Chitosan-Integrated Curcumin–Graphene Oxide/Copper Oxide Hybrid Nanocomposites for Antibacterial and Cytotoxicity Applications
Abstract
:1. Introduction
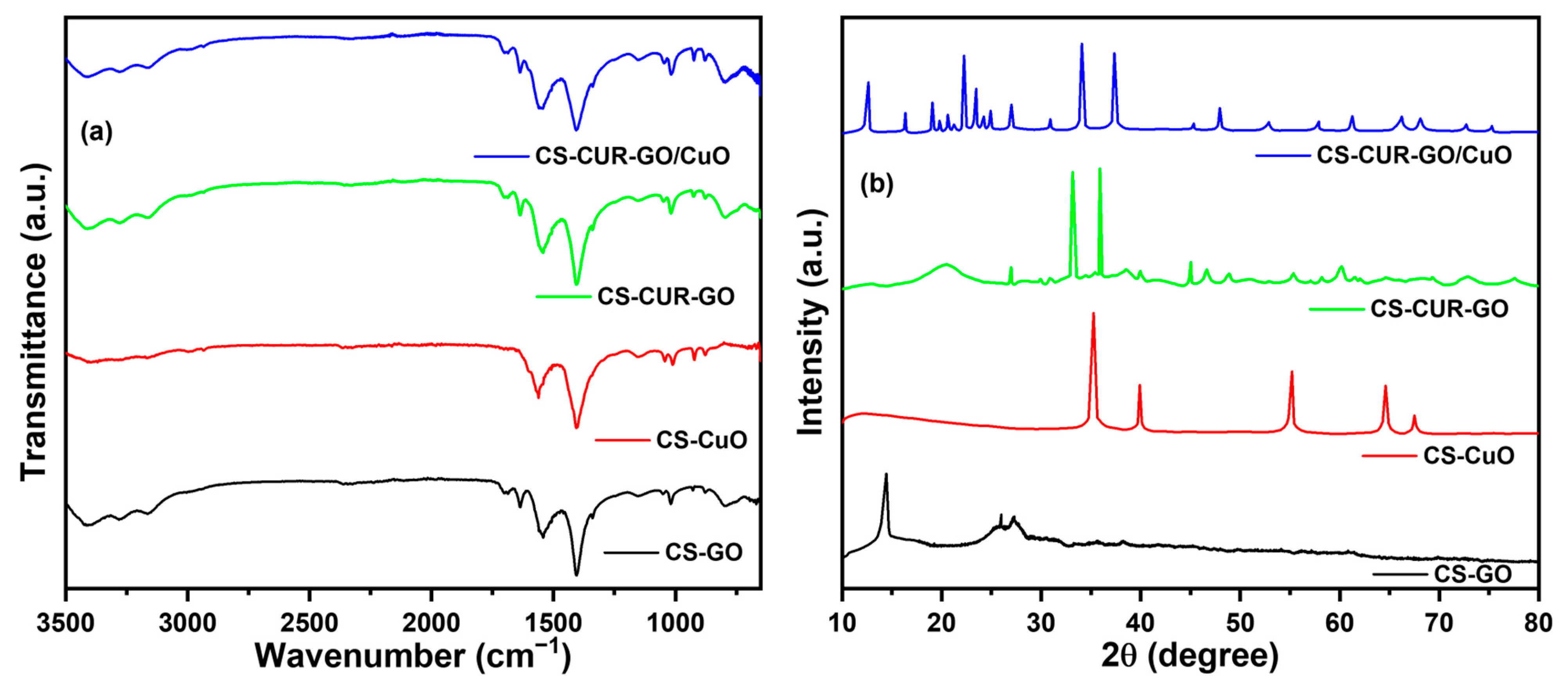
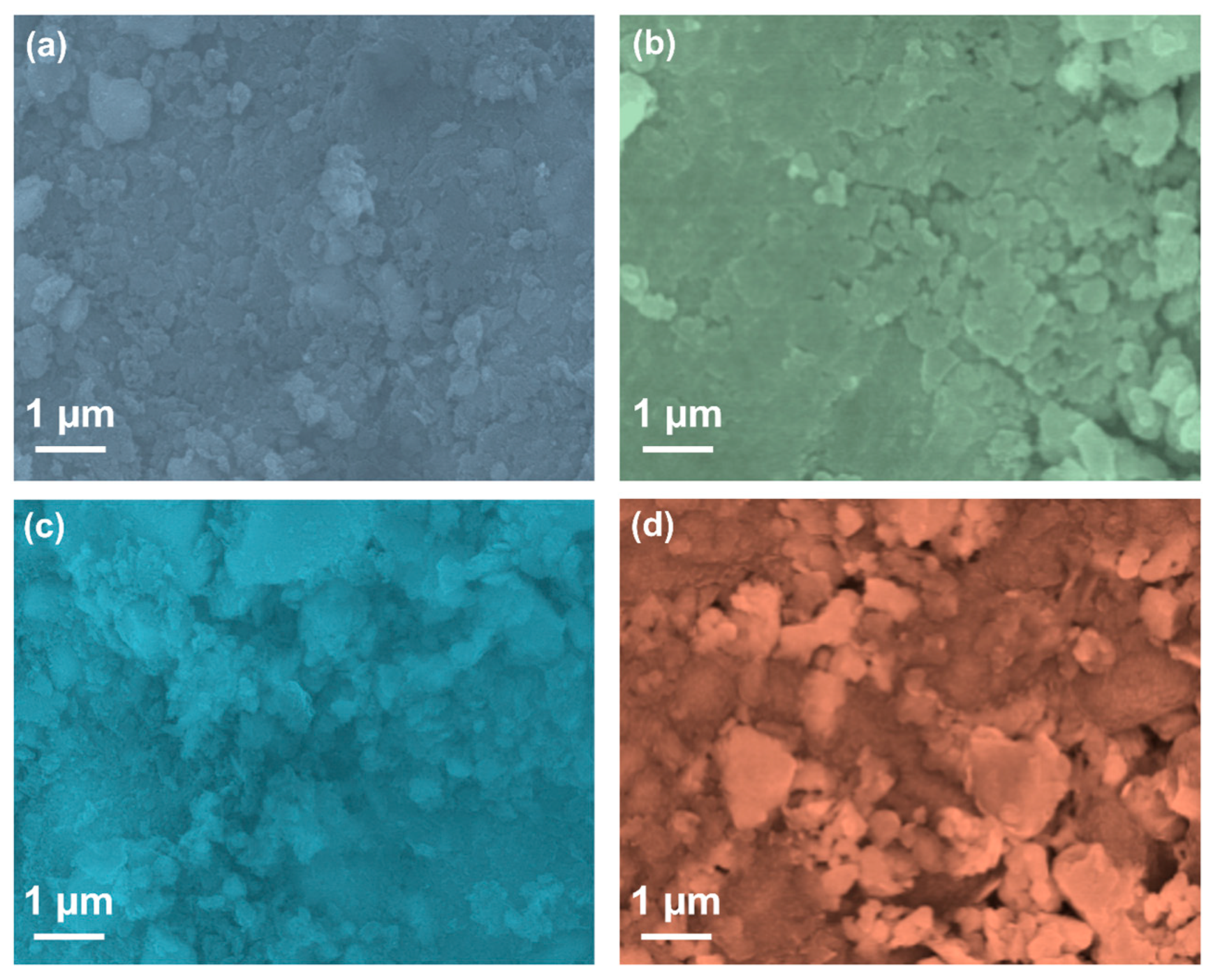
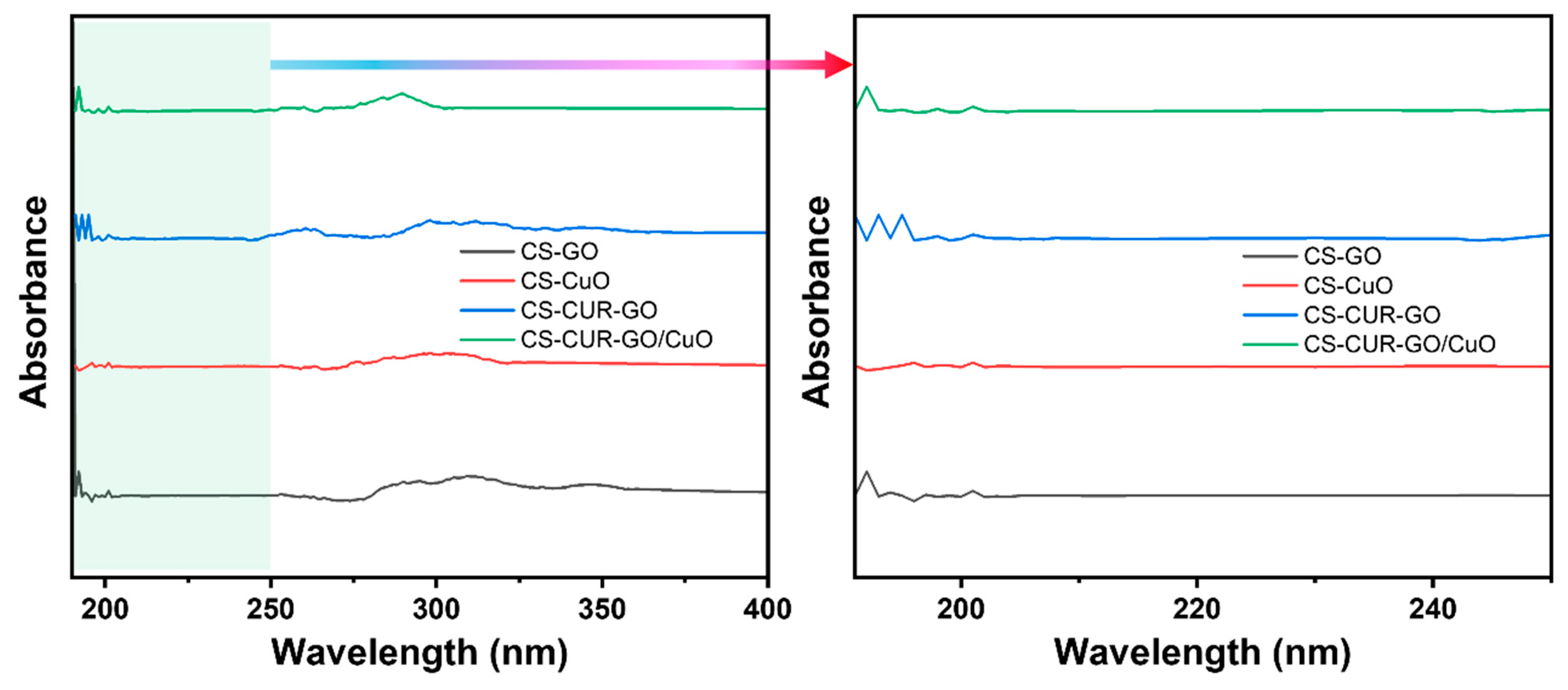
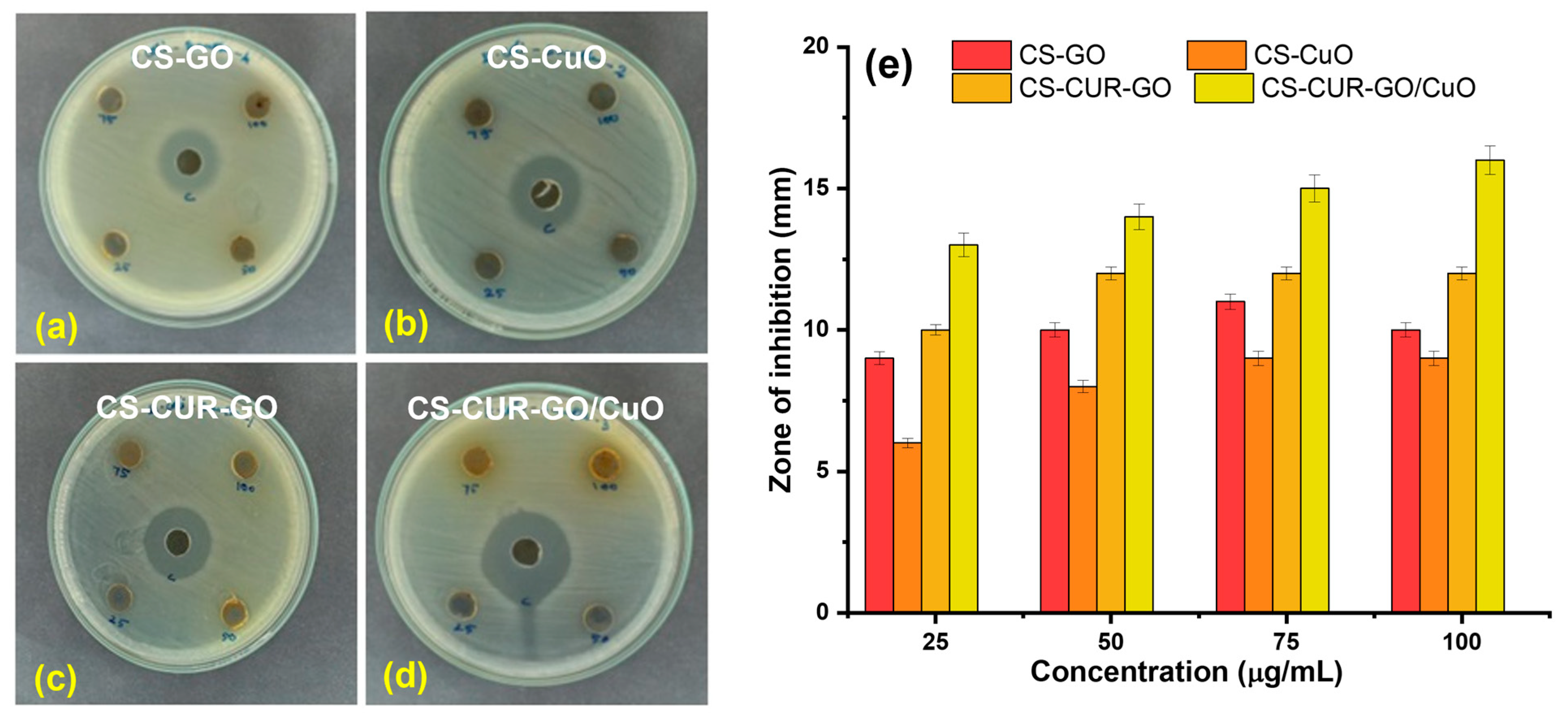
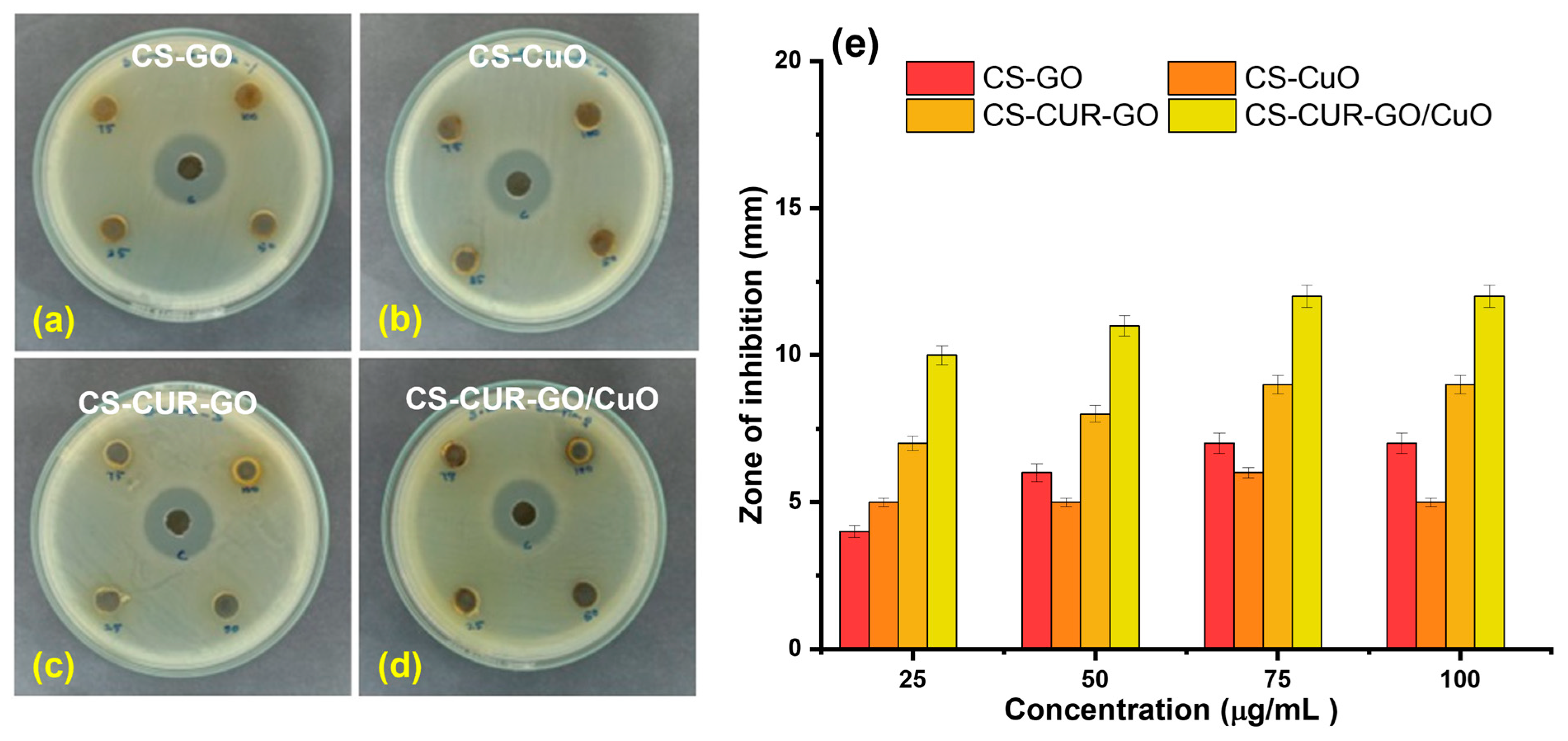
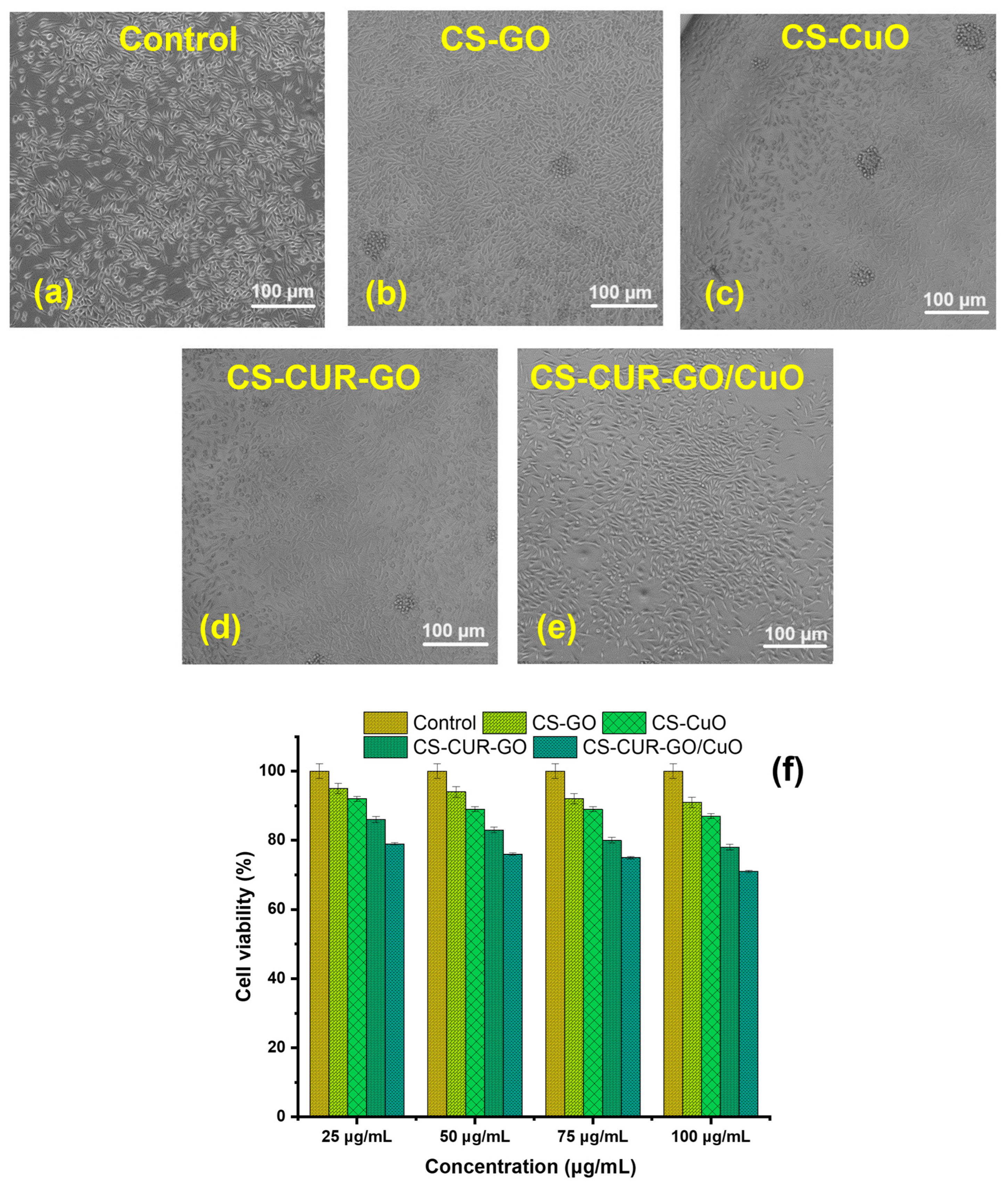
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Curcumin
4.3. Synthesis of Graphene Oxide
4.4. Synthesis of CS-CuO Nanocomposite
4.5. Synthesis of CS-GO-Based Nanocomposites
4.6. Physiochemical Characterization
4.7. In Vitro Drug Release
4.8. Antibacterial Studies
4.9. Cytotoxicity Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Moosavi Basri, S.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Packirisamy, R.G.; Govindasamy, C.; Sanmugam, A.; Venkatesan, S.; Kim, H.-S.; Vikraman, D. Synthesis of novel Sn1−xZnxO-chitosan nanocomposites: Structural, morphological and luminescence properties and investigation of antibacterial properties. Int. J. Biol. Macromol. 2019, 138, 546–555. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Sreekumar, S.; Wattjes, J.; Niehues, A.; Mengoni, T.; Mendes, A.C.; Morris, E.R.; Goycoolea, F.M.; Moerschbacher, B.M. Biotechnologically produced chitosans with nonrandom acetylation patterns differ from conventional chitosans in properties and activities. Nat. Commun. 2022, 13, 7125. [Google Scholar] [CrossRef]
- Ali, G.; Sharma, M.; Salama, E.-S.; Ling, Z.; Li, X. Applications of chitin and chitosan as natural biopolymer: Potential sources, pretreatments, and degradation pathways. Biomass Convers. Biorefinery 2024, 14, 4567–4581. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Sanmugam, A.; Shanthi, D.; Sairam, A.B.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kavitha, A.; Kim, H.-S.; Vikraman, D. Fabrication of chitosan/fibrin-armored multifunctional silver nanocomposites to improve antibacterial and wound healing activities. Int. J. Biol. Macromol. 2024, 257, 128598. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Al-Zahrani, S.S.; Bora, R.S.; Al-Garni, S.M. Antimicrobial activity of chitosan nanoparticles. Biotechnol. Biotechnol. Equip. 2021, 35, 1874–1880. [Google Scholar] [CrossRef]
- Bellier, N.; Baipaywad, P.; Ryu, N.; Lee, J.Y.; Park, H. Recent biomedical advancements in graphene oxide- and reduced graphene oxide-based nanocomposite nanocarriers. Biomater. Res. 2022, 26, 65. [Google Scholar] [CrossRef]
- Sanmugam, A.; Abbishek, S.; Kumar, S.L.; Sairam, A.B.; Palem, V.V.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Vikraman, D. Synthesis of chitosan based reduced graphene oxide-CeO2 nanocomposites for drug delivery and antibacterial applications. J. Mech. Behav. Biomed. Mater. 2023, 145, 106033. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, C.; Gan, L.; Owens, G.; Chen, Z. Antibacterial activity of reduced graphene oxide prepared by microbe. Mater. Today Sustain. 2023, 22, 100341. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M.; Ismael, S.; Al-Hazmi, N.E.; El-Sayyad, G.S.; Tantawy, H. Potential Impact of Reduced Graphene Oxide Incorporated Metal Oxide Nanocomposites as Antimicrobial, and Antibiofilm Agents Against Pathogenic Microbes: Bacterial Protein Leakage Reaction Mechanism. J. Clust. Sci. 2023, 34, 823–840. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of Curcumin Nanoparticles from Raw Turmeric Rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef]
- Chang, R.; Chen, L.; Qamar, M.; Wen, Y.; Li, L.; Zhang, J.; Li, X.; Assadpour, E.; Esatbeyoglu, T.; Kharazmi, M.S.; et al. The bioavailability, metabolism and microbial modulation of curcumin-loaded nanodelivery systems. Adv. Colloid Interface Sci. 2023, 318, 102933. [Google Scholar] [CrossRef]
- Hou, G.; Li, Y.; Wang, Q.; Zhang, H.; Liang, S.; Liu, B.; Shi, W. iRGD-grafted N-trimethyl chitosan-coated protein nanotubes enhanced the anticancer efficacy of curcumin and melittin. Int. J. Biol. Macromol. 2022, 222, 348–359. [Google Scholar] [CrossRef]
- Mujahid, M.H.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Park, M.N.; Sharangi, A.B.; Saeed, M.; Upadhye, V.J.; Kim, B. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed. Pharmacother. 2022, 155, 113791. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.-K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef]
- Elbadawy, M.; Hayashi, K.; Ayame, H.; Ishihara, Y.; Abugomaa, A.; Shibutani, M.; Hayashi, S.-M.; Hazama, S.; Takenouchi, H.; Nakajima, M.; et al. Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids. Biomed. Pharmacother. 2021, 142, 112043. [Google Scholar] [CrossRef]
- Zou, P.; Helson, L.; Maitra, A.; Stern, S.T.; McNeil, S.E. Polymeric Curcumin Nanoparticle Pharmacokinetics and Metabolism in Bile Duct Cannulated Rats. Mol. Pharm. 2013, 10, 1977–1987. [Google Scholar] [CrossRef]
- Hu, L.; Jia, Y.; Niu, F.; Jia, Z.; Yang, X.; Jiao, K. Preparation and Enhancement of Oral Bioavailability of Curcumin Using Microemulsions Vehicle. J. Agric. Food Chem. 2012, 60, 7137–7141. [Google Scholar] [CrossRef]
- Sinha, S.; Kumar, R.; Anand, J.; Gupta, R.; Gupta, A.; Pant, K.; Dohare, S.; Tiwari, P.; Kesari, K.K.; Krishnan, S.; et al. Nanotechnology-Based Solutions for Antibiofouling Applications: An Overview. ACS Appl. Nano Mater. 2023, 6, 12828–12848. [Google Scholar] [CrossRef]
- Packirisamy, R.G.; Govindasamy, C.; Sanmugam, A.; Karuppasamy, K.; Kim, H.-S.; Vikraman, D. Synthesis and Antibacterial Properties of Novel ZnMn2O4–Chitosan Nanocomposites. Nanomaterials 2019, 9, 1589. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Amal, R. Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts. ACS Nano 2011, 5, 7214–7225. [Google Scholar] [CrossRef]
- Sanmugam, A.; Sellappan, L.K.; Manoharan, S.; Rameshkumar, A.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kim, H.-S.; Vikraman, D. Development of chitosan-based cerium and titanium oxide loaded polycaprolactone for cutaneous wound healing and antibacterial applications. Int. J. Biol. Macromol. 2024, 256, 128458. [Google Scholar] [CrossRef]
- Jihad, K.M.; Roknabadi, M.R.; Mohammadi, M.; Goharshadi, E.K. Reduced graphene oxide/TiO2/NiFe2O4 nanocomposite as a stable photocatalyst and strong antibacterial agent. Sustain. Environ. Res. 2023, 33, 43. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Park, H.J.; Kim, S.-I.; Thaiyan, M. Magnetic, structural and optical behavior of cupric oxide layers for solar cells. J. Alloys Compd. 2016, 686, 616–627. [Google Scholar] [CrossRef]
- Sanmugam, A.; Vikraman, D.; Karuppasamy, K.; Lee, J.Y.; Kim, H.S. Evaluation of the corrosion resistance properties of electroplated chitosan-Zn1−xCuxO composite thin films. Nanomaterials 2017, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Ślosarczyk, A.; Klapiszewska, I.; Parus, A.; Balicki, S.; Kornaus, K.; Gapiński, B.; Wieczorowski, M.; Wilk, K.A.; Jesionowski, T.; Klapiszewski, Ł. Antimicrobial action and chemical and physical properties of CuO-doped engineered cementitious composites. Sci. Rep. 2023, 13, 10404. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Jadhav, S.; Gaikwad, S.; Nimse, M.; Rajbhoj, A. Copper Oxide Nanoparticles: Synthesis, Characterization and Their Antibacterial Activity. J. Clust. Sci. 2011, 22, 121–129. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, C.; Ji, F.; Chen, M.; Zhong, Z.; Xing, W. Micro-Octahedron Cu2O-Based Photocatalysis-Fenton for Organic Pollutant Degradation: Proposed Coupling Mechanism in a Membrane Reactor. Ind. Eng. Chem. Res. 2022, 61, 7255–7265. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Bidram, E.; Zarrabi, A.; Amini, A.; Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 2020, 10, 18052. [Google Scholar] [CrossRef]
- Rochman, R.A.; Wahyuningsih, S.; Ramelan, A.H.; Hanif, Q.A. Preparation of nitrogen and sulphur Co-doped reduced graphene oxide (rGO-NS) using N and S heteroatom of thiourea. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012119. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Guo, H.-L.; Wang, X.-F.; Qian, Q.-Y.; Wang, F.-B.; Xia, X.-H. A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Wampande, E.M.; Moja, T.N.; Nxumalo, E.; Maaza, M.; Sackey, J.; Ejobi, F.; Kirabira, J.B. Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 2021, 11, 4116. [Google Scholar] [CrossRef] [PubMed]
- Ananda Murthy, H.C.; Zeleke, T.D.; Tan, K.B.; Ghotekar, S.; Alam, M.W.; Balachandran, R.; Chan, K.-Y.; Sanaulla, P.F.; Anil Kumar, M.R.; Ravikumar, C.R. Enhanced multifunctionality of CuO nanoparticles synthesized using aqueous leaf extract of Vernonia amygdalina plant. Results Chem. 2021, 3, 100141. [Google Scholar] [CrossRef]
- Silva Filho, J.C.; Venancio, E.C.; Silva, S.C.; Takiishi, H.; Martinez, L.G.; Antunes, R.A. A thermal method for obtention of 2 to 3 reduced graphene oxide layers from graphene oxide. SN Appl. Sci. 2020, 2, 1450. [Google Scholar] [CrossRef]
- Aragaw, B.A. Reduced graphene oxide-intercalated graphene oxide nano-hybrid for enhanced photoelectrochemical water reduction. J. Nanostruct. Chem. 2020, 10, 9–18. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 5282. [Google Scholar] [CrossRef]
- Xie, H.; Ma, L.; Li, Y.; Fu, J.; Li, Z.; Yu, X.; Gao, Q. Preparation and Characterizations of Curcumin Protection and Delivery System Using Linear Dextrin. Compounds 2022, 2, 353–366. [Google Scholar] [CrossRef]
- Dhanasekaran, V.; Mahalingam, T.; Rajendran, S.; Rhee, J.K.; Eapen, D. Electroplated cuo thin films from high alkaline solutions. J. New Mater. Electrochem. Syst. 2012, 15, 49–55. [Google Scholar] [CrossRef]
- Dhanasekaran, V.; Mahalingam, T. Surface modifications and optical variations of (−1 1 1) lattice oriented CuO nanofilms for solar energy applications. Mater. Res. Bull. 2013, 48, 3585–3593. [Google Scholar] [CrossRef]
- Abdolrahimi, M.; Seifi, M.; Ramezanzadeh, M.H. Study the effect of acetic acid on structural, optical and mechanical properties of PVA/chitosan/MWCNT films. Chin. J. Phys. 2018, 56, 221–230. [Google Scholar] [CrossRef]
- Aisyah, N.; Rifai, H.; Maisonneuve, C.B.D.L.; Oalmann, J.; Forni, F.; Eisele, S.; Phua, M.; Putra, R. Scanning electron microscope (SEM) imaging and analysis of magnetic minerals of lake Diatas peatland section DD REP B 693. J. Phys. Conf. Ser. 2020, 1481, 012025. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Mohamed, A.; Ali Abdelnaby, M. Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of end-life ultrafiltration polymer nanocomposite membranes. Chem. Eng. J. 2022, 428, 131181. [Google Scholar] [CrossRef]
- Khan, A.M.; Abid, O.u.R.; Mir, S. Assessment of biological activities of chitosan Schiff base tagged with medicinal plants. Biopolymers 2020, 111, e23338. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Tantawy, H.; Hashem, A.H. Promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Adv. 2021, 11, 25961–25975. [Google Scholar] [CrossRef]
- Choudhary, P.; Das, S.K. Bio-Reduced Graphene Oxide as a Nanoscale Antimicrobial Coating for Medical Devices. ACS Omega 2019, 4, 387–397. [Google Scholar] [CrossRef]
- Pandey, A.; Chauhan, P. Chapter 1—Functionalized graphene nanomaterials: Next-generation nanomedicine. In Functionalized Carbon Nanomaterials for Theranostic Applications; Mallakpour, S., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–18. [Google Scholar]
- Kazemi-Andalib, F.; Mohammadikish, M.; Divsalar, A.; Sahebi, U. Hollow microcapsule with pH-sensitive chitosan/polymer shell for in vitro delivery of curcumin and gemcitabine. Eur. Polym. J. 2022, 162, 110887. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Assis, M.; da Silva, J.S.; Gonçalves, M.O.; de Almeida Rodolpho, J.M.; de Lima Fragelli, B.D.; Corte, A.B.P.; Ribeiro, L.K.; Teodoro, M.D.; de Freitas Anibal, F.; de Sousa, C.P.; et al. Bactericidal activity of Ag4V2O7/β-AgVO3 heterostructures against antibiotic-resistant Klebsiella pneumoniae. Biomater. Adv. 2022, 141, 213097. [Google Scholar] [CrossRef]
- Jin, C.-Y.; Zhu, B.-S.; Wang, X.-F.; Lu, Q.-H. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmugam, A.; Sellappan, L.K.; Sridharan, A.; Manoharan, S.; Sairam, A.B.; Almansour, A.I.; Veerasundaram, S.; Kim, H.-S.; Vikraman, D. Chitosan-Integrated Curcumin–Graphene Oxide/Copper Oxide Hybrid Nanocomposites for Antibacterial and Cytotoxicity Applications. Antibiotics 2024, 13, 620. https://doi.org/10.3390/antibiotics13070620
Sanmugam A, Sellappan LK, Sridharan A, Manoharan S, Sairam AB, Almansour AI, Veerasundaram S, Kim H-S, Vikraman D. Chitosan-Integrated Curcumin–Graphene Oxide/Copper Oxide Hybrid Nanocomposites for Antibacterial and Cytotoxicity Applications. Antibiotics. 2024; 13(7):620. https://doi.org/10.3390/antibiotics13070620
Chicago/Turabian StyleSanmugam, Anandhavelu, Logesh Kumar Sellappan, Abbishek Sridharan, Swathy Manoharan, Ananda Babu Sairam, Abdulrahman I. Almansour, Subha Veerasundaram, Hyun-Seok Kim, and Dhanasekaran Vikraman. 2024. "Chitosan-Integrated Curcumin–Graphene Oxide/Copper Oxide Hybrid Nanocomposites for Antibacterial and Cytotoxicity Applications" Antibiotics 13, no. 7: 620. https://doi.org/10.3390/antibiotics13070620




