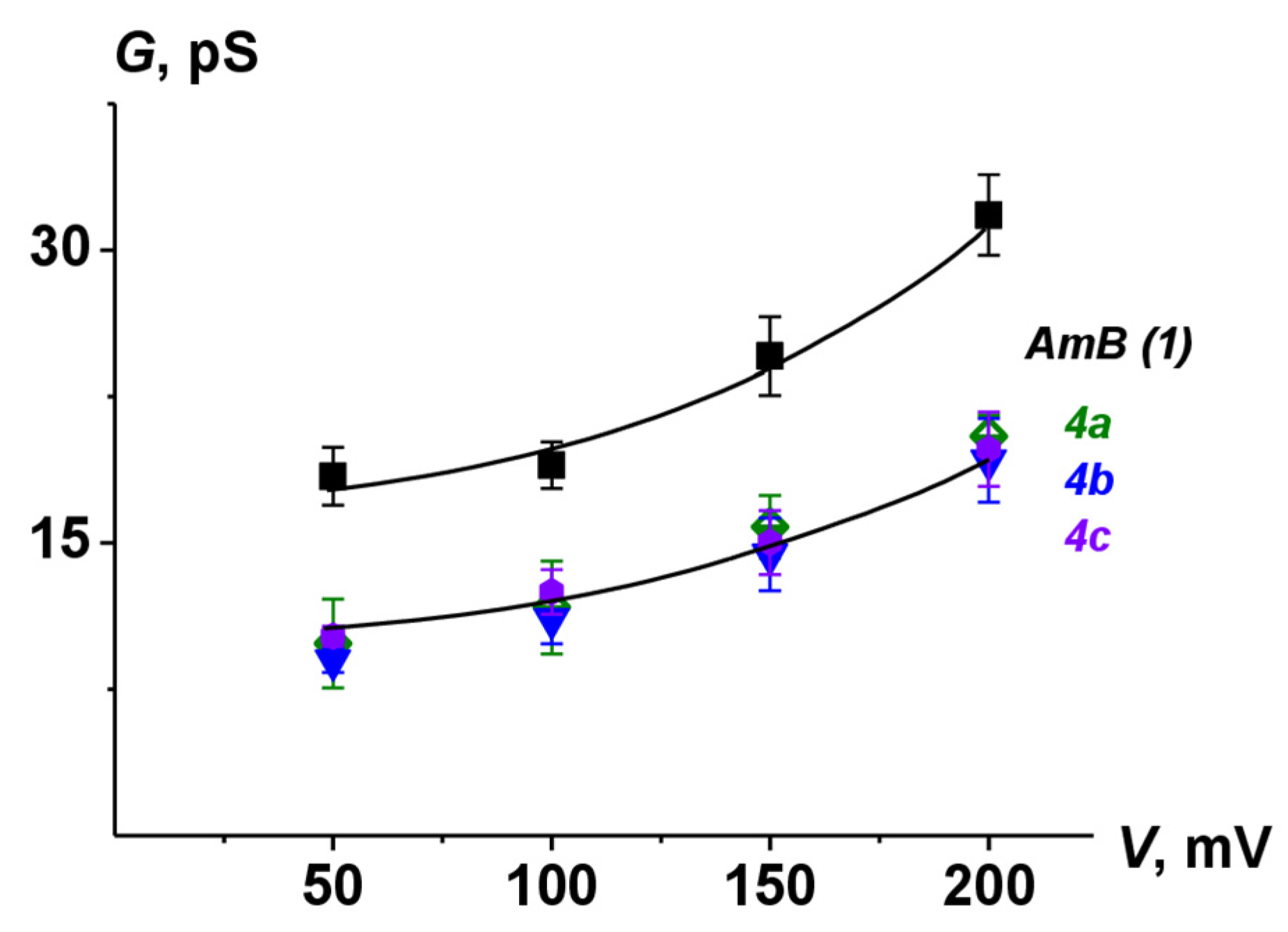
2.8. Ion Channel Recording
3. Discussion
4. Materials and Methods
4.1. General
4.2. Quaternized Derivatives of Amphotericin B, Nystatin, and Natamycin (4a-c, 5a-c, 6a-c) (General Method)
4.3. Antifungal Activity Testing
4.4. Cell Culture and Antiproliferative Activity
4.5. Hemolysis Assay
4.6. Antibiofilm Activity
4.7. Studying the Effect of Drugs on Biofilms by Scanning Ion Conductance Microscopy (SICM)
4.8. Fluorescence Microscopy
4.9. Statistical Analysis (Investigations of Biofilms)
4.10. Calcein Release from Large Unilamellar Vesicles
4.11. Registration of Ion Channels in Planar Lipid Bilayers
4.12. Differential Scanning Microcalorimetry
4.13. The Confocal Fluorescence Microscopy of Giant Unilamellar Vesicles
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- von Lilienfeld-Toal, M.; Wagener, J.; Einsele, H.; Cornely, O.A.; Kurzai, O. Invasive Fungal Infection. Dtsch. Arztebl. Int. 2019, 116, 271–278. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Logan, A.; Wolfe, A.; Williamson, J.C. Antifungal Resistance and the Role of New Therapeutic Agents. Curr. Infect. Dis. Rep. 2022, 24, 105–116. [Google Scholar] [CrossRef]
- Madhavan, Y.; Sai, K.V.; Shanmugam, D.K.; Manimaran, A.; Guruviah, K.; Mohanta, Y.K.; Venugopal, D.C.; Mohanta, T.K.; Sharma, N.; Muthupandian, S. Current Treatment Options for COVID-19 Associated Mucormycosis: Present Status and Future Perspectives. J. Clin. Med. 2022, 11, 3620. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty Years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Streekstra, H.; Verkennis, A.E.E.; Jacobs, R.; Dekker, A.; Stark, J.; Dijksterhuis, J. Fungal Strains and the Development of Tolerance against Natamycin. Int. J. Food Microbiol. 2016, 238, 15–22. [Google Scholar] [CrossRef]
- Sousa, F.; Nascimento, C.; Ferreira, D.; Reis, S.; Costa, P. Reviving the Interest in the Versatile Drug Nystatin: A Multitude of Strategies to Increase Its Potential as an Effective and Safe Antifungal Agent. Adv. Drug Deliv. Rev. 2023, 199, 114969. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; Levin-Khalifa, M.; Dvash, T. Water-Soluble Nystatin and Derivative. ACS Med. Chem. Lett. 2022, 13, 182–187. [Google Scholar] [CrossRef]
- Ma, H.; Qian, A.; Zheng, Y.; Meng, X.; Wang, T.; Zhang, Y.; Sun, L.; Zou, F.; Zhao, B.; Zhang, S.; et al. Design, Synthesis, and Structure–Activity Relationship Studies of Bisamide Derivatives of Amphotericin B with Potent Efficacy and Low Toxicity. J. Med. Chem. 2022, 65, 8897–8913. [Google Scholar] [CrossRef]
- Haro-Reyes, T.; Díaz-Peralta, L.; Galván-Hernández, A.; Rodríguez-López, A.; Rodríguez-Fragoso, L.; Ortega-Blake, I. Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications. Membranes 2022, 12, 681. [Google Scholar] [CrossRef]
- Welscher, Y.M.; Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- te Welscher, Y.M.; van Leeuwen, M.R.; de Kruijff, B.; Dijksterhuis, J.; Breukink, E. Polyene Antibiotic That Inhibits Membrane Transport Proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11156–11159. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin Forms an Extramembranous and Fungicidal Sterol Sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.M.; Cioffi, A.G.; Burke, M.D. Our Path to Less Toxic Amphotericins. Synlett 2016, 27, 337–354. [Google Scholar]
- Wilcock, B.C.; Endo, M.M.; Uno, B.E.; Burke, M.D. C2′-OH of Amphotericin B Plays an Important Role in Binding the Primary Sterol of Human Cells but Not Yeast Cells. J. Am. Chem. Soc. 2013, 135, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin Primarily Kills Yeast by Simply Binding Ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Paquet, V.; Carreira, E.M. Significant Improvement of Antifungal Activity of Polyene Macrolides by Bisalkylation of the Mycosamine. Org. Lett. 2006, 8, 1807–1809. [Google Scholar] [CrossRef]
- Janout, V.; Schell, W.A.; Thévenin, D.; Yu, Y.; Perfect, J.R.; Regen, S.L. Taming Amphotericin B. Bioconjug. Chem. 2015, 26, 2021–2024. [Google Scholar] [CrossRef]
- Boros-Majewska, J.; Salewska, N.; Borowski, E.; Milewski, S.; Malic, S.; Wei, X.-Q.; Hayes, A.J.; Wilson, M.J.; Williams, D.W. Novel Nystatin A1 Derivatives Exhibiting Low Host Cell Toxicity and Antifungal Activity in an in Vitro Model of Oral Candidosis. Med. Microbiol. Immunol. 2014, 203, 341–355. [Google Scholar] [CrossRef]
- Davis, S.A.; Vincent, B.M.; Endo, M.M.; Whitesell, L.; Marchillo, K.; Andes, D.R.; Lindquist, S.; Burke, M.D. Nontoxic Antimicrobials That Evade Drug Resistance. Nat. Chem. Biol. 2015, 11, 481–487. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Efimova, S.S.; Alexandrov, A.I.; Ghazy, E.S.M.O.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.B.; Grammatikova, N.E.; Dezhenkova, L.G.; Pereverzeva, E.R.; et al. Semisynthetic Amides of Polyene Antibiotic Natamycin. ACS Infect. Dis. 2023, 9, 42–55. [Google Scholar] [CrossRef]
- Antillón, A.; de Vries, A.H.; Espinosa-Caballero, M.; Falcón-González, J.M.; Flores Romero, D.; González–Damián, J.; Jiménez-Montejo, F.E.; León-Buitimea, A.; López-Ortiz, M.; Magaña, R.; et al. An Amphotericin B Derivative Equally Potent to Amphotericin B and with Increased Safety. PLoS ONE 2016, 11, e0162171. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.; Efimova, S.; Alexandrov, A.; Omelchuk, O.; Ghazy, E.; Bychkova, E.; Zatonsky, G.; Grammatikova, N.; Dezhenkova, L.; Solovieva, S.; et al. Semisynthetic Amides of Amphotericin B and Nystatin A1: A Comparative Study of In Vitro Activity/Toxicity Ratio in Relation to Selectivity to Ergosterol Membranes. Antibiotics 2023, 12, 151. [Google Scholar] [CrossRef]
- Hervé, M.; Debouzy, J.C.; Borowski, E.; Cybulska, B.; Gary-Bobo, C.M. The Role of the Carboxyl and Amino Groups of Polyene Macrolides in Their Interactions with Sterols and Their Selective Toxicity. A 31P-NMR Study. Biochim. Biophys. Acta Biomembr. 1989, 980, 261–272. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.V.; Grammatikova, N.E.; Isakova, E.B.; Mirchink, E.P.; Treshchalin, I.D.; Pereverzeva, E.R.; Bykov, E.E.; et al. Discovery of Amphamide, a Drug Candidate for the Second Generation of Polyene Antibiotics. ACS Infect. Dis. 2020, 6, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, E.I.; Grammatikova, N.E.; Shchekotikhin, A.E. Eremomycin Picolylamides and Their Cationic Lipoglycopeptides: Synthesis and Antimicrobial Properties. Macroheterocycles 2019, 12, 98–106. [Google Scholar] [CrossRef]
- Li, L.; Ji, Y.; Tang, X. Quaternary Ammonium Promoted Ultra Selective and Sensitive Fluorescence Detection of Fluoride Ion in Water and Living Cells. Anal. Chem. 2014, 86, 10006–10009. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Pert, H.; Guinea, J. EUCAST Definitive Document E.DEF 7.3.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts; EUCAST: Copenhagen, Denmark, 2020. [Google Scholar]
- Arendrup, M.C.; Guinea, J.; Cuenca-Estrella, M.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Pert, H. EUCAST Definitive Document E.DEF 9.3.2 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds; EUCAST: Copenhagen, Denmark, 2020. [Google Scholar]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, Epidemiology, Pathogenicity and Antifungal Resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Prado, C.G.; Carvalho, R.R.; Dias, K.S.T.; Dias, A.L.T. Candida albicans and Non-C. albicans Candida Species: Comparison of Biofilm Production and Metabolic Activity in Biofilms, and Putative Virulence Properties of Isolates from Hospital Environments and Infections. Mycopathologia 2013, 175, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Miyazaki, T.; Makau, J.N.; Mizuta, S.; Tanaka, Y.; Ishikawa, T.; Makimura, K.; Hirayama, T.; Takazono, T.; Saijo, T.; et al. Novel and Potent Antimicrobial Effects of Caspofungin on Drug-Resistant Candida and Bacteria. Sci. Rep. 2020, 10, 17745. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Costa, C.; Carneiro Nassiazene Lima Marreto, L.; Lopes Rocha, V.; Cristina da Silva, T.; do Rosário Rodrigues Silva, M. Biofilm Forming Capability and Antifungal Susceptibility Profile of Candida spp. from blood. Rev. Patol. Trop. 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Slaninová, J.; Putnová, H.; Borovičková, L.; Šácha, P.; Čeřovský, V.; Monincová, L.; Fučík, V. The Antifungal Effect of Peptides from Hymenoptera Venom and Their Analogs. Cent. Eur. J. Biol. 2011, 6, 150–159. [Google Scholar] [CrossRef]
- Savin, N.; Erofeev, A.; Kolmogorov, V.; Salikhov, S.; Efremov, Y.; Timashev, P.; Grammatikova, N.; Levshin, I.; Edwards, C.; Korchev, Y.; et al. Scanning Ion-Conductance Microscopy Technique for Studying the Topography and Mechanical Properties of Candida parapsilosis Yeast Microorganisms. Biomater. Sci. 2023, 11, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.A.; Martini, A.K.; Pruskowski, K.A.; Rowan, M.P.; Niece, K.L.; Akers, K.S. Thermal Stability of Mafenide and Amphotericin B Topical Solution. Burns 2018, 44, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Silvius, J.R. Thermotropic Phase Transitions of Pure Lipids in Model Membranes and Their Modifications by Membrane Proteins. Lipid Protein Interact. 1982, 2, 239–281. [Google Scholar]
- Juhasz, J.; Davis, J.H.; Sharom, F.J. Fluorescent Probe Partitioning in Giant Unilamellar Vesicles of ‘Lipid Raft’ Mixtures. Biochem. J. 2010, 430, 415–423. [Google Scholar] [CrossRef]
- Chulkov, E.G.; Efimova, S.S.; Schagina, L.V.; Ostroumova, O.S. Direct Visualization of Solid Ordered Domains Induced by Polyene Antibiotics in Giant Unilamellar Vesicles. Chem. Phys. Lipids 2014, 183, 204–207. [Google Scholar] [CrossRef]
- Efimova, S.S.; Tevyashova, A.N.; Olsufyeva, E.N.; Bykov, E.E.; Ostroumova, O.S. Pore-Forming Activity of New Conjugate Antibiotics Based on Amphotericin B. PLoS ONE 2017, 12, e0188573. [Google Scholar] [CrossRef]
- Kleinberg, M.E.; Finkelstein, A. Single-Length and Double-Length Channels Formed by Nystatin in Lipid Bilayer Membranes. J. Membr. Biol. 1984, 80, 257–269. [Google Scholar] [CrossRef]
- Bruzzese, T.; Cambieri, M.; Recusani, F. Synthesis and Biological Properties of Alkyl Esters of Polyene Antibiotics. J. Pharm. Sci. 1975, 64, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Umegawa, Y.; Yamamoto, T.; Dixit, M.; Funahashi, K.; Seo, S.; Nakagawa, Y.; Suzuki, T.; Matsuoka, S.; Tsuchikawa, H.; Hanashima, S.; et al. Amphotericin B Assembles into Seven-Molecule Ion Channels: An NMR and Molecular Dynamics Study. Sci. Adv. 2022, 8, eabo2658. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M. Recent Progress in the Study of the Interactions of Amphotericin B with Cholesterol and Ergosterol in Lipid Environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef]
- Biernasiuk, A.; Berecka-Rycerz, A.; Gumieniczek, A.; Malm, M.; Łączkowski, K.Z.; Szymańska, J.; Malm, A. The Newly Synthesized Thiazole Derivatives as Potential Antifungal Compounds against Candida albicans. Appl. Microbiol. Biotechnol. 2021, 105, 6355–6367. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef]




| MIC (μg/mL) 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4a 2 | 4b 3 | 4c 4 | AmB | 5a 2 | 5b 3 | 5c 4 | Nys | 6a 2 | 6b 3 | 6c 4 | Nata | |
| C. albicans ATCC 10231 | 2/2 1 | 1/2 | 1/1 | 1/1 | 8/8 | 1/2 | 4/4 | 2/2 | 16/16 | 4/4 | 16/32 | 4/4 |
| C. albicans 604M | 2/2 | 1/1 | 1/1 | 1/2 | 8/8 | 2/2 | 4/8 | 2/2 | 32/32 | 8/8 | 32/32 | 4/4 |
| C. albicans 8R | 2/2 | 2/2 | 2/2 | 2/2 | 8/16 | 2/2 | 4/8 | 2/4 | 16/32 | 4/8 | 16/32 | 4/4 |
| C. parapsilosis ATCC 22019 | 2/2 | 1/2 | 1/1 | 1/1 | 4/8 | 2/4 | 2/4 | 1/2 | 16/16 | 4/8 | 16/32 | 4/8 |
| C. parapsilosis 58L | 2/2 | 1/2 | 2/2 | 1/2 | 8/16 | 2/4 | 4/8 | 2/4 | 16/32 | 8/8 | 32/32 | 8/8 |
| C. glabrata 61L | 2/2 | 1/1 | 1/1 | 1/1 | 4/8 | 2/2 | 2/4 | 2/4 | 4/4 | 2/2 | 4/4 | 4/4 |
| C. tropicalis 3010 | 2/2 | 1/1 | 1/1 | 1/1 | 4/8 | 2/2 | 2/4 | 2/4 | 4/4 | 2/2 | 4/4 | 4/4 |
| C. krusei 432M | 4/4 | 2/2 | 2/2 | 2/2 | 16/16 | 2/4 | 8/16 | 2/4 | 16/16 | 4/4 | 16/16 | 4/4 |
| A. fumigatus ATCC 46645 | 4 | 4 | 4 | 2 | 8 | 4 | 4 | 8 | >32 | 16 | >32 | 4 |
| IC50, μM | ||
|---|---|---|
| HEK293 | hFB-hTERT6 | |
| 4a | 5.2 ± 0.7 | 4.2 ± 0.5 |
| 4b | >50 | 9.8 ± 1.4 |
| 4c | 14.1 ± 1.8 | 4.3 ± 0.6 |
| AmB (1) | 18.2 ± 4.5 | 2.1 ± 0.3 |
| 5a | >100 | >100 |
| 5b | >100 | >100 |
| 5c | >100 | >100 |
| Nys (2) | 25.3 ± 3.4 | 45.6 ± 6.4 |
| 6a | >500 | 174.7 ± 21.8 |
| 6b | 443.0 ± 57.0 | 82.1 ± 10.3 |
| 6c | >500 | 144.1 ± 18.2 |
| Nata (3) | 78.6 ± 9.1 | 81.0 ± 11.0 |
| Doxorubicin | 0.11 ± 0.02 | 0.12 ± 0.03 |
| MIC, μg/mL | SMIC50, μg/mL | SMIC80, μg/mL | |
|---|---|---|---|
| C. albicans 604M | |||
| AmB (1) | 0.5/1 | 1.30 ± 0.05 | 8.9 ± 0.91 |
| 4b | 1/1 | 1.20 ± 0.03 | 7.94 ± 1.1 |
| C. tropicalis 56-05 | |||
| AmB (1) | 0.5/2 | 3.10 ± 0.17 | 9.3 ± 0.52 |
| 4b | 1/1 | 2.76 ± 0.19 | 6.4 ± 0.62 |
| C. parapsilosis 58L | |||
| AmB (1) | 0.5/2 | 1.60 ± 0.06 | 4.46 ± 0.24 |
| 4b | 1/2 | 1.60 ± 0.14 | 4.20 ± 0.24 |
| Biofilm Height, Microns | Biofilm Eradication (% of Control) | The Value of Cell Indentation on the Upper Layers of the Biofilm δ (nm) | Density Reduction (% of Control) | |
|---|---|---|---|---|
| Control | 45.2 ± 1.9 | - | 11.0 ± 1.4 | - |
| AmB (1) | 37.1 ± 1.6 | 17.7 | 13.1 ± 2.6 | 1.19 |
| 4b | 16.2 ± 1.2 | 64.4 | 31.9 ± 8.0 | 65.5 |
| POPC/Chol (67/33 mol%) | POPC/Erg (67/33 mol%) | RFErg/RFChol | |||||
|---|---|---|---|---|---|---|---|
| Polyene | RFmax, % | t1, min | t2, min | RFmax, % | t1, min | t2, min | |
| 1 | 6 ± 1 | 0.6 ± 0.3 | 12.4 ± 8.1 | 9 ± 1 | 0.3 ± 0.1 | 5.8 ± 1.1 | 1.5 ± 0.4 |
| 4a | 22 ± 3 | 0.2 ± 0.1 | 3.6 ± 1.3 | 28 ± 4 | 0.2 ± 0.1 | 4.1 ± 0.9 | 1.3 ± 0.4 |
| 4b | 25 ± 5 | 1.9 ± 0.7 | 14.1 ± 1.6 | 18 ± 6 | 1.2 ± 0.5 | 5.5 ± 1.9 | 0.7 ± 0.4 |
| 4c | 14 ± 1 | 3.1 ± 0.6 | 9.1 ± 0.8 | 24 ± 4 | 0.2 ± 0.1 | 3.4 ± 0.8 | 1.7 ± 0.4 |
| 2 | 10 ± 2 | 0.9 ± 0.3 | 5.7 ± 1.9 | 19 ± 3 | 0.7 ± 0.1 | 5.4 ± 0.5 | 1.9 ± 0.1 |
| 5a | 4 ± 2 | – | – | 8 ± 3 | 0.2 ± 0.1 | 4.9 ± 0.8 | 2.0 ± 0.4 |
| 5b | 3 ± 1 | – | – | 6 ± 2 | – | – | 2.0 ± 0.3 |
| 5c | 9 ± 1 | 0.2 ± 0.1 | 8.1 ± 3.3 | 16 ± 3 | 0.7 ± 0.6 | 4.1 ± 0.9 | 1.8 ± 0.5 |
| 3 | 3 ± 1 | – | – | 4 ± 2 | – | – | 1.3 ± 0.3 |
| 6a | 2 ± 1 | – | – | 2 ± 2 | – | – | 1.0 ± 0.2 |
| 6b | 5 ± 2 | – | – | 24 ± 6 | 5.1 ± 1.1 | 26 ± 3 | 4.8 ± 1.5 |
| 6c | 5 ± 2 | – | – | 5 ± 2 | – | – | 1.0 ± 0.2 |
| Compounds | Lipid/Polyene Ratio | ΔTm, °C | ΔT1/2, °C |
|---|---|---|---|
| AmB (1) | 50:1 | −0.1 ± 0.1 | 0 |
| 10:1 | 0.2 ± 0.1 | 0.3 ± 0.1 | |
| 4a | 50:1 | 0 | 0 |
| 10:1 | 0 | 0 | |
| 4b | 50:1 | 0 | 0 |
| 10:1 | 0 | 0 | |
| 4c | 50:1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| 10:1 | 0.3 ± 0.1 | 2.6 ± 0.2 | |
| Nys (2) | 50:1 | −0.1 ± 0.1 | 0 |
| 10:1 | 0.4 ± 0.2 | 0.3 ± 0.1 | |
| 5a | 50:1 | 0 ± 0.1 | 0.2 ± 0.1 |
| 10:1 | 0.4 ± 0.2 | 0.4 ± 0.1 | |
| 5b | 50:1 | 0 ± 0.1 | 0.2 ± 0.1 |
| 10:1 | 0.2 ± 0.1 | 0.5 ± 0.1 | |
| 5c | 50:1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| 10:1 | 0.5 ± 0.2 | 0.7 ± 0.1 |
| Polyene | G200 mV, pS | τ, ms | Pop |
|---|---|---|---|
| AmB | 31.7 ± 2.1 | 23 ± 4 | 0.49 ± 0.09 |
| 4a | 20.5 ± 1.1 | 6 ± 2 | 0.33 ± 0.05 |
| 4b | 19.2 ± 2.2 | 6 ± 1 | 0.29 ± 0.07 |
| 4c | 19.8 ± 1.8 | 7 ± 2 | 0.31 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omelchuk, O.; Tevyashova, A.; Efimova, S.; Grammatikova, N.; Bychkova, E.; Zatonsky, G.; Dezhenkova, L.; Savin, N.; Solovieva, S.; Ostroumova, O.; et al. A Study on the Effect of Quaternization of Polyene Antibiotics’ Structures on Their Activity, Toxicity, and Impact on Membrane Models. Antibiotics 2024, 13, 608. https://doi.org/10.3390/antibiotics13070608
Omelchuk O, Tevyashova A, Efimova S, Grammatikova N, Bychkova E, Zatonsky G, Dezhenkova L, Savin N, Solovieva S, Ostroumova O, et al. A Study on the Effect of Quaternization of Polyene Antibiotics’ Structures on Their Activity, Toxicity, and Impact on Membrane Models. Antibiotics. 2024; 13(7):608. https://doi.org/10.3390/antibiotics13070608
Chicago/Turabian StyleOmelchuk, Olga, Anna Tevyashova, Svetlana Efimova, Natalia Grammatikova, Elena Bychkova, George Zatonsky, Lyubov Dezhenkova, Nikita Savin, Svetlana Solovieva, Olga Ostroumova, and et al. 2024. "A Study on the Effect of Quaternization of Polyene Antibiotics’ Structures on Their Activity, Toxicity, and Impact on Membrane Models" Antibiotics 13, no. 7: 608. https://doi.org/10.3390/antibiotics13070608
APA StyleOmelchuk, O., Tevyashova, A., Efimova, S., Grammatikova, N., Bychkova, E., Zatonsky, G., Dezhenkova, L., Savin, N., Solovieva, S., Ostroumova, O., & Shchekotikhin, A. (2024). A Study on the Effect of Quaternization of Polyene Antibiotics’ Structures on Their Activity, Toxicity, and Impact on Membrane Models. Antibiotics, 13(7), 608. https://doi.org/10.3390/antibiotics13070608







